Translate this page into:
Stability in Vitiligo: Is there a Perfect Way to Predict it?
Address for correspondence: Dr. Davinder Parsad, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012, India. E-mail: parsad@me.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Stability is a hard-to-define concept in the setting of vitiligo, but is nonetheless extremely crucial to the planning of treatment regimens and also in prognosticating for the patient. There are several ways to judge stability in vitiligo, which include clinical features and, recently, many biochemical, cytological and ultrastructural correlates of the same. These recent advances help in not only in prognosticating individual patients but also in elucidating some of the mechanisms for the pathogenesis of vitiligo, including melanocytorrhagy and oxidative damage to melanocytes.
Keywords
Concept
stability
vitiligo
INTRODUCTION
The concept of stability in vitiligo is multifaceted, and no consensus has yet been reached on defining the criteria for this so far. It includes not only clinical aspects of stability but also many recently identified biochemical and ultrastructural correlates of the same. The exact definition of stability in vitiligo is still elusive, and a number of difficulties arise when examining individual patients to decide on the stability of this disease. This concept gains utmost importance when selecting appropriate patients of refractory vitiligo for surgical interventions.
Apart from clinical features that help in deciding stability of the disease, a number of studies have focussed on evaluating ultrastructural, serological and biochemical parameters to distinguish between stable and active disease. Most of these parameters have evolved as a result of understanding the pathogenesis of the disease and the various hypotheses for the same: Autoimmune hypothesis (serum levels of autoantibodies, T cell subset dysregulation and histological changes), neural hypothesis (serum catecholamines and their metabolites and neuropeptide Y), oxidative stress (oxidant-antioxidant levels) and melanocytorrhagy (ultrastructural studies).
CONCEPT OF CLINICAL STABILITY IN VITILIGO
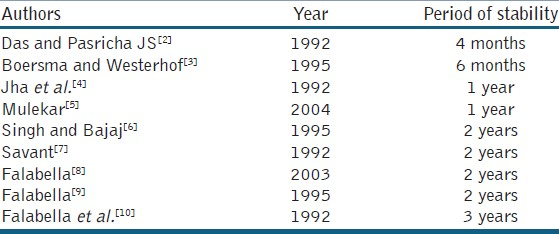
Although no universal consensus exists on the optimum duration of non-progression for the disease to be labelled as stable clinically, recently, the Indian Association of Dermatologists and Venereologists (IADVL) taskforce for standard guidelines of care for dermatosurgical procedures in their consensus recommendations defined stability as ‘a patient reporting no new lesions, no progression of existing lesions, and absence of Koebner phenomenon during the past 1 year’.[1] The stark difference in the minimum required period of stability in different studies is depicted in Table 1. However, in a recent study comparing the results of suction blister grafting in patients with varying periods of stability, successful repigmentation (> 75%) was seen in 0% of patients with period of stability ranging from 3 months to 1 year, 37.5% in 1–2 year group and 77.8% when period of stability was > 2 years (P = 0.005). This new study may lend greater credence to supporters of a 2 year minimum stability period.[11]
In many cases, a clinician’s expertise and certain characteristics of the disease may help in deciding the likely course of the vitiligo. A study on 400 patients found that significantly greater progression of disease was seen in patients with a longer disease duration, positive family history, nonsegmental clinical type, Koebner›s phenomenon and mucous membrane involvement.[12] It is important to not completely rely on the patient’s reporting of disease activity as this may be erroneous and the clinician must rely on his own documentation or photographic records in doubtful cases.
Clinical parameters for establishing stability
Various authors have emphasized on different clinical criteria for defining stable vitiligo. The most commonly described clinical criteria include the following:
-
History of progression: Absence of new lesions
-
Extension of old lesions: No extension of pre-existing lesions
-
Koebner phenomenon: It is defined as ‘the development of vitiligo at sites of aspecifically traumatized skin’. It has frequently been proposed to indicate active disease. Recently, it has been further classified into Koebner phenomenon by history (KP-h) and experimentally induced Koebner phenomenon (KP-e) by Njoo et al., who studied the correlation between KP-h and KP-e and the responsiveness to ultraviolet light therapy (UVA plus fluticasone propionate or NBUVB) in 61 patients of vitiligo vulgaris off any topical or systemic therapies for at least 6 months. KP-e was assessed by inducing an epidermodermal injury by extracting a 2-mm punch biopsy specimen from clinically uninvolved skin. The outcome was evaluated 3 months later to look for depigmentation in the scar or surrounding it (KP-e positive). They found a significant difference between KP-h and KP-e and reported the positive and negative predictive value, respectively, of KP-h when taking KP-e as gold standard to be 89% and 52%, respectively. Surprisingly, the responsiveness to UV-B (311 nm) therapy among KP-e-positive or KP-e-negative patients was not significantly different (P = 0.66). However, in the fluticasone propionate plus UV-A group, KP-e-positive patients showed a better response than did KP-e-negative patients (P = 0.01).[13] Boersma et al.,[3] found a correlation between KP-h and minipunch test grafting; in all patients with positive KP-h, depigmentation of the punch grafts was observed.
-
VIDA score (vitiligo disease activity score) was described by Njoo et al.,[13] to grade the level of activity of disease in individual vitiligo patients off any therapies for atleast 6 months. They scored the activity of disease from +4 to −1, indicating gradually decreasing levels of activity. Patients with score of 0 were those with disease stable for 1 year and those with score −1 had, in addition, spontaneous repigmentation. In a study to correlate the VIDA score with KP-e, it was observed that patients with VIDA scores of +1 and +2 did not necessarily show a positive KP-e. However, all the patients with VIDA scores of +3 and +4 showed a positive KP-e.[12]
-
Minigrafting or test grafting
This test was described by Falabella et al.,[14] to aid in selecting appropriate patients for surgical intervention in vitiligo. They performed the test grafting on 57 patients of vitiligo, which was stable for atleast 2 years. The test consists of placing a few grafts (1.0–1.2 mm) in the centre of the depigmented lesion to be scrutinized followed by dressing with Micropore® adhesive tape, which is kept for a couple of weeks. This is followed by sun exposure for 15 min daily for a period of 3 months, following which, the test sites are visualized under Wood’s light. The test is considered positive if unequivocal repigmentation takes place beyond 1 mm from the border of the implanted grafts.
However, a number of exceptions exist to the above said rules. There is some evidence to suggest that stability may be site-dependent. In the same patient, some lesions may show evidence of progression, while other lesions may repigment well with surgical therapy. In addition, there have been instances of simultaneous donor site depigmentation and recipient site repigmentation and also vice-versa reported following surgery for vitiligo. Even test grafting has not been found to be fool proof in detecting ideal cases for surgical intervention. There have been instances of failed surgical attempts in patients with positive test grafting and also reports of successful repigmentation in patients with negative test grafting.[15–17] In fact, Falabella himself observed that test grafting is not an absolute indication for successful surgical repigmentation.[11] Hence, it has been suggested that each patient must be treated on an individualized basis rather than based on the above criteria.
Recently, an attempt has been made to study the lesional, perilesional and repigmented skin of vitiligo patients by in vivo reflectance confocal microscopy. Depigmented skin showed disappearance of the bright rings normally seen at the dermo-epidermal junction, while even the non-lesional skin of vitiligo patients showed unexpected changes as the presence of half-rings or scalloped border-like features of the bright papillary rings. Activated dendritic melanocytes were observed in re-pigmented areas after phototherapy. These data are preliminary, but may prove useful in the future to predict the possible course of disease for individual patients.[18]
Apart from clinical criteria of stability, various studies have shown several biochemical, histopathological and ultrastructural features, which differ in active versus stable vitiligo. At present, however, they are not sufficiently well characterized so as to definitively differentiate active from stable vitiligo. To completely understand the concept of stability, it is essential to have an in-depth knowledge of the pathomechanisms of the disease.
BIOCHEMICAL PARAMETERS
Morrone, et al., were among the first to report significant increases in the urinary concentrations of the catecholamine metabolites homovanillic acid (HVA) and vanillylmandelic acid (VMA) in active vitiligo patients.[19] Their study has been followed by other similar studies reporting increase in plasma as well as urine concentrations of catecholamines and their metabolites in vitiligo patients having active disease. It has been proposed that the synthesis as well as metabolism of catecholamines is increased in vitiligo patients. LePoole et al., demonstrated higher levels of catechol-o-methyl transferase (COMT) activity in epidermal homogenates from vitiligo patients as compared to healthy controls. Such differences were, however, not found at the systemic level.[20] In accordance with these findings, Cucchi et al., found that, as compared to patients with stable nonsegmental vitiligo, the patients with active disease (in last 3 months) showed significantly increased plasma levels of norepinephrine (NE), normetanephrine (NMN), 3-methoxy-4-hydroxyphenylglycol (MHPG) and HVA, all of which are COMT products of catecholamines. In addition, they also observed that, among patients with active disease, statistically significant differences were found in values of NE, epinephrine (E) and metanephrine (MN) depending on the disease duration, with levels being much higher in patients with recent onset disease.[21] The same group of authors later demonstrated that 24-h urinary NE levels were significantly higher in patients with active vitiligo as compared to stable disease and controls. Epinephrine (E), DA (dopamine), NMN, MN (metanephrine), VMA, 5-HIAA (5-hydroxy indole acetic acid) and HVA levels were significantly higher in patients in an active phase than in controls. They propose that urinary metabolite levels give more mediated information, referring to 24-h period of activity as compared to the plasma levels, which reflect the actual real-time activity, and thus are more likely to be affected by momentary emotional events and even the drawing of blood. Hence, urinary levels of these metabolites may be more accurately used in the assessment of stability of disease.
Serum analysis of oxidant-antioxidant status in vitiligo patients may also give a clue to the status of stability of disease. The oxidative stress hypothesis of vitiligo proposes that certain intermediates in the process of melanin biosynthesis, such as 3, 4-dihydroxyphenylalanine (dopa), dopachrome and 5, 6-dihydroxyindole, are toxic to melanocytes through the increased production of free radicals. Deficient antioxidant activity has also been found in vitiligo patients.
Ines, et al.,[22] compared serum and red blood cell (RBC) oxidant-antioxidant status in patients of active and stable vitiligo with that in controls and found higher serum levels of malondialdehyde (an end product of lipid peroxidation) and selenium and higher RBC levels of superoxide dismutase (SOD) in patients of active vitiligo as compared to that in controls. These levels were also higher than those in patients with stable disease; however, no comment was made whether these results reached statistical significance. They also observed RBC glutathione peroxidase levels to be lower in active vitiligo patients as compared to controls and stable vitiligo patients. The higher SOD level in active vitiligo patients has been proposed to occur as an adaptation to the increased oxidative stress evident in these individuals.
In few small studies, the role of elevated serum homocysteine levels has been proposed in the pathogenesis of vitiligo. Singh et al.,[23] studied homocysteine (Hcy) levels in serum of active and stable vitiligo patients and controls. They found that there was a significant relationship between Hcy level and activity of vitiligo. The mean Hcy level in patient with active vitiligo was significantly higher than in stable vitiligo cases. It has been proposed that patients in the active stages of vitiligo are deficient in vitamin B12 and folate, which are cofactors for the enzyme Hcy methyl transferase, which is important for the regeneration of methionine from Hcy. This leads to increased serum levels of Hcy, which is responsible for the production of reactive oxygen species and also the inhibition of the enzyme tyrosinase by binding with the copper at its active site.
Tu et al.,[24] in their study on plasma levels of neuropeptide Y (NPY) found the levels to be significantly higher in patients with vitiligo (of the generalized, local and segmental types) as compared to normal controls. In addition, in both local and segmental type, but not in the generalized type, the levels in progressive stage were significantly higher than in those in the stable stage. They also documented that the levels of NPY in the tissue fluids from skin lesions were significantly higher than in those from uninvolved skin in both the local type and segmental type (P < 0.05), while, in the generalized type, there was no significant difference (P > 0.05) between the NPY level in the tissue fluid from skin lesion and that from uninvolved skin. Nerve endings lie close in contact with melanocytes and the release of mediators such as neuropeptide Y has been proposed to cause melanocyte loss in vitiligo by both immunological and non-immunological means. The above data points to the greater role of neurological mediators in segmental as compared to non segmental vitiligo and may allow for an assessment of stability of segmental vitiligo, about which not much data exists so far.
In another study, it was found that the serum levels of granulocyte macrophage colony stimulating factor (GM-CSF) in both focal and generalized type and the IL-6 level in the generalized type were significantly higher in those with progressive disease (in last 1 month) than in those with stable disease. However, such differences depending on activity of disease did not exist either for IL-lβ, IL-8 and TNF-α or for any cytokine in segmental vitiligo.[25] However, these findings are contradictory to the results of the study of Yu et al., who showed a decrease in the production of GM-CSF and proposed GM-CSF to be an intrinsic factor for melanocyte growth.[26]
Apart from these biochemical markers, peripheral T cell subset imbalance has also been found in patients of vitiligo as compared to controls. However, the only study that compared T cell subsets between active and stable vitiligo patients failed to find a significant difference.[27]
SEROLOGICAL STUDIES
One of the most popular theories regarding the pathogenesis of vitiligo is the autoimmune theory. According to this theory, antibodies targeted against melanocyte antigens lead to melanocyte destruction. Antibodies against melanocyte antigens as well as enzymes involved in melanogenesis have been found in vitiligo patients, but not in controls. However, it is not entirely clear whether the presence of these autoantibodies is a pathomechanism of vitiligo or just an epiphenomenon related to melanocyte loss and the exposure of cryptic autoantigens. Whether vitiligo autoantibodies are the cause or the result of the disease, they do have the capacity to injure pigment cells. Different techniques have been used to detect these antibodies including immunoprecipitation,[28] indirect immunofluorescence,[29] immunoblotting,[30] and conventional ELISA,[31] and their functions have been further investigated with complement-dependent cytotoxicity[32] and antibody-dependent cellular cytotoxicity assays or passive transfer experiments.[3334]
Titres of these autoantibodies have been found to correlate with disease activity in vitiligo. One of the first studies in this regard was conducted by Cui et al., who documented that complement-mediated cytolysis of melanocytes by patient sera was found to be much more common in patients with active disease than in those with inactive disease.[32] In a previous study, using modified live-cell ELISA, the same authors had reported that IgG pigment cell antibodies were present in 80% (8 of 10) of patients with active vitiligo, but in none of those with inactive disease or in normal individuals.[35]
However, the presence of these antibodies was found to be also related to the extent of disease being detected in 50% of patients with minimal vitiligo (< 2% of skin area involved) as compared to 93% of patients with greater depigmentation (5–10% of skin area involved).[36]
Autoantibodies in vitiligo have been described to a plethora of autoantigens, the earliest ones described were pigment cell antigens with molecular weights of 35, 40–45, 75, 90 and 150 kDa. Of these, 35 and 90 kDa were found to be preferentially expressed on pigment cells.[3738]
This was followed by the identification of tyrosinase,[3940] tyrosine hydroxylase[41] and tyrosinase-related proteins (TRP-1 and TRP-2)[4243] as putative autoantigens.
Among these, only antibody titres against tyrosine hydroxylase (TH) have been found to correlate with activity of disease. Kemp et al., studied the prevalence of anti-TH antibodies in a large cohort of vitiligo patients (n = 87), patients with other autoimmune diseases without concomitant vitiligo (n = 91) and healthy controls (n = 28). Of the 79 patients with non-segmental vitiligo, 15 had stable disease (no progression for > 6 months), while 64 had active disease. TH antibodies were evaluated in a radioimmunoassay (RIA) using [35S]-labelled TH and titres were determined by testing serum dilutions of 1:100, 1:200, 1:500, 1:1000 and 1:2000. In addition, for each sample, a TH antibody (TH Ab) index was calculated as counts per minute (cpm) immunoprecipitated by tested serum⁄mean cpm immunoprecipitated by healthy control sera. The upper limit of normal for the RIA was estimated as a TH Ab index of 1.09. Any serum sample with a TH Ab index above the upper limit of normal was designated as positive for TH antibody reactivity. The authors observed that 18 of 79 (23%) sera from patients with non-segmental vitiligo had a TH Ab index above 1.09 and were considered positive, while none of the sera of patients with segmental vitiligo or controls were TH antibody positive (P = 0.003). Eighteen of the 67 (27%) patients with active vitiligo demonstrated TH antibodies in the serum, while none of the 20 (0%) patients with stable disease demonstrated the same (P = 0.009). This study may have a high clinical relevance, since the presence of TH antibodies had a 100% positive predictive value and specificity for recognition of active disease. However, 77% of patients with active disease did not show the antibodies and hence the sensitivity in diagnosing active disease is low.[44]
However, in another study, Xie et al., using immunoblotting found no significant difference in the prevalence of antibodies to an antigen that comigrated with tyrosinase between patients with active vitiligo (50%), stable vitiligo (64.3%) and controls (55.8%). In addition, by immunoprecipitation DOPA stain and sandwich ELISA, none of the vitiligo or control individuals had antibodies to tyrosinase. They concluded that the antibodies detected using immunoblotting were not targeted against tyrosinase.[45]
One of its kind study by Hann et al.,[46] measured the percent cytotoxicity of melanocyte mediated by autoantibody and complement in normal controls (n = 31) and in the patients with active vitiligo (n = 37) and followed up with repeat measurements after a course of systemic steroids. They found a significant reduction in percent cytotoxicity between pre- and post-treatment groups (P = 0.0243). These findings lend favour to the hypothesis that a decrease in the antibody-mediated cytotoxicity against melanocytes may play a role in the improvement of vitiliginous lesions after systemic steroid treatment.
MICROSCOPIC AND ULTRASTRUCTURAL CORRELATES
The end result of all the pathogenetic factors in vitiligo is to create a local environment that is unfavourable to the survival of melanocytes, which are believed to be more sensitive than other cells to these deleterious stimuli because of a defective membrane structure and functionality. It has long been debated whether the ultimate loss of melanocytes in vitiligo occurs via necrosis, apoptosis or melanocytorrhagy, with the evidence in favour of melanocytorrhagy fast gaining momentum.[4748] A number of ultrastructural studies have been done on the skin of vitiligo patients (lesional, perilesional and normal) in order to gain an insight into the pathogenesis of the disease. In the process, a number of findings have come forth that may allow differentiation of active from stable disease.
Light and electron microscopy studies were performed on the vitiliginous and adjacent normal skin from 97 patients with actively spreading vitiligo and 19 patients with stable vitiligo. Epidermis of vitiliginous skin revealed complete loss of pigment and melanocytes, while perilesional skin showed vacuolar changes of basal cells and epidermal and dermal infiltration of lymphocytes and melanophages in the upper dermis. All these changes were more prominent in the skin of actively spreading vitiligo than in stable vitiligo.[49] In another study, apart from the above changes, focal spongiosis was found to be present in 48% of vitiliginous lesions and this was largely limited to the marginal areas of the lesions.[50] In another case series of trichrome vitiligo, the number of Langerhans cells was found to be increased in the epidermis of light brown skin and perilesional normal skin as compared with vitiliginous and normal skin. These may be responsible for the immune dysregulation implicated in the spread of trichrome vitiligo.[51] Abnormal expression of MHC class II and a six-fold increased expression of ICAM-1 has also been demonstrated in perilesional melanocytes of vitiligo patients. These data emphasize on the role of antigen presentation in the damage to melanocytes in active vitiligo.[52]
Kumar et al.,[53] studied 14 patients of vitiligo (7 each with stable and active disease) and 5 controls. Stable vitiligo was defined as having no new lesions and no progression of existing lesions for at least 2 years, while unstable vitiligo was defined as new lesions or progression of existing lesions over the past 6 weeks. Perilesional skin melanocytes from these patients were cultured and studied for morphological changes, markers of adhesion and apoptosis. It was documented that melanocytes from patients with active disease were morphologically different from those with stable disease and normal controls in demonstrating a larger perinuclear zone and small dendrites with clubbed ends (retracted dendrites). Significantly low adhesion to collagen type IV was also observed in melanocytes from unstable vitiligo patients as compared to control and stable vitiligo patients. In addition, caspase 3 and annexin V (both markers of apoptosis) staining was significantly greater in unstable vitiligo cultured melanocytes treated with okadaic acid (an inducer of apoptosis) as compared with the melanocytes from controls. All these findings lend credence to the hypothesis of defective melanocyte attachment and melanocytorrhagy as pathogenetic in progressive vitiligo and also imply that unstable vitiligo melanocytes are more prone to apoptosis as compared to controls.
Similar to the above data on serum oxidant-antioxidant levels in vitiligo described above, Ines et al., followed up their study with assessment of antioxidant enzymes and lipid peroxidation status at the tissue level in stable and active vitiligo cases.[54] They observed that levels of superoxide dismutase, glutathione peroxidase and malondialdehyde in tissues were increased significantly, while catalase levels were decreased significantly in patients with active vitiligo as compared to stable vitiligo patients and controls. They concluded that, although oxidative stress is involved in the pathophysiology of both active and stable vitiligo, an increased imbalance of antioxidants is observed in the tissues of patients with active vitiligo.
Hann et al.,[50] conducted light and electron microscopy studies on the vitiligo and adjacent, normal appearing skin from 97 patients with actively spreading vitiligo and 19 patients with stable vitiligo. Epidermal and dermal changes in perilesional skin were found to be more prominent in the skin of actively spreading vitiligo than in stable vitiligo. These changes included vacuolar changes of basal cells, degenerative changes in melanocytes, epidermal and dermal infiltration of lymphocytes, and melanophages in the upper dermis. These findings suggest that the biopsy from adjacent, normal appearing skin of vitiligo may give a clue to the status of stability of disease in an individual patient. Further supporting the role of inflammation in the spread of vitiligo lesions, Ahn et al., in an immunohistochemical study from the marginal skin of vitiligo lesions found enhanced expression of epidermal ICAM-1 and CD4 lymphocytes in samples from active vitiligo as compared to stable disease.[55]
In another study, highly significant increases in CD3+, CD4+ and CD8+ T cells were found in margin of vitiligo lesions and these cells were mostly activated CD45RO+ cells of the memory subset. Most intense infiltration was present within 0.6 mm of the edge of the lesion.[56] In addition, deficient expression of CCL22, a skin homing chemokine for Treg (regulatory T cells) has been found in vitiligo skin on immunohistochemistry. This was hypothesized to explain the failure of circulating, functional Treg to home to the skin in vitiligo that was proposed to be responsible for perpetual anti-melanocyte reactivity in progressive disease.[57]
Benzekri et al., biopsied 50 patients of vitiligo classified them clinically into hypomelanotic with poorly defined borders (HPDB, 29 cases) or amelanotic with sharply demarcated borders (ASDB, 21 cases) and followed them for a period of 1 year. One year after the biopsy, of the 48 patients still in the study, 20 had lesions that were considered to be stable and 28 had active lesions. The HPDB and ASDB lesions were correlated respectively with active and stable status (P < 0·001) and correlations were also obtained between clinical aspects, histological findings and vitiligo activity.[58]
Most of the other studies on ultrastructural changes in vitiligo do not provide a head-to-head comparison of findings in active as compared to stable vitiligo. Extensive research is still needed to identify definitive ultrastructural and molecular features, which may allow reliable differentiation of vitiligo that is expected to progress from vitiligo that has reached stability.
CORRELATION OF BIOCHEMICAL AND CYTOLOGICAL CORRELATES WITH SUCCESS OF REPIGMENTATION
One of the only studies of its kind by Rao et al., studied the correlation of results of repigmentation following suction blister grafting in vitiligo with serum catalase levels and lesional immunohistochemistry for CD4, CD8, CD45RO, CD45RA and FoxP3. There was no correlation of serum catalase levels with either the period of stability or the results of surgical repigmentation. Similarly, CD45RA, CD4 and Fox P3 were also not found to correlate with response to surgical treatment. However, CD8 T cell counts were found to be lower in responders [median 1% (range 0–4%)] as compared to the non-responders [median 3% (range 1–7%)] (P = 0.04). Percentage of repigmentation was found to correlate inversely with percentage of CD8+ cells. CD45RO cells were seen exclusively in some non-responders, while the responders showed complete absence of these cells.[11]
WHERE DO WE STAND?
Most studies on the biochemical/serological aspects of stability in vitiligo are cross-sectional and hence do not shed light on the correlation of these parameters with the course and prognosis of the disease. More studies need to be conducted to follow the course of disease and to correlate it with the levels of humoural factors. Another aspect on which further research is needed is the correlation of these tests with the outcome of medically and/or surgically induced repigmentation. Second, most studies have so far not been able to establish cut-off values that may be helpful to classify disease as active or stable in an individual patient. It may also be useful to correlate the change in level of serum autoantibodies with the introduction of immunosuppressive therapy or phototherapy. A landmark study in this regard was the study by Hann et al., who found that response of vitiligo lesions to systemic steroid therapy correlates with the finding of a significant reduction in percent cytotoxicity of melanocyte mediated by autoantibody and complement.[46]
Lesional stability as a concept needs to be investigated in greater detail, since, in the same patient, at the same time, different lesions may show stability, regression or progression. Ultrastructural changes evaluated at the site of induced Koebner phenomenon may lead to further breakthroughs in understanding both the pathogenesis of disease and the concept of stability. In addition, there is a need to set uniform clinical criteria for defining stability before embarking on biochemical and other investigative findings so as to be able to compare results between studies and draw definitive conclusions.
Also, so far these tests are only research tools available in very few centres and extensive work needs to be done to make these tests sufficiently reliable, cheap and widely available, so as to enable decision making in individual patients.
Extensive research on the concept of stability is essential in order to prognosticate individual patients affected by this socially devastating disease that would help us in counselling them as to the expected course of their disease. More emphasis also needs to be laid on the detection of genetic defects that may correlate with the severity of disease and enable genetic counselling.
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Standard guidelines of care for vitiligo surgery. Indian J Dermatol Venereol Leprol. 2008;74:S37-45.
- [Google Scholar]
- Punch grafting as a treatment for residual lesions in vitiligo. Ind J Dermatol Venereol Leprol. 1992;58:315-9.
- [Google Scholar]
- Repigmentation in vitiligo vulguris by autologous minigrafting: Results in nineteen patients. J Am Acad Dermatol. 1995;33:990-5.
- [Google Scholar]
- Long-term follow-up study of segmental and focal vitiligo treated by autologous, noncultured melanocyte-keratinocyte cell transplantation. Arch Dermatol. 2004;140:1211-5.
- [Google Scholar]
- Autologous miniature skin punch grafting in vitiligo. Indian J Dermatol Venereol Leprol. 1995;61:77-80.
- [Google Scholar]
- Autologous miniatures punch grafting in vitiligo. Ind J Dermatol Venereol Leprol. 1992;58:310-4.
- [Google Scholar]
- Surgical treatment of Vitiligo: Why, when and how. J Eur Acad Dermatol Venereol. 2003;17:518-20.
- [Google Scholar]
- Treatment of refractory and stable vitiligo by transplantation of in vitro cultured epidermal autografts bearing melanocytes. J Am Acad Dermatol. 1992;26:230-6.
- [Google Scholar]
- Study of clinical, biochemical and immunological factors determining stability of disease in patients with generalized vitiligo undergoing melanocyte transplantation. Br J Dermatol. 2012;166:1230-6.
- [Google Scholar]
- Association of the Köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135:407-13.
- [Google Scholar]
- The minigrafting test for vitiligo: Detection of stable lesions for melanocyte transplantation. J Am Acad Dermatol. 1995;32:228-32.
- [Google Scholar]
- Clinico-cellular stability of vitiligo in surgical repigmentation: An unexplored frontier. Dermatology. 2004;209:170-1.
- [Google Scholar]
- How unstable is the concept of stability in surgical repigmentation of vitiligo? Dermatology. 2000;201:182-3.
- [Google Scholar]
- The concept of stability of vitiligo: A reappraisal. Indian J Dermatol. 2012;57:83-9.
- [Google Scholar]
- Preliminary evaluation of vitiligo using in vivo reflectance confocal microscopy. J Eur Acad Dermatol Venereol. 2007;21:1344-50.
- [Google Scholar]
- Catecholamines increase in the urine of non-segmental vitiligo especially during its active phase. Pigment Cell Res. 2003;16:111-6.
- [Google Scholar]
- A comparative study of oxidant-antioxidant status in stable and active vitiligo patients. Arch Dermatol Res. 2006;298:147-52.
- [Google Scholar]
- Levels of neuropeptide-Y in the plasma and skin tissue fluids of patients with vitiligo. J Dermatol Sci. 2001;27:178-82.
- [Google Scholar]
- Increased interleukin-6 and granulocyte-macrophage colony stimulating factor levels in the sera of patients with non-segmental vitiligo. J Dermatol Sci. 2003;31:73-8.
- [Google Scholar]
- Granulocyte/macrophate colony-stimulating factor is an intrinsic keratinocyte-derived growth factor for human melanocytes in UVA-induced melanosis. Biochem J. 1996;313:625-31.
- [Google Scholar]
- Detection of antibodies to melanocytes in vitiligo by specific immunoprecipitation. J Invest Dermatol. 1983;81:540-2.
- [Google Scholar]
- Coexistence and relationship of antikeratinocyte and antimelanocyte antibodies in patients with non-segmental-type vitiligo. J Invest Dermatol. 1993;100:823-8.
- [Google Scholar]
- Detection of antibodies to melanocytes in vitiligo by western immunoblotting. Yonsei Med J. 1996;37:365-70.
- [Google Scholar]
- Cytolytic antibodies to melanocytes in vitiligo. J Invest Dermatol. 1993;100:812-5.
- [Google Scholar]
- Enhanced susceptibility of melanocytes to different immunologic effector mechanisms in vitro: Potential mechanisms for postinflammatory hypopigmentation and vitiligo. Pigment Cell Melanoma Res. 1988;1:113-23.
- [Google Scholar]
- In vivo destruction of melanocytes by the IgG fraction of serum from patients with vitiligo. J Invest Dermatol. 1995;105:683-6.
- [Google Scholar]
- Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol. 1991;97:1078-80.
- [Google Scholar]
- Correlation between vitiligo antibodies and extent of depigmentation in vitiligo. J Am Acad Dermatol. 1986;15:978-81.
- [Google Scholar]
- Identification of pigment cell antigens defined by vitiligo antibodies. J Invest Dermatol. 1992;98:162-5.
- [Google Scholar]
- Tyrosinase as an autoantigen in patients with vitiligo. Clin Exp Immunol. 1996;105:84-8.
- [Google Scholar]
- Autoantigens in vitiligo identified by the serological selection of a phage-displayed melanocyte cDNA expression library. J Invest Dermatol. 2010;130:230-40.
- [Google Scholar]
- Anti-tyrosinase-related protein-2 immune response in vitiligo and melanoma patients receiving active-specific immunotherapy. J Invest Dermatol. 1998;111:1034-9.
- [Google Scholar]
- Autoantibodies to tyrosinase-related protein-1 detected in the sera of vitiligo patients using a quantitative radiobinding assay. Br J Dermatol. 1998;139:798-805.
- [Google Scholar]
- Autoantibodies against tyrosine hydroxylase in patients with non-segmental (generalised) vitiligo. Exp Dermatol. 2011;20:35-40.
- [Google Scholar]
- Vitiligo antibodies are not directed to tyrosinase. Arch Dermatol. 1999;135:417-22.
- [Google Scholar]
- The change of melanocyte cytotoxicity after systemic steroid treatment in vitiligo patients. J Dermatol Sci. 1993;6:201-5.
- [Google Scholar]
- A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res. 2006;19:406-11.
- [Google Scholar]
- A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res. 2003;16:322-32.
- [Google Scholar]
- Inflammatory changes in vitiligo: Stage I and II depigmentation. Am J Dermatopathol. 2004;26:108-12.
- [Google Scholar]
- Clinical and histopathologic characteristics of tri+chrome vitiligo. J Am Acad Dermatol. 2000;42:589-96.
- [Google Scholar]
- Abnormal expression of MHC class II and ICAM-1 by melanocytes in vitiligo. J Pathol. 1993;169:203-6.
- [Google Scholar]
- Role of apoptosis and melanocytorrhagy: A comparative study of melanocyte adhesion in stable and unstable vitiligo. Br J Dermatol. 2011;164:187-91.
- [Google Scholar]
- Antioxidant enzymes and lipid peroxidation at the tissue level in patients with stable and active vitiligo. Int J Dermatol. 2009;48:476-80.
- [Google Scholar]
- Immunohistochemical studies from vitiligo: Comparison between active and inactive lesions. Yonsei Med J. 1994;35:404-10.
- [Google Scholar]
- An immunohistological study of cutaneous lymphocytes in vitiligo. J Pathol. 1993;170:149-55.
- [Google Scholar]
- Reduced skin homing by functional treg in vitiligo. Pigment Cell Melanoma Res. 2010;23:276-86.
- [Google Scholar]
- Clinical features and histological findings are potential indicators of activity in lesions of common vitiligo. Br J Dermatol. 2013;168:265-71.
- [Google Scholar]






