Translate this page into:
Giant Epithelial Malignancies (Basal Cell Carcinoma, Squamous Cell Carcinoma): A Series of 20 Tumors from a Single Center
Address for correspondence: Prof. Dr. Uwe Wollina, Department of Dermatology and Allergology, Academic Teaching Hospital Dresden-Friedrichstadt, Friedrichstrasse 41, 01067 Dresden, Germany. E-mail: wollina-uw@khdf.de
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Among nonmelanoma skin cancer (NMSC), basal cell carcinoma (BCC), and squamous cell carcinoma (SCC) are the most common. Giant NMSCs have occasionally reported in the medical literature with particular problems related to diagnosis and treatment. The aim of this study was to analyze patients, treatment, and outcome with giant BCC/SCC.
Materials and Methods:
We analyzed our files between January 1, 2008, and December 31, 2011, of an academic teaching hospital in the dermatology department. Patients were analyzed according to demographic factors, clinical presentation, histopathology, treatment, and outcome. American Society of Anesthesiology physical status system was used to assess the fitness of patients before surgery.
Results:
The frequency of giant NMSC was estimated as 0.4% for both tumor entities. 80% of giant BCC patients were female and 100% of giant SCC patients were male. The mean age was 81.5 ± 8.5 years for BCC) and 79.5 ± 11.4 years for SCC. The major anatomical site was the scalp. Four of 10 BCCs were classified metatypic (basosquamous). Perineural infiltration was seen in 5 NMSCs. Seventy percent of patients had an ASA score ≥3. Surgery was performed in general anaesthesia in 5 (BCC) and 6 (SCC) patients, respectively. All other patients were operated in local or tumescence anesthesia. Blood transfusions were necessary in five patients. The primary treatment was delayed Mohs technique. Defect closure was realized with rotational flaps in most cases. Neoadjuvant chemoimmune therapy and adjuvant combined cetuximab/radiotherapy have been performed in three patients. We observed three deaths, all unrelated to NMSC. 75% of patients achieved complete remission.
Conclusions:
Giant NMSC is uncommon but not rare. These tumors are high-risk subtypes. Treatment needs an interdisciplinary approach.
Keywords
Basal cell carcinoma
giant tumors
nonmelanoma skin cancer
squamous cell carcinoma
treatment
INTRODUCTION
Nonmelanoma skin cancer (NMSC) is the most common malignancy in Caucasians worldwide. The two major types of NMSC are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). The incidence of BCC is about 80 per 100,000 inhabitants each year for Germany and reaches 884 in Australia. The incidence of SCC reaches 2.3 for The Netherlands and 387 for Australia.[1–4]
Despite all efforts in skin cancer campaigns, there is a subpopulation of patients presenting with giant NMSC.[56] By definition, NMSCs with a diameter of at ≥5 cm are considered as giant. This subtype of giant cancer bears a higher risk of complication and mortality. They are also a great challenge considering surgical treatment.[7–9]
We analyzed our data from 2008 to 2011 for giant BCC and SCC.
MATERIALS AND METHODS
We analyzed data from the files of the Department of Dermatology and Allergology, Academic Teaching Hospital Dresden-Friedrichstadt, Germany. The hospital primarily cares for patients in eastern Saxony, but patients from neighboring states Brandenburg, Thuringia, and Saxony-Anhalt also belong to the catchment area of the hospital. BCC and SCC with a diameter ≥5 cm were selected.[7] Data for these patients were analyzed in detail. Surgical risk was assessed by American Society of Anesthesiology (ASA) physical status classification system.[10] The ASA differentiates six scores:
-
A normal healthy patient.
-
A patient with mild systemic disease.
-
A patient with severe systemic disease.
-
A patient with severe systemic disease that is a constant threat to life.
-
A moribund patient who is not expected to survive without the operation.
-
A declared brain-dead patient whose organs are being removed for donor purposes.
Follow-up was performed according to the German guidelines once a year in cooperation with outpatient dermatologists.[1112]
RESULTS
Patients and clinical findings [Tables 1 and 2]
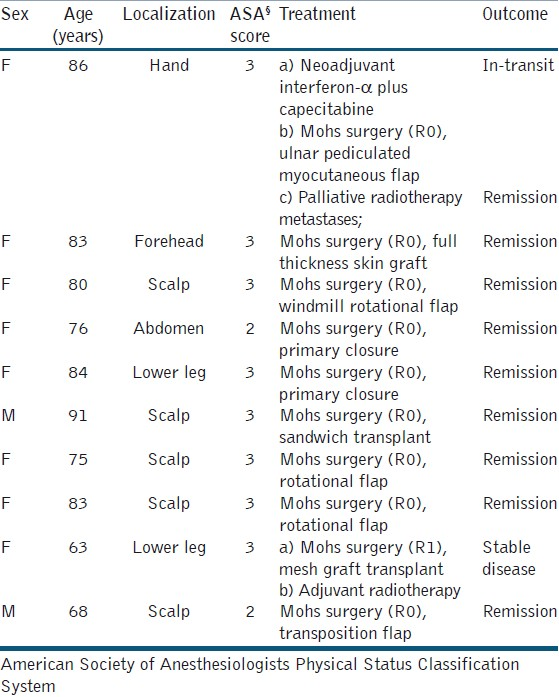
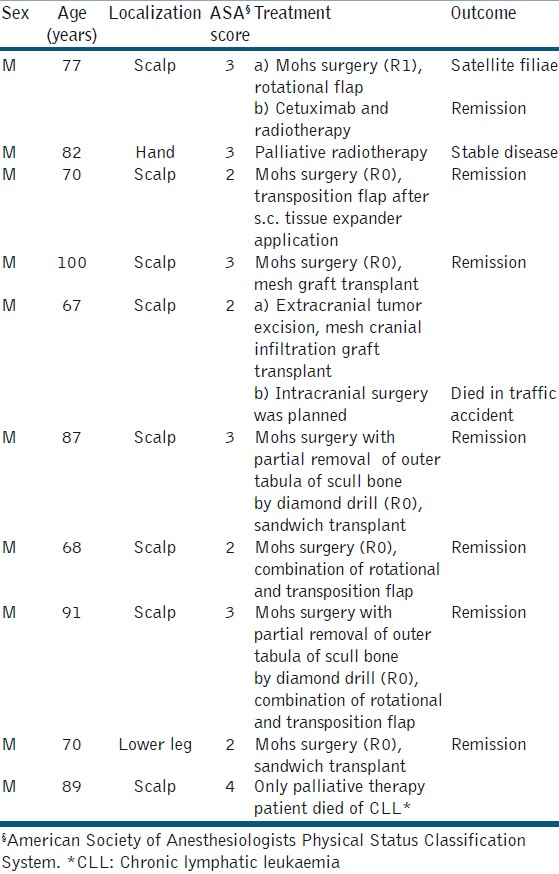
During the 3-year period, about 2,500 NMSCs were treated at the department of dermatology and allergology based on published data of the Regional Tumour Centre, Dresden. We identified a total of 20 patients, 10 with giant BCC and 10 with giant SCC. The frequency of giant NMSC among our patients was estimated as 0.4% for each tumor entity.
The gender distribution was different in both groups: 80% of giant BCC patients were female. In contrast, 100% of giant SCC patients were male. The age distribution was not different between the two groups, i.e., 81.5 ± 8.5) years (mean ± standard deviation) for BCC and 79.5±11.4 years for SCC. The delay of treatment was caused mainly by the patients themselves. The longest period documented was 30 years for a BCC. A rough estimation of mean delay was 7.5 ± 3.1 years (BCC) and 2.1 ± 1.2 years (SCC), respectively.
The BCCs were localized on the scalp (6×), the extremities (3×), and the abdominal skin (1×). SCCs were localized on the scalp (8×), the lower leg (1×), and the hand (1×). The size was 35.0 ± 65.8 cm2 (BCC) and 38.0 ± 76.0 cm2 (SCC). Ulceration was evident in six (BCC) and five (SCC) patients. Bleeding was seen in one BCC and three SCC. Two patients with SCC presented with myiasis.
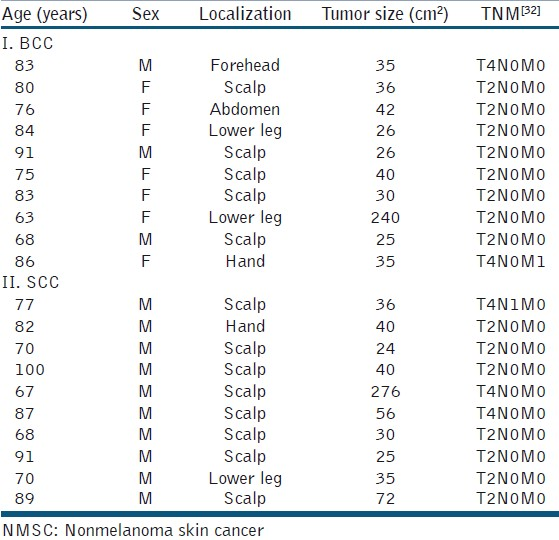
Among the scalp tumors, scull infiltration was suspected in preoperative imaging in four SCCs, in one patient with cranial infiltration. A single SCC patient presented with two cutaneous satellite metastases at the first visit. For TNM classification [Table 3].
Histopathologic findings
BCCs were classified either basosquamous (metatypic; 4x), two with perineural infiltration, or nodular (6x). One SCC was classified tricholemmal and two tumors were desmoplastic. Three SCCs demonstrated perineural infiltration, one in combination with infiltration of lymphatic vessels. Differentiation was graded intermediate in three and low in seven tumors. Further details are summarized in Tables 1 and 2.
ASA physical status classification
Thirteen of 20 patients were classified ≥3 (65%). Comparing BCC and SCC, the rate of ASA ≥3 was 80% and 60%, respectively. One SCC patients was classified ASA 4. Only palliative treatment was performed on him [Tables 1 and 2].
Treatment and outcome
All patients had a detailed clinical examination, imaging to exclude metastatic spread, and laboratory investigations as needed. Imaging techniques had been selected on an individual base with diagnostic ultrasound as primary technique, and high-resolution X-ray and magnetic resonance imaging when necessary. For scalp tumors with suspicion of scull infiltration, cranial computerized tomography (CT) and vascular CT were used. Preoperative antibiosis was used in all cases. Surgery has been performed in general anaesthesia in five (BCC) and six (SCC) patients, respectively. Time for surgery was between 2 and 6 hours in these patients. All other patients were operated in local or tumescence anesthesia. Blood transfusions were necessary in five patients, who had pre-existent anemia [Tables 1 and 2].
BCC patients
All BCCs were excised with delayed Mohs technique to assess R0 resection with tumor-free surgical margin [Table 1]. R0 resection was achieved in all but one patient with a large metatypic BCC in a longstanding leg ulcer.
Defect closure was realized with rotational flaps in most cases [Figures 1 and 2]. Neoadjuvant chemoimmune therapy with capecitabine and interferon-α was performed in one patient with an advanced metatypic BCC of the hand to reduce the tumor mass. Capecitabine 950 mg m-2 body surface on days 1–14 was combined with interferon alpha 3 × 3 mioU s.c. three times a week. The chemotherapy was repeated on day 22.[13] After three courses, delayed Mohs surgery was performed and the defect was closed by a pedicled ulnar myocutaneous flap based on superior ulnar collateral artery. During follow-up, this patient developed cutaneous metastases (primary tumor of the hand, metastases on the ipsilateral arm) treated by palliative radiotherapy. There was no BCC-related death in the follow-up.
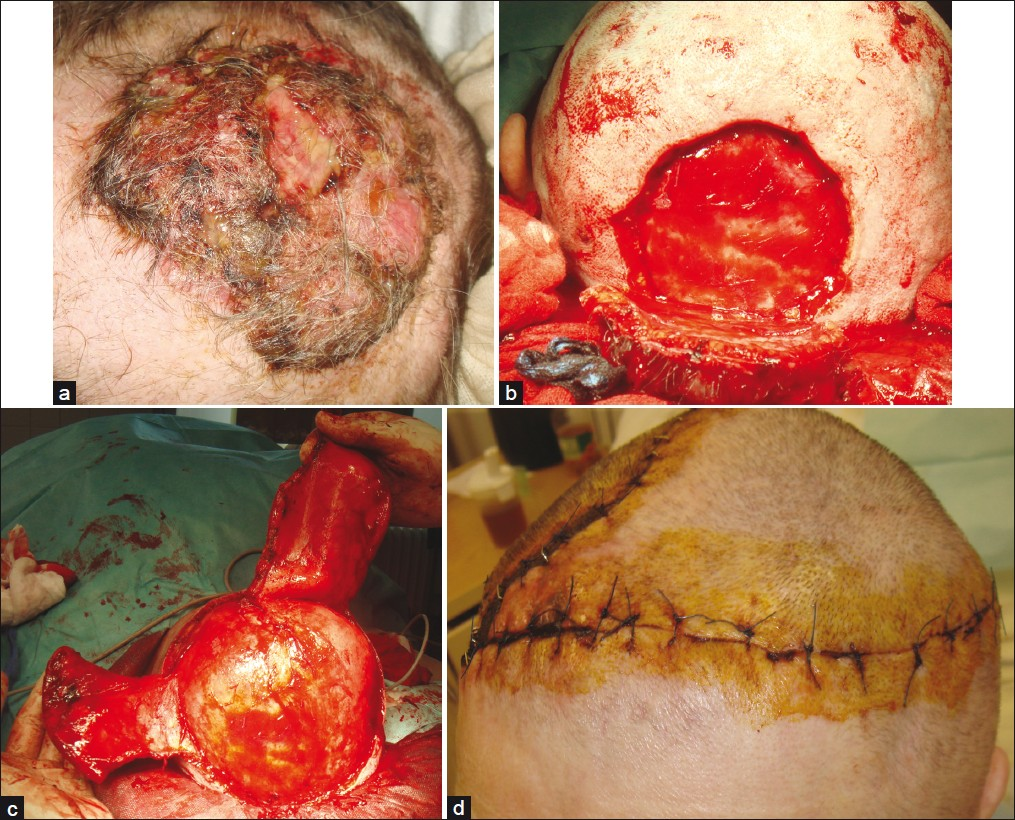
- Giant metatypic (basosquamous) basal cell carcinoma of the scalp; (a) Clinical presentation; (b) Defect after complete excision; (c) Preparation of dual rotational flaps; (d) Eight days after surgery
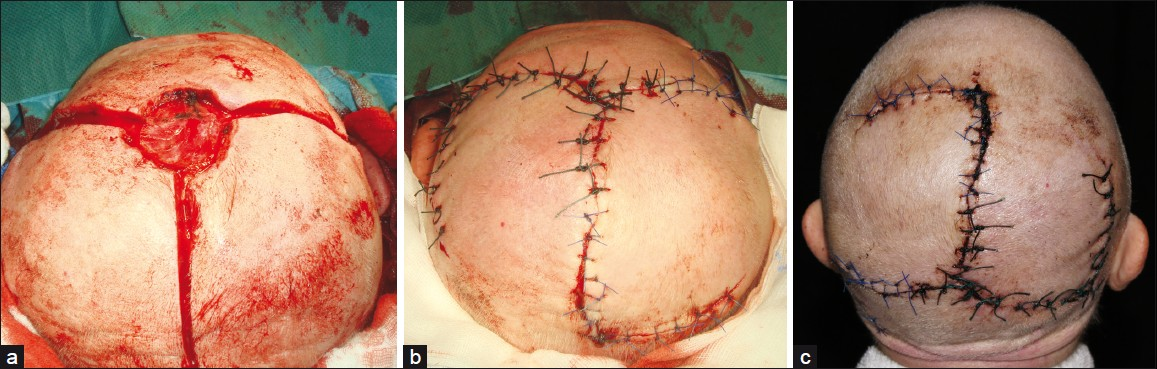
- Giant basal cell carcinoma of the scalp (a) Defect after tumor removal and preparation of windmill flaps; (b) Defect closure; (c) Seven days after surgery
SCC patients
In one SCC patient, no major surgery was possible because of significant comorbidities (chronic lymphatic leukemia with anemia needing regular transfusions, colon cancer, and heart disease). The patient refused any treatment of his skin cancer except the tumor-associated myiasis causing repeated bleeding. Another patient with a giant SCC of his left hand was referred to our hand surgeons for second opinion. Eventually, he denied amputation. Palliative radiotherapy was performed.
In six of the remaining eight patients, delayed Mohs surgery achieved R0 resection status. Defect closure was realized by rotation or transposition flaps (n = 4) or split skin mesh graft transplantation in sandwich technique, in two patients in combination with osmotic tissue expanders [Figures 3–6]. One patient reported previously had cranial infiltration.[14] Surgery was realized in cooperation with the neurosurgery unit in a multiple-step approach. Just before the last operation, the patient died in a car traffic accident.
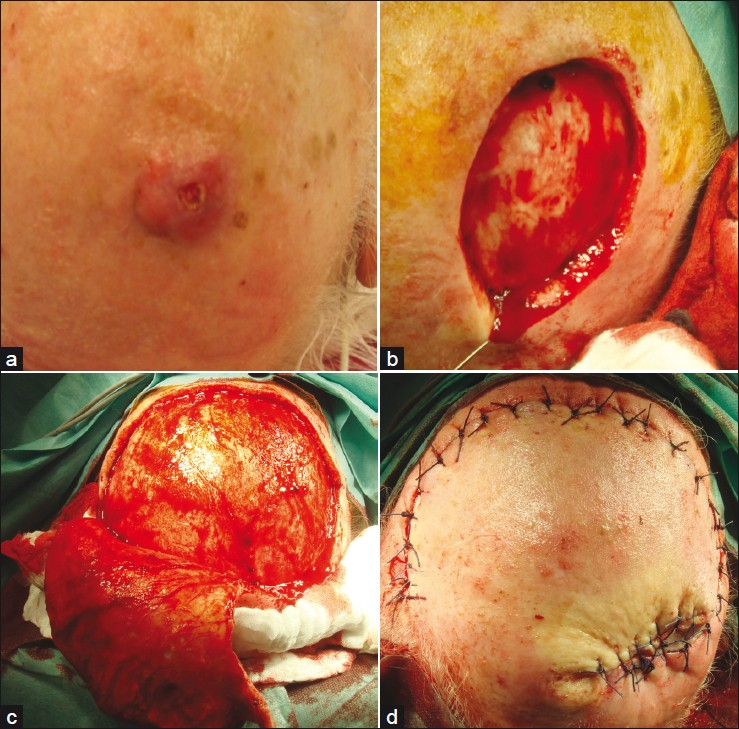
- Desmoplastic squamous cell carcinoma of the scalp; (a) Clinical presentation of an ill defined nodular tumor; (b) Defect after Mohs delayed surgery; (c) Preparation of a rotational flap; (d) Defect closure
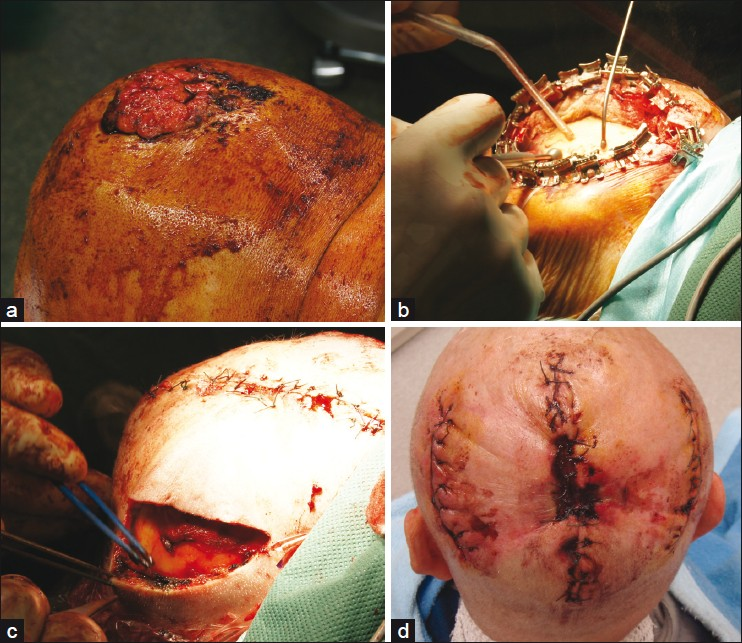
- Giant squamous cell carcinoma of the scalp; (a) Clinical presentation; (b) After complete excision preparation of the outer tabula by diamond drill; (c) Defect closure was achieved by combined tissue advancement and extension. The primary defect has already been closed; (d) Five days after surgery
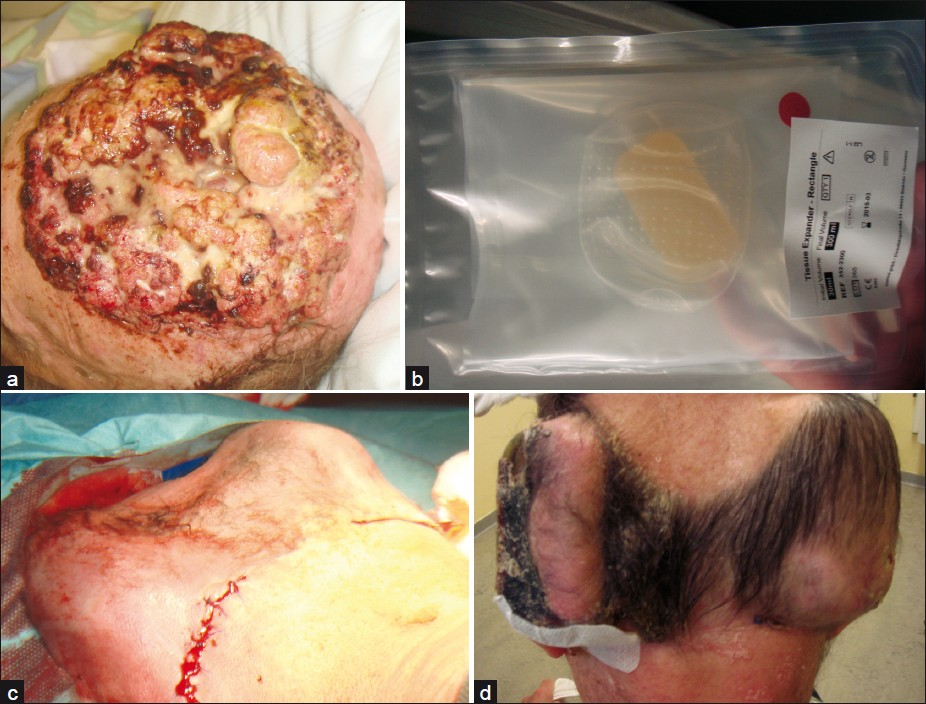
- Giant tricholemmal squamous cell carcinoma; (a) Clinical presentation; (b) Osmotic expander; (c) Implantation of two expanders in the occipital region; (d) After 6 weeks of tissue expansion with a final volume of 300 mL each
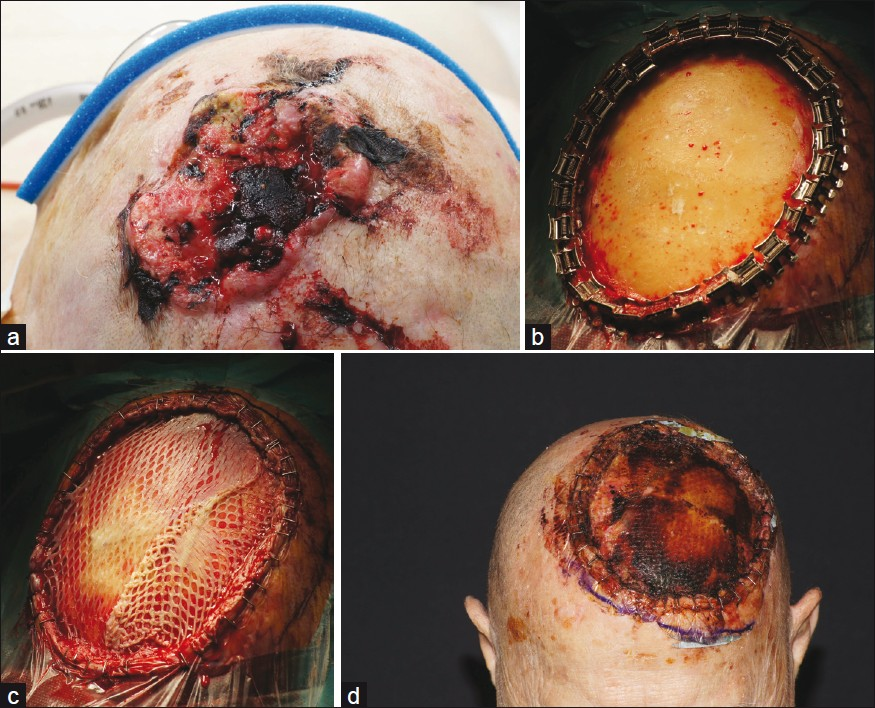
- Giant squamous cell carcinoma of the scalp; (a) Ulcerated, ill-defined tumor; (b) After complete excision with removal of periost and partial removal of the outer tabula. Bleeding was stopped by bony wax. Only some minor bleedings can be seen to feed the transplant; (c) Sandwich transplantation: elastin-collagen template was placed above the bone and a split skin mesh-graft transplant was fixed above in the same session; (d) Stable transplant after 8 days
Among all 20 patients, there was a single patient with primary failure of split skin mesh graft. The grafting was repeated. There was no flap failure or infection and no need for secondary interventions.
Two patients with high-risk scalp tumors were treated with adjuvant combined cetuximab/radiotherapy. Targeted therapy with monoclonal anti-epidermal growth factor receptor (EGFR) antibody cetuximab (Erbitux®; Bristol-Meyers Sqibb) was started with a loading dose of 400 mg/m2, thereafter 250 mg/m2 was given once a week for 6 weeks. Pre-medication consisted of 100 mg prednisolone i.v., 4 mg dimetindene maleate i.v., 50 mg ranitidine i.v. and 8 mg ondansetron p.o.[15]
Causes of death
We observed two deaths in the SCC group–one traffic accident and a death related to chronic lymphatic leukemia. In the BCC group, one female patient died from a heart attack.
DISCUSSION
Giant NMSC (BCC and SCC) is defined as tumors with a diameter ≥5 cm. All of the patients were older than 60 years of age. Neglect by patients was the most common cause of marked delay to primary treatment. The presence of myiasis in some tumors can be considered a symptom of the neglect.[1516]
Most of the tumors we observed were localized on the scalp. Scalp NMSC is most often seen in elderly patients.[17] Among 51 cases of giant BCCs, the most common location was on the back.[7] Another study from Italy reported more cases in the head and neck region as we observed.[18] The difference may in part be explained by the different gender. The majority of our patients were females in contrast to Archontaki et al.[7]
Giant BCC fulfil the criteria of high-risk BCC.[19] Primary treatment is surgically with complete examination of excision margins to ensure R0 resection. We perform the technique of delayed Mohs surgery. For defect closure various flaps and transplants have been used.[7–919–24]
Since after removal of periostal the scull bone is not a useful place for mesh grafts as exposed tendons as well, we used a sandwich technique to overcome these problems. In the case of scull, a small rose drill was used to reach diploe veins for transplant nutrition before sandwich transplantation. In all cases, a dermal template was used to cover exposed bone or tendons. The split-thickness mesh graft placed over the template in the same session. The technique has been described in detail recently.[2526]
In contrast to BCC, there is no accepted system for defining high-risk SCC.[27] Multivariate analysis of SCC identified major risk factors for metastasis: tumor thickness >2 mm, tumor size >6 mm, and desmoplastic growth pattern.[28] Perineural invasion is a risk factor for local recurrence and metastatic spread. The risk factors for metatypic (basosquamous) BCC resemble those for SCC.[29]
A part of the patients with giant NMSC needs an adjuvant treatment, i.e., patients with R1 resection, rapid relapse after primary surgery, and high-risk tumors. EGFR-antagonists and radiotherapy offer a treatment option in particular for the group of elderly patients with significant comorbidities, who could not tolerate classical chemotherapy.[152830]
It becomes obvious that patients with giant NMSC often need an intensive care due to the complexity of the surgical procedures, anemia, and other comorbidities. The physical status before surgery was classified ASA ≥3 in 70% of patients.[10] An interdisciplinary approach meets the needs of these patients in the most appropriate way.
CONCLUSION
In conclusion, giant NMSCs are not as rare as one would expect. Neglect of patients is the major reason for delayed diagnosis and treatment. Obviously, the primary care by GPs is not efficient to detect such tumors earlier. An improved education in NMSC diagnosis is necessary. The minSKIN protocol might serve as a template.[3132]
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- Basal cell carcinoma: Histological classification and body-site distribution. Br J Dermatol. 2006;155:401-7.
- [Google Scholar]
- Incidence and trends of cutaneous malignancies in the Netherlands, 1989-2005. J Invest Dermatol. 2010;130:1807-12.
- [Google Scholar]
- Non-melanoma skin cancer in Australia: The 2002 national survey and trends since 1985. Med J Aust. 2006;184:6-10.
- [Google Scholar]
- Skin cancer - Primary and secondary prevention (information campaigns and screening) - with a focus on children and sunbeds. Prog Biophys Mol Biol. 2011;107:473-6.
- [Google Scholar]
- Population education in preventing skin cancer: From childhood to adulthood. J Drugs Dermatol. 2010;9:112-6.
- [Google Scholar]
- Giant basal cell carcinoma: Clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-63.
- [Google Scholar]
- Giant basal cell carcinoma surgical management and reconstructive challenges. Ann Plast Surg. 2007;58:250-4.
- [Google Scholar]
- Surgical management of giant nonmelanoma skin neoplasia. South Med J. 1998;91:190-5.
- [Google Scholar]
- Short German guidelines: Squamous cell carcinoma. J Dtsch Dermatol Ges. 2008;6(Suppl 1):S5-8.
- [Google Scholar]
- Short German guidelines: Basal cell carcinoma. J Dtsch Dermatol Ges. 2008;6(Suppl 1):S2-4.
- [Google Scholar]
- Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-4.
- [Google Scholar]
- Giant tricholemmal squamous cell carcinoma with cranial infiltration. J Clin Aesthet Dermatol. 2011;4:34-7.
- [Google Scholar]
- Combined cetuximab and volumetric modulated arc-radiotherapy in advanced recurrent squamous cell carcinoma of the scalp. Dermatol Rep. 2011;3:e57.
- [Google Scholar]
- Scalp myiasis associated with advanced basal cell carcinoma. Dermatol Surg. 2009;35:1539-40.
- [Google Scholar]
- Massive scalp myiasis with bleeding in a patient with multiple malignancies. Int Wound J. 2010;7:297-9.
- [Google Scholar]
- malignant cutaneous tumors of the scalp: A study of demographic characteristics and histologic distributions of 398 Taiwanese patients. J Am Acad Dermatol. 2007;56:448-52.
- [Google Scholar]
- Giant basal cell carcinoma of the skin: Literature review and personal experience. J Eur Acad Dermatol Venereol 2011 [In press] [DOI: 10.1111/j.1468-3083.2011.04427.x]
- [Google Scholar]
- Big bad BCCs: Craniofacial resection and reconstruction for atypical basal cell carcinoma. J Plast Reconstr Aesthet Surg. 2010;63:e433-41.
- [Google Scholar]
- Can we put a simplified algorithm for reconstruction of large scalp defects following tumor resection? World J Surg Oncol. 2011;9:129.
- [Google Scholar]
- Reconstruction of a large scalp defect by the sequential use of dermal substitute, self-filling osmotic tissue expander and rotational flap. J Cutan Aesthet Surg. 2010;3:106-10.
- [Google Scholar]
- Reconstruction of large scalp defects by expander technique. Z Hautkrankh. 1991;66:776-82.
- [Google Scholar]
- Use of a collagen-elastin matrix for hard to treat soft tissue defects. Int Wound J. 2011;8:291-6.
- [Google Scholar]
- One-stage reconstruction of soft tissue defects with the sandwich technique: Collagen–elastin dermal template and skin grafts. J Cutan Aesthet Surg. 2011;4:176-82.
- [Google Scholar]
- Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthet Dermatol. 2010;3:39-48.
- [Google Scholar]
- Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008;9:713-20.
- [Google Scholar]
- Basosquamous carcinoma: Analysis of prognostic factors influencing recurrence. Cancer. 2000;88:1365-9.
- [Google Scholar]
- Targeting epidermal growth factor receptor in head and neck cancer: Lessons learned from cetuximab. Exp Biol Med (Maywood). 2010;235:907-20.
- [Google Scholar]
- Min SKIN does a multifaceted intervention improve the competence in the diagnosis of skin cancer by general practitioners.? Study protocol for a randomised controlled trial. Trials. 2011;12:165.
- [Google Scholar]
- AJCC Cancer Staging Manual (7th ed). New York, NY: Springer; 2010. p. :301-14.






