Translate this page into:
10% Thioglycolic Acid Peel in the Treatment of Pigmented Purpuric Dermatoses: A Pilot Study
Address for correspondence: Dr. Arunima Ray, Department of Dermatology, IMS and SUM Hospital, Bhubaneswar, Odisha, India. E-mail: arunima.roma@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Pigmentary purpuric dermatosis (PPD) is a chronic dyspigmentation characterized by reddish-brown, irregular maculae with dermal hemosiderin deposition, usually affecting the legs. The –SH group in the thioglycolic acid (TGA) strongly binds to iron molecule, solubilizes it, and clears the pigment. We conducted a longitudinal, right-left leg study for assessing the effectiveness and side effects of TGA 10% peel in treating PPD. For preparation of 10% TGA peel, 80% TGA was diluted with distilled water to 10% concentration by mixing 0.5 mL of acid with 3.5 mL of water before every peel session. The peel was applied on the left leg, weekly for 6 weeks. Assessment was done at baseline and at weeks 3 and 6. Any improvement was noted by the patient and another independent dermatologist. The right leg was untreated. Any side effects during peel application and afterwards were noted. According to the patient assessment, 4/10 patients observed mild improvement, 5/10 patients had moderate improvement, and only a single patient had marked improvement. In the physician assessment, 2/10 patients had >50% improvement, 5/10 patients had 30–40% improvement, and 3/10 patients had 10–20% improvement. Side effects included slight burning during application and foul odor. A single patient had intense erythema and mild swelling of the leg after peel application. 10% TGA is effective in the partial clearance of PPD dyspigmentation with weekly sessions for 6 weeks without any serious side effects.
Keywords
10% Thioglycolic acid
chemical peeling
pigmented purpuric dermatoses
PPD
INTRODUCTION
Pigmented purpuric dermatoses (PPD) are chronic vascular dermatoses which appear as multiple pinpoint petechiae or ecchymoses and occasionally as papules. Lesional color is due to dermal deposition of hemosiderin and may appear purple, red, or brown to yellow depending on the timeline of deposit. Though benign, it can be a cause for considerable cosmetic concern.[1] Factors that may accelerate the causation of purpura are capillary fragility, longstanding venous hypertension, exercise, prolonged standing, and possible contact allergy.[2]
Though a common impairment, there is no single effective treatment available for PPD, and newer modalities of treatment need to be explored. There are very few reported cases using 10% thioglycolate gel in pigmentary dermatoses. Thioglycolic acid (TGA) is effective in treating hemosiderotic hyperchromias because of its ability to chelate the iron in the hemosiderin. We conducted a pilot study with the objective of assessing the efficacy of TGA in the clearance of PPD and to understand the possible side effects.
MATERIALS AND METHODS
An open-label, prospective, left-right comparative, pilot study was conducted at a tertiary hospital in Eastern India. Prior to conducting the study, permission of the Institutional Ethical Committee was taken. Patients presenting with bilateral PPD over the legs were included in the study after obtaining an informed written consent for the procedure.
We excluded patients who were on anticoagulants, had varicosities, had local inflammation or infections, with any known hypersensitivity to TGA, pregnant or lactating, and any history of pulmonary disease or acute respiratory disease. The peeling sessions were conducted as office procedures in the outpatient department.
Photographic and dermoscopic images were recorded at baseline and at weeks 3 and 6. Dermoscopy was done from the same site with a Dermlite DL3 attached to an iPhone X. For preparation of 10% thioglycolate peel, 80% TGA was diluted with distilled water to 10% concentration, by mixing 0.5 mL of acid with 3.5 mL of water. The peel was freshly prepared before every session [Figure 1]. It was applied on the left leg only. The patients were allowed to use bland emollients on both the legs. Prior to applying the peel and taking photographs, the patient was asked to wash his legs thoroughly. The peel was uniformly applied in a single layer over the affected areas only and kept for 20 min, after which the peel was wiped off with a saline gauze and the patient was asked to wash their legs. Any side effects during and after the peel application were recorded. Any improvement was assessed by the patient and an independent dermatologist. Patient’s assessment of improvement was recorded as mild/moderate/marked, and the observer assessment was in terms of percentage decrease in pigment color. Final assessments were done at the end of study period for 6 weeks.
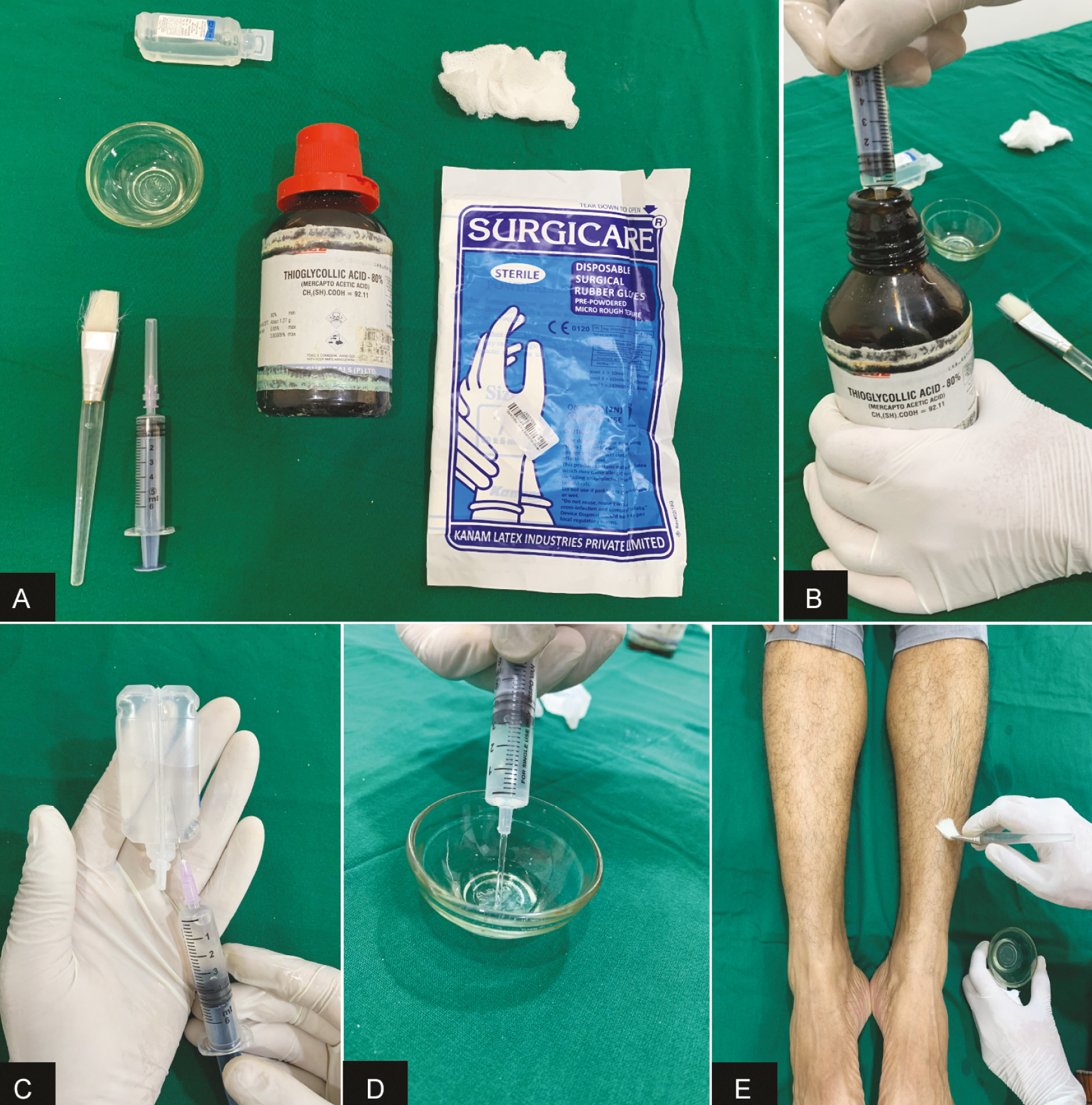
- (A) Components of 10% thioglycolate peel. (B) 0.5 mL of 80% TGA is drawn with a disposable 5 mL syringe. (C) In the same syringe, 3.5 mL of distilled water is taken. (D) The mixture is poured into a sterile glass bowl. (E) Using a brush, the mixture is applied in a single layer over the desired area.
RESULTS
A total of 10 patients completed the study [Table 1]. According to patients’ self-assessment, four (40% patients) had mild improvement, five had moderate improvement (50% patients), and a single patient had marked improvement. According to independent physician’s assessment, two patients had >60% improvement, five had 30–60% improvement, and three had <30% improvement [Figures 2 and 3]. Graphic illustration of improvement is seen in Figure 4.
| No. | Patient initials | Age/sex | Duration | Patients’ self-assessment | Physician’s assessment | ||
|---|---|---|---|---|---|---|---|
| Follow-up at week 3 | Follow-up at week 6 | Follow-up at week 3 (%) | Follow-up at week 6 (%) | ||||
| 1 | SM | 54/F | 4 months | None | Mild | <30 | <30 |
| 2 | NSM | 48/M | 1 year | None | Moderate | <30 | 30–60 |
| 3 | BCR | 43/M | 6 months | Mild | Moderate | 30–60 | 30–60 |
| 4 | SM | 28/F | 8 months | Mild | Moderate | <30 | 30–60 |
| 5 | AS | 31/M | 1 year | None | Moderate | 30–60 | >60 |
| 6 | PKS | 41/M | 6 months | Mild | Mild | <30 | <30 |
| 7 | MB | 35/F | 2 years | Mild | Moderate | <30 | 30–60 |
| 8 | GR | 55/F | 3 years | Moderate | Marked | 30–60 | >60 |
| 9 | KCP | 38/M | 4 months | None | Mild | <30 | 30–60 |
| 10 | SRK | 36/F | 2 years | None | Mild | <30 | <30 |
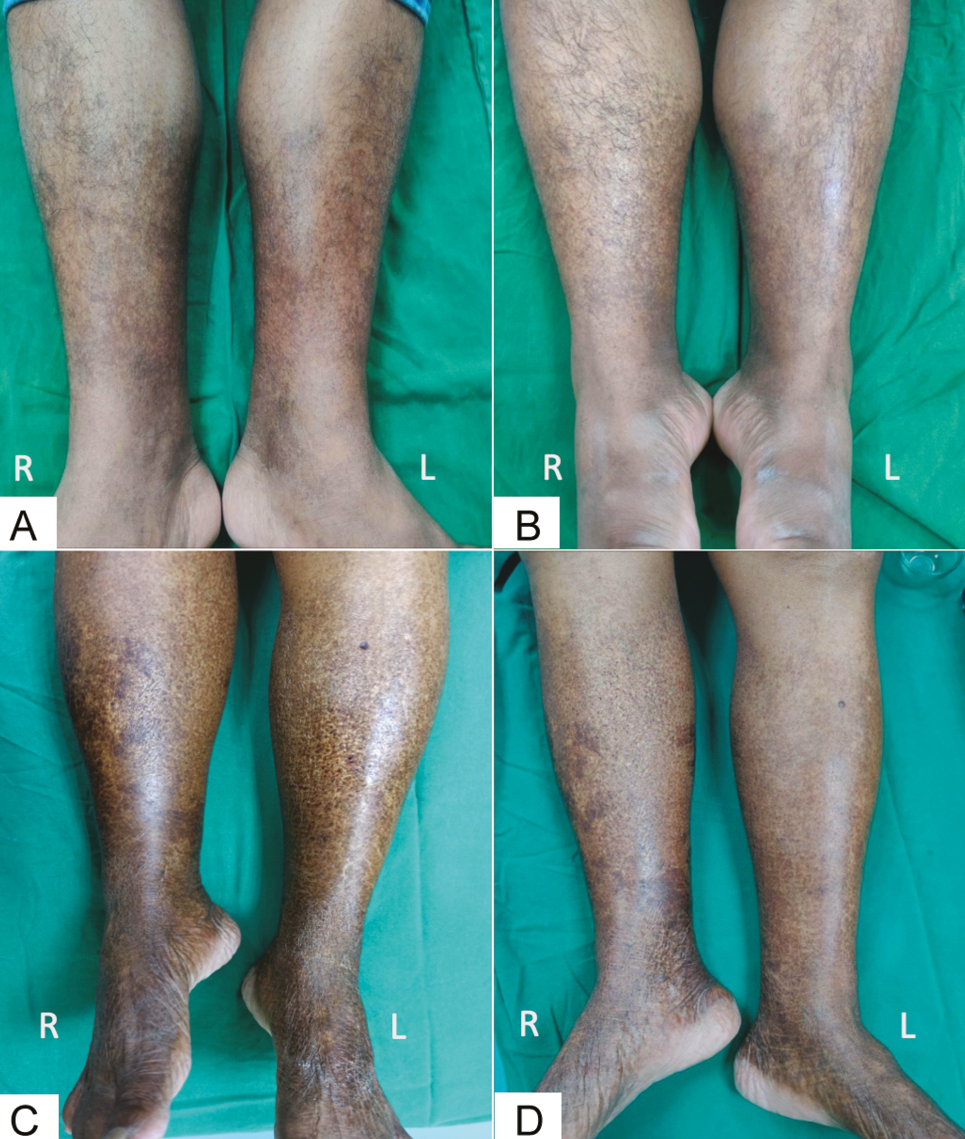
- Baseline photograph (A and C) and post-peel photograph (B and D) at week 6 showing >60% improvement in two patients
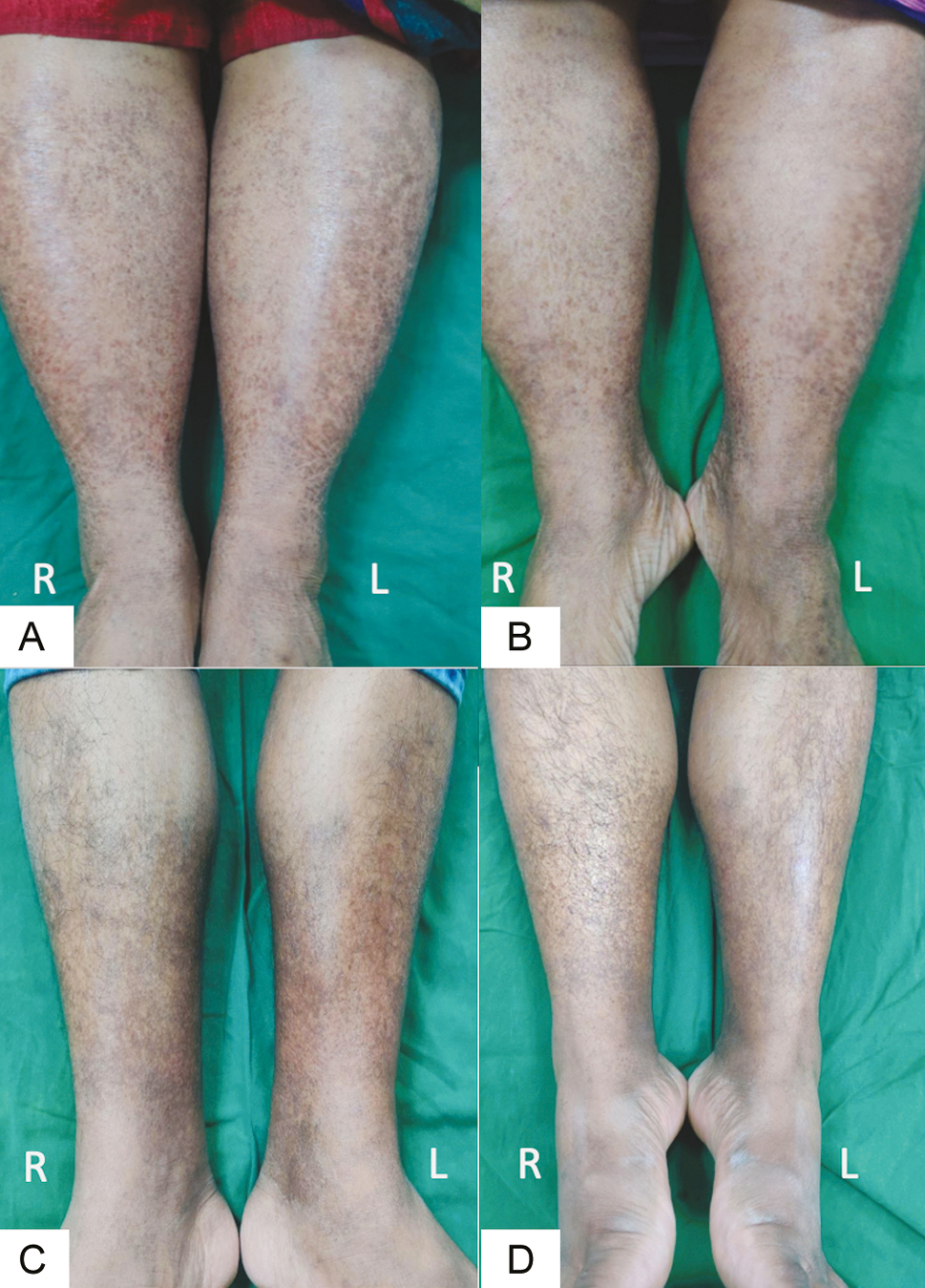
- Baseline photograph (A and C) and post-peel photograph (B and D) at week 6 showing 30–60% improvement in two patients
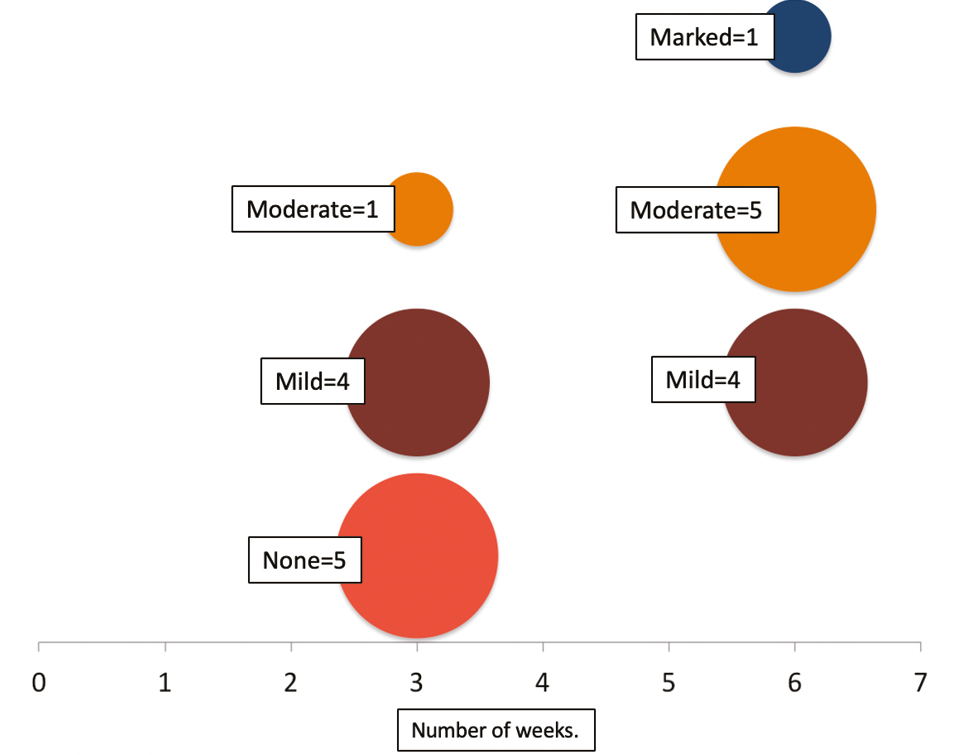
- Bubble chart illustrating the improvement in patient’s assessment
Side effects observed were slight burning sensation during peel application in two patients, which lasted about 2 h after the peel was cleaned. Eight out of 10 patients complained of a tolerable foul odor during the peel application and 2 patients complained of persistent foul smell from their clothes for few hours after procedure. All the patients had superficial desquamation on the peeling site, starting 2 days after the procedure and until the next peeling date, a week after. In her last session, one patient had erythema, pain, and swelling of the peeling site for 5 days after the procedure and this resolved with topical mometasone applied twice daily for 3 days.
Dermoscopic assessment showed decrease in red dots and intensity of brownish pigment at 6 weeks in all the patients with a noticeable change in patients who had moderate and marked improvement. An increase in scaling on dermoscopy was seen in the post-peel period in all the patients [Figure 5].
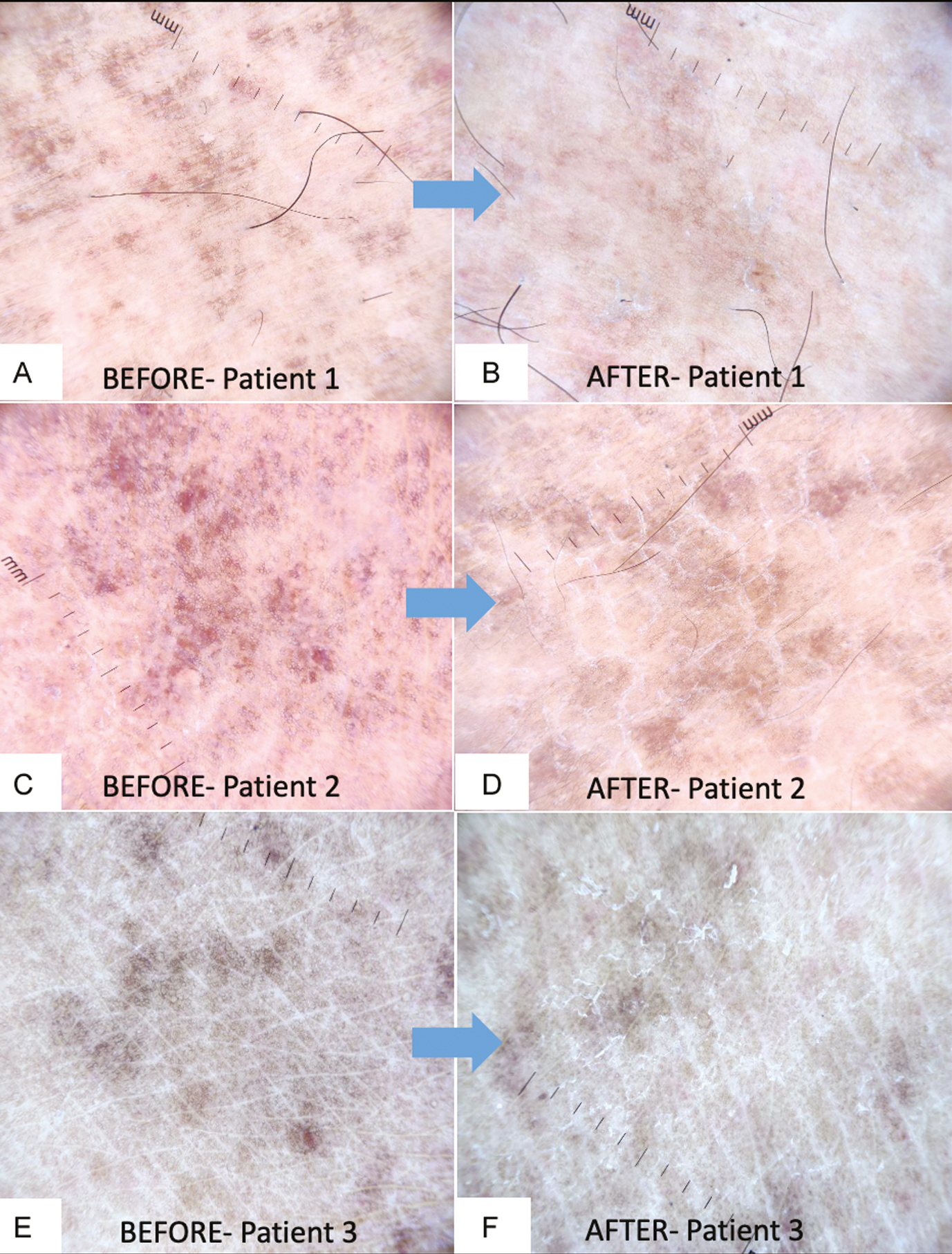
- Dermoscopic comparison of baseline features (A, C, E) and final post-peel features (B, D, F) shows a decrease in red dots, lightening of brownish background, and increased scaling (10×, non-polarized mode)
DISCUSSION
TGA (active ingredient: 2-mercaptoacetic acid) causes depigmentation in spots of ferric origin. Unlike chelators, like EDTA and desferrioxamine, TGA has a direct solubilizing effect on the dermal iron. The –SH group in TGA also shows a strong affinity for iron ions in the skin which required fewer applications with limited side effects. This puts TGA at an advantage from other solubilisers like glycolic acid and trichloro-acetic acid, which need prolonged and multiple applications with more side effects. Reported side effects of topical TGA are rare cases of low intensity burns and erythema. Our patients did not show any significant side effects and had good compliance with weekly peel sessions. The single instance of acute dermatitis in our study was well controlled with topical mometasone.[3]
TGA peel is commercially unavailable in India and topical TGA can be safely used at less than 15% concentration. Successful use of 10% TGA in PPD has been demonstrated in a single case by Hammerschmidt et al., in which improvement was seen in five to six fortnightly sessions, without any side effects except mild desquamation.[4]
Costa et al.[5] also demonstrated the efficacy of 10% TGA in treating periorbital hypermelanosis. Long-term use of thioglycolates as a cosmetic industry additive has proven it to be safe and non-toxic with a safety margin in concentrations from 12% to 15%.
A single case report of systemic toxicity showed pulmonary edema, acute respiratory distress syndrome, and multisystem failure. However, this was by inhalation of concentrated thioglycolic acid (>80%) and not dermal contact.
Our study uses thioglycolic acid at a much lower concentration (10%) and careful inclusion of only healthy consenting adult subjects with detailed post-peel counseling will prevent any serious side effects.[6]
CONCLUSION
Our study demonstrates that 10% TGA is effective and safe in clearing PPD with weekly sessions for 6 weeks. However, like use of peels in other pigmentary dermatoses, the results are variable and marked improvement may be seen infrequently. Further studies with more peeling sessions and combination of other topical and oral treatment modalities will help develop a more effective treatment plan.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- A cross-sectional study of clinico-etiological profile and associated comorbidities in Indian patients of pigmented purpuric dermatoses. Indian J Dermatol. 2020;65:187-92.
- [Google Scholar]
- Pigmented purpuric dermatitis [Updated December 13, 2020] 2021. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; Available from: https://www.ncbi.nlm.nih.gov/books/NBK519562/
- [Google Scholar]
- The use of thioglycolic acid as depigmenting agent. Patent WO/1998/047466, October 29, 1998. Available from: http://www.freepatentsonline.com/WO1998047466.html
- Peeling de ácido tioglicólico na doença de Schamberg. Surg Cosmet Dermatol. 2013;5:165-8.
- [Google Scholar]
- 10% thioglycolic acid gel peels: A safe and efficient option in the treatment of constitutional infraorbital hyperpigmentation. Surg Cosmet Dermatol. 2010;2:29-33.
- [Google Scholar]
- Thioglycolic acid: Understanding the risk of specific chemicals of interest [Internet] 2014. Thioglycolic Acid Risk. Available from: https://www.prevor.com/en/understanding-the-risk-of-specific- chemicals-of-interest-thioglycolic-acid
- [Google Scholar]






