Translate this page into:
Treatment of Lipoma by Injection Lipolysis
Address for correspondence: Dr. Soni Nanda, 40 Prayag Appartments, Vasundara Enclave, Delhi-110 096, India. E-mail: soni@shineandsmile.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Injection lipolysis or lipodissolve is the practice of injecting phosphatidyl choline/ sodium deoxycholate (PDC/DC) compounds in the subcutaneous fat. Though this practice is being used extensively for nonsurgical contouring of body and dissolving localized collections of excess fat, it's use as a treatment modality for lipomas needs further evaluation. We present a case where this technique was used for treating a lipoma, with no recurrence after 9 months of follow up. Injection lipolysis as a treatment modality for lipomas needs to be evaluated for safety and efficacy in trials on larger population. This could prove to be a very valuable adjunct to the current practice of excision, if done by a trained person in a properly selected patient. Also the side effects and the controversies regarding this procedure have been discussed in detail in the present paper.
Keywords
Deoxycholate
injection lipolysis
phophatidylcholine
INTRODUCTION
Lipomas are a commonly encountered entity in dermatology practice. Multiple lipomas in a patient are also frequently encountered. Injection lipolysis is a rapidly growing technique for dissolving fat for non-surgical body contouring.[1] A case of solitary lipoma, treated with phosphatidylcholine/sodium deoxycholate without any recurrence even after 9 months is hereby presented. A review of literature regarding this controversial technique is also included.
CASE REPORT
A 35-year-old healthy male with complaints of a gradually increasing growth of 9 months duration on right wrist presented to Dermatology OPD. The lesion became more prominent on movement of wrist. There was a history of similar growth in the father. No abnormality was detected on general physical examination. Local area examination revealed a 3 × 3 cm, soft to firm, hemispherical, non-tender, mobile lesion on the medial aspect of right wrist [Figure 1]. Clinically, a diagnosis of lipoma was made and confirmed by FNAC. The patient was not open to any surgical intervention and was given the option of injection lipolysis. After discussing the possible side effects like hyperpigmentation, skin loss, prolonged pain, swelling, or tenderness, late onset hives or itching and skin contour irregularities in detail, an informed written consent for three sessions at 6-8 weeks gap was taken.
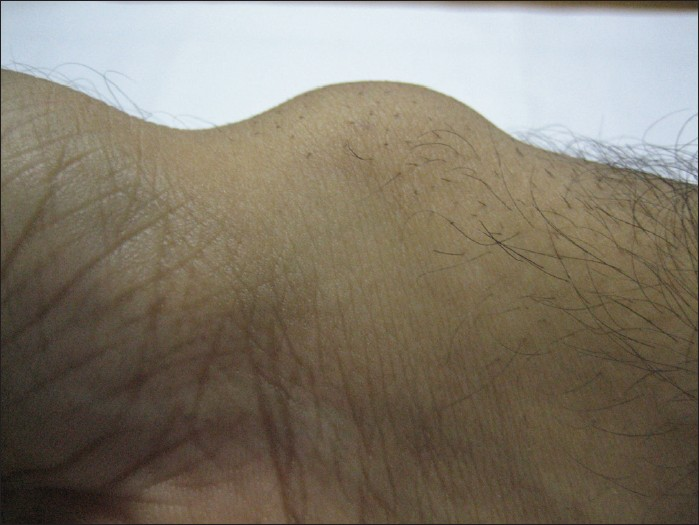
- A firm, mobile, 3 × 3 cm growth on right wrist
The lesion was cleaned and draped. The area was divided into small grids (1.5 cm apart) [Figure 2]. Solution of phosphatidyl choline and deoxycholate (50 mg/ml) was filled in insulin syringes (4 injections of 16 units of formula). The pinch and pull technique was used. The lipoma was pulled away from the underlying structures and four units were injected in each of four grids (total of 16 units). Care was taken not to inject any material while withdrawing the needle to avoid any intradermal deposition of the drug. The injections were not perceived as painful by the patient. Immediately after the injections there was swelling, redness and burning sensation which persisted for around half an hour [Figure 3]. No external compression was used and gradually the swelling settled. In the next 48 h, the lesion shrunk to half of its original size and disappeared completely within next 3 weeks, after the first injection itself. The patient is under follow up for the last 9 months with no evidence of any recurrence [Figure 4].

- Grids of 1.5 cm
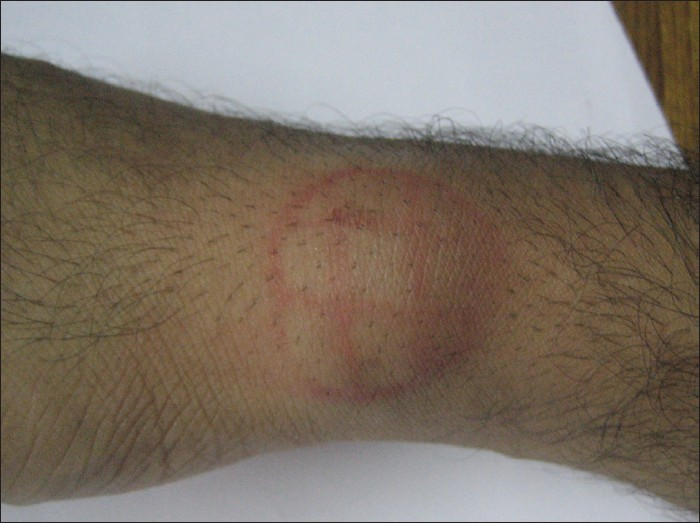
- Swelling and redness immediately after the injection
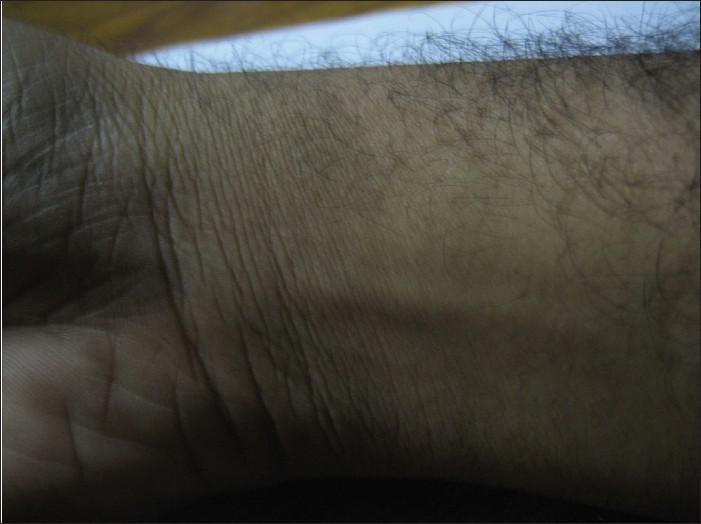
- Follow up at 9 months after the injection
DISCUSSION
Injection lipolysis or lipodissolve is the practice of injecting phosphatidyl choline/sodium deoxycholate (PDC/DC) compounds in the subcutaneous fat.[1] Though the use of this technique for non surgical body contouring (e.g., reduction of areas of localized fat like double chin, love handles and abdominal fat) has been increasing rapidly over the past few years, very few studies have been carried out to determine its efficacy for dissolution of commonly encountered lipomas.[23] This practice is growing rapidly but there are still controversies attached with this technique.[4–6]
Various injection techniques have been described including use of a syringe (as was done in our case), use of multi-injector device such as mesorelle, and use of mesogun. Indications for injection lipolysis include small, soft areas of localized fat, lipomas,[23] post-liposuction deformities, skin contour irregularities due to traumatic fat necrosis, cellulite, post-fat grafting deformities, and depressed scar with adjoining areas of protruding fat. Larger fat deposits (>500 ml), fat pad more than 3 cm. thick,[1] fibrous fat or thinner fat deposits spread over a broad surface area should not be treated with this technique.
PDC and DC are the commonly used compounds. The exact role of PDC is not clear as DC is supposed to lyse the adipocytes by detergent action. Additives such as vasodilators (procaine, lidocaine, pentoxyphilline), local anaesthetics (bupivacaine) and vitamins such as alpha tocopherol or vitamin B complex are used in various formulas besides the PDC/DC. But none of these have been scientifically proven to improve the results. Currently the standard maximum dose of PDC per injection session is considered as 2500 mg. Average number of treatments required is 3 at a gap of 6-8 weeks.[1] However, in the present case only a single session was required.
In the past years many physicians worldwide have decried the use of injection lipolysis, claiming a lack of both scientific data and longevity of treatment experience. Source of controversy worldwide is the lack of safety and efficacy recognition by Food and Drug Administration (FDA), Medicines and Healthcare Products Regulatory Agency (MHRA), or any other health regulatory agency.[57] A ban on the use of Lipostabil in Brazil was a source of major controversy.[1] The side effects that can be encountered include poor outcome, atypical mycobacterial infection or an uneven contour. These injections could also cause skin loss, hematoma, muscle injury, or nerve damage if the injector is not aware of the underlying anatomy of the injection area. There have been reports of skin necrosis or multiple areas of skin ulceration and blistering after self injections. Intradermal injections of large amounts of formula can lead to skin loss. Majority of these side effects have been reported when the procedure was done by untrained individuals or in cases of self injections.
Bechara et al, studied the effect of lipolysis in cases of multiple familial lipomatosis in 2006. They achieved a reduction up to 45.8% after four injections at 6-8 weeks intervals.[2] In the current study, we have seen a complete dissolution with a single injection and no recurrence in 9 months. There is no scarring and can be used to effectively treat multiple lipomas where surgery may not be a viable option. Dermastabilion is available as a 5 ml ampule costing Rs. 500. It can be used for treating multiple lipomas at the same time. Hence, it would turn out to be a very cost-effective treatment modality.
Absolute contraindications include age <18 years, pregnancy, breast feeding, patient, on anti-coagulant therapy, current serious, or significant illness or active infection, known allergy to soy products, injections for breast reduction, unstable diabetic control, or impaired circulation, body mass index BMI>30, previous adverse reaction to this treatment, severe needle phobia, immunocompromised patients such as transplant recipients and those undergoing chemotherapy.[1] Vascular insufficiency should be ruled out.
CONCLUSION
If performed by a trained doctor in the right patient, injection lipolysis could prove to be a safe and cost effective treatment modality for lipomas. In the current case there was no recurrence even after 9 months. A study with larger number of patients is needed to establish this technique as a safe and effective treatment modality for lipomas. Also, a comparative study between PDC plus DC versus DC alone for lipolysis would help in clarifying the effect of PDC in adipocyte destruction.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Fat reduction using phosphatidylcholine/ sodium deoxycholate injections: Standard of practice. Aesth Plast Surg. 2008;32:858-72.
- [Google Scholar]
- Lipolysis of lipomas in patients with familial multiple lipomatosis: An ultrasonography-controlled trial. J Cutan Med Surg. 2006;10:155-9.
- [Google Scholar]
- Lipomas treated with subcutaneous injections of sodium deoxycholate. J Am Acad Dermatol. 2005;53:973-8.
- [Google Scholar]
- Lipodissolve for subcutaneous fat reduction and skin retraction. Aesth Surg J. 2005;25:530-43.
- [Google Scholar]
- The American Society of Aesthetic Plastic Surgery. In: Fat melting fad: too good to be true?. New York: American Society for Aesthetic Plastic Surgery; 2004.
- [Google Scholar]
- The American Society of Aesthetic Plastic Surgery. In: Position Statement on Injection lipolysis. New York: American Society of aesthetic plastic surgery; 2007.
- [Google Scholar]






