Translate this page into:
Fat Ful‘fill’ment: A Review of Autologous Fat Grafting
Address for correspondence: Dr. Kiran Godse, Department of Dermatology, Dr. D.Y. Patil Medical College, Navi Mumbai, Maharashtra, India. E-mail: drgodse@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
For more than a century, clinicians have attempted to utilise fat for the treatment of tissue deficiencies and contour abnormalities. Autologous fat transplantation for soft-tissue augmentation has become increasingly popular in recent years. The popularity of tumescent liposuction has brought renewed interest and accessibility of fat for transplantation. Newer techniques and approaches to augmentation have provided more predictable and reproducible results. Fat augmentation has become an effective, safe and reliable method for restoring volume and correcting the atrophy that accompanies senescence. In this review, the authors have described their approach to fat transplantation.
Keywords
Autologous fat grafting
autologous dermal fillers
fat transplantation
INTRODUCTION
Dermal fillers are an important tool in the armamentarium of an aesthetic dermatologist in the management of ageing skin. A surge in the use of fillers has been witnessed owing to increasing awareness among people, easy availability of fillers and increased enthusiasm amongst the Dermatologists and plastic surgeons to use this modality. Fat has the potential to be the ideal soft-tissue filler because it is abundant, easily accessible, inexpensive, host compatible and because it can be harvested repeatedly. It also offers a similar long-term durability with a low-cost compared to dermal fillers.[1]
A large array of indications have been reported previously: Cosmetic enhancement and rejuvenation, body contour improvement and reconstruction of scarred sites, periocular rejuvenation, fat atrophy in human immunodeficiency virus positive patients, Parry-Romberg syndrome and radiation-damaged sites, among others. Multiple contributions and discoveries have also been made in this field and tumescence anaesthesia is the pillar of all these fat graft surgeries.[2] Use of platelet rich plasma for longer survival of grafts and the realization that fat contains stem cells are few of the new concepts that have gained importance nowadays.[3]
Even though, fat grafting has become widely used by plastic surgeons, most surgeons choose their method of fat grafting based almost entirely on anecdotal evidence. As more and more scientific studies appear in the literature, we now may have a more objective, scientific evidence to support the use of specific techniques. This paper focuses on the evolution of autologous fat grafting and giving a rationalised approach to it in present times.
HISTORICAL PERSPECTIVE
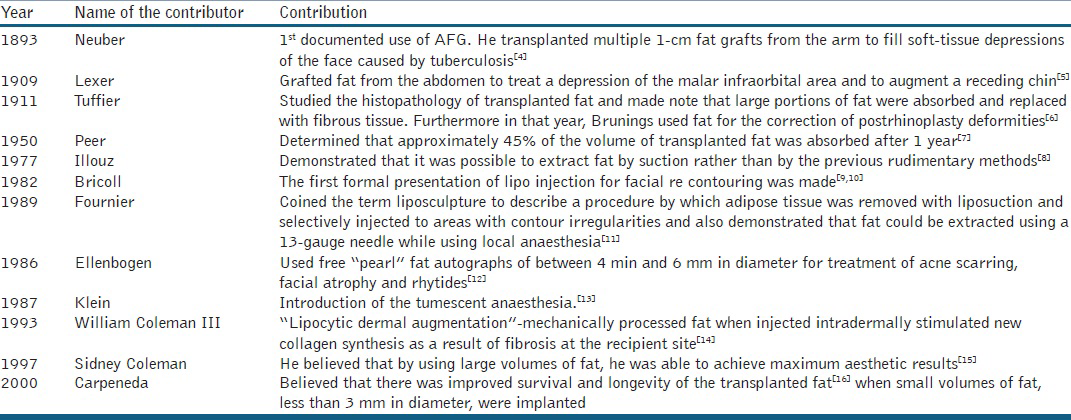
Autologous fat transplantation has been attempted for over a century with varying degrees of success. During this time, it has been alternately embraced and abandoned Table 1 shows the historical contributions made in the field of Autologous fat grafting.
In the early 2000s, appreciation of the potentials of adipose tissue and its related stromal elements, led to examination of the adipose-derived adult mesenchymal stem cell content.[21718] Evidence has clearly shown the key importance of the progenitor cells, stromal vascular fraction and extracellular matrix as integral contributors to the tissue maintenance and healing processes.[3]
TECHNIQUE
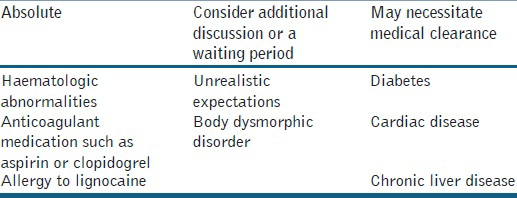
Pre-operative planning is critical when performing fat transplantation. During the pre-operative consultation, medications and allergies are reviewed, with specific concern given to medications that interfere with lidocaine metabolism. Patient is instructed to stop all medications that interfere with the platelet function, 2 weeks prior to surgery and can restart them 1 week after the procedure. Contraindications [Table 2][19] to fat transplantation should be noted and the procedure avoided in certain patients. Appropriate laboratory tests are obtained approximately 1 week before the procedure. Patient is instructed to take 500 mg of Cefadroxil the evening before and continue twice daily to 1 week after the procedure. In cases with a history of previous herpes labialis infection, 400 mg of acyclovir was given in the morning of the procedure and then twice daily for 1 week). Diazepam may be given to any anxious patient, approximately, 1 hr before the procedure.
More recently, the atraumatic technique has been popularized and known to many surgeons. This technique emphasises on atraumatic method of fat harvesting, proper centrifugation and injection aimed at maximising nutrition and structural integrity at the recipient site.[20] This basic fat grafting procedure was arbitrarily divided into four parts: Donor site selection, harvesting, processing and placement.
Donor site selection
Studies by Rohrich et al.[21] and Ullmann et al.[22] have shown that there is no evidence of a favourable donor site for the harvest of fat grafts. According to these two studies, the viability of lipocytes within the fat grafts from different donor sites may be considered equal. However, adipose tissue has recently been identified as a source of processed lipoaspirate cells or adipose-derived stem cells (ADSCs).[23] Padoin et al. evaluated that lower abdomen and inner thigh have higher concentrations of these processed lipoaspirate cells and the inner thigh and lower abdomen may be the better donor sites of adult ADSCs compared with other common donor sites.[24] Hence, fat grafts may not only serve as fillers, but also improve the quality of aged and scarred skin.
With what we know about the potential role of ADSCs in autologous fat grafting, the lower abdomen and inner thighs should; therefore, be chosen as the better donor sites for fat transplantation. We prefer using the thigh as a donor site as in the Indian setting, women usually wear saris and accidental asymmetry of the lower abdomen would be unacceptable. These donor sites are not only easily accessible by the surgeons with a patient in the supine position, but also scientifically sound because they have a higher concentration of stem cells than other donor sites as long as patients have an adequate amount of adipose tissue in those areas.[25]
Once the donor site is decided, the surgeon should also estimate approximate amounts that will be transplanted into each area so that the appropriate amount of fat is harvested. The area for liposuction is marked with a patient in the standing position after a sterile preparation. Often in a thinner patient, several areas have to be prepared and marked in order to accomplish the full harvest.
Method of harvest
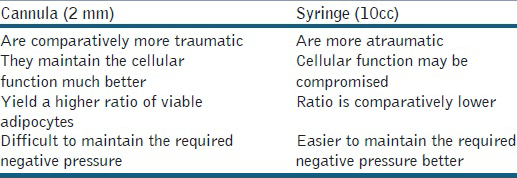
In our series, all patients were given short general anaesthesia as this helps to reduce anxiety and pain as per our experience. Then area to be suctioned was infiltrated with 500 ml of ringer lactate with 0.5cc of adrenaline. If small infiltrate is required then the procedure can be carried out under tumescent anaesthesia and in larger areas, tumescent anaesthesia with sedation is given [Table 3]. Once the area is infused, it is best to wait 15 min in order to allow epinephrine to take full effect and for the anaesthesia to infuse evenly through the tissues. If one suctions too quickly, the area tends to give an extract that has a greater percentage of tumescent fluid and is blood-tinged. There are various methods as options for harvesting the fat; Most of these comprehensive studies consistently support that the atraumatic technique for the harvest of fat grafts is superior to conventional liposuction.[26] We prefer using blunt atraumatic fat grafting cannulas since they are considered superior to the conventional liposuction as a preferred method of choice for fat graft harvesting.[27] Comparison between the cannula and syringe harvesting is given in Table 4.[2728]

The next question to tackle is, what is the proper size of the cannula and syringe favourable for aspiration to harvest fat grafts? A comprehensive study (Viable cell count, a cell proliferation assay, an enzyme assay and Oil Red O stain) conducted by Gonzalez et al. conclude that the viability of fat grafts is significantly better when fat graft is harvested by 2 mm diameter cannula with a blunt tip and several side holes connected to a 10cc syringe as compared with a 3 mm diameter blunt tipped cannula connected to a 60cc syringe.
To start with the harvesting, once the cannula attached to 10cc syringes is ready, the left hand makes a fold on the extraction site and the needle is pushed through the skin in the middle of the adipose tissue. The left hand is then placed flat on the extraction zone and will stay that way throughout the procedure. The right hand pulls out the syringe plunger completely and the surgeon begins the extraction [Figure 1]. Throughout the procedure, the plunger has to be kept in the same position; hence, syringe locks available in the market can be used for same. At different levels back and forth movements are made in a fan shape almost the length of the cannula. Four or five of these are made in one direction before going to neighbouring region, without taking out the cannula, which must remain under the skin. This process is then repeated with multiple syringes until the required amount of fat is harvested.
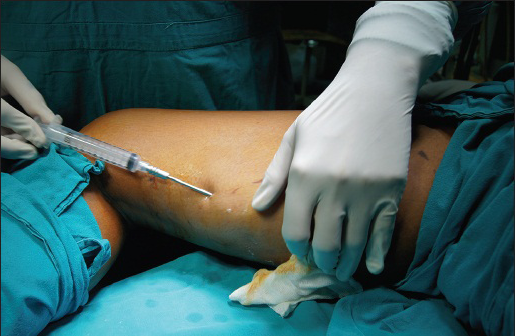
- Harvesting of fat grafts seen from the inner thigh
Method of process
Most surgeons believe that fat grafts harvested with syringe aspiration or conventional liposuction need to be processed in some way in order to limit the blood or oil within the lipoaspirates so that only pure fat as a soft-tissue filler will be used for injection. However, this has become a highly controversial issue and currently there is no agreement among surgeons in terms of which is the best method for processing fat grafts. Three primary methods (Sedimentation by gravity, filtering technique and centrifugation) have been used clinically to process fat grafts. Many experimental studies designed to compare these three refinement techniques were evaluated only by a single measurement selected by the investigators and thus, which method is better still remains debatable. Although comprehensive studies have now proved that centrifugation, may be more aggressive on adipocytes, but it clears the fat from most blood remnants are able to possibly maintain the highest concentration of stem cells within the processed lipoaspirates.[29] Since stem cell or angiogenic growth factor may play a role in fat graft survival, centrifugation at 3000 rpm (about 1289 g) for 3 min appears to offer more benefits and should be a better method of choice for processing fat grafts.[3031] After the centrifugation, layers are seen in the syringe, yellow supernatant and blood stained infranatant, this blood stained fluid collected in the lower layer is wicked away prior to injection [Figure 2]. We prefer using a manual centrifuge since it is not too harsh on the adipocytes and also gives a fairly good separation of blood and fat. In this centrifuge machine, the surgeon rotates the lever and this leads to desirable separation of the fat in approximately 5 min. Sedimentation technique relies mainly on gravity and is time consuming. It can be used when adjunct procedures are planned. Washing of grafts and filtration can also be carried out with commercially available systems.
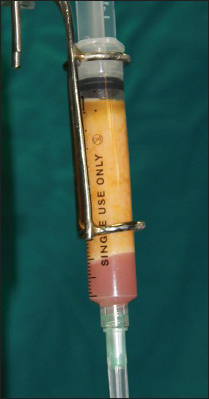
- Layers of supernatant yellow fat and infranatant blood-tinged fluid seen post-centrifugation
Anaesthesia of recipient site and method of placement of graft
For recipient site anaesthesia, the entry sites are anaesthetised and a regional nerve block is given. A standard 18 gauge needle is used to create the entry site and local anaesthesia is infiltrated with a blunt cannula. Now, a 10cc syringe full of adipose tissue is used and the injection begins. Approximately, 0.1cc is injected with every pass. This is carried out in an intermittent retrograde pattern while constantly verifying the evacuation of the syringes contents by its graduated scale. This fan shaped reinjection should be carried out at various different levels to obtain a harmonious result and restore the previous anatomy of the site [Figure 3]. This technique also makes sure that fat grafts have a maximal amount of contact with the vascularised tissue in the grafted area for better survival.[32] The placement of fat grafts in different tissue planes was also studied by Karacaoglu et al. in a rabbit face model. By measuring transplanted fat grafts morphometrically and histologically, the results reveal the survival of fat grafts is significantly higher if they are placed in supra muscular layer than in subcutaneous or submuscular layer. The findings of the study support the placement of fat grafts indifferent tissue planes to achieve a better result clinically.[33] We prefer, injecting the fat in the subcutaneous layer as the author feels the survival of the grafts is better in this layer.
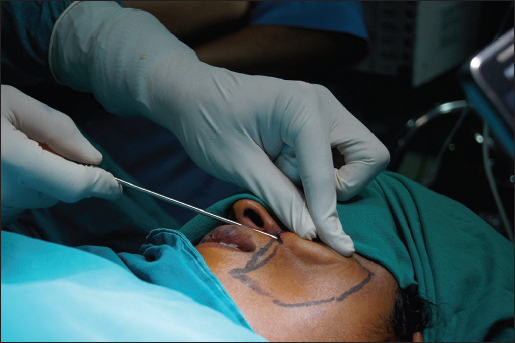
- Placement of the fat grafts in the recipient site
POST-OPERATIVE
Oedema varies from one patient to another. There are rarely ecchymoses and patients do not mention any pain or discomfort. Patients have a normal appearance after 3-5 days. However, the extraction zones take longer to return to normal. They have oedema and are indurated for several weeks.
When the filling is carried out in an ambulatory basis without general anaesthesia, the whole operation can be completed in 1.5-2 hrs. Antibiotics and anti-inflammatories are prescribed post-operatively, analgesics are not required [Figures 4a and b].
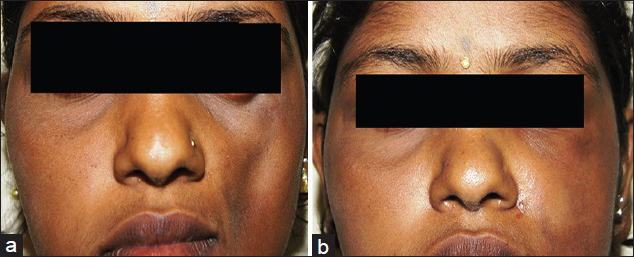
- (a) Hemifacial atrophy seen on the left side of the face. (b) Defect is filled post-operatively giving clinically acceptable symmetry
COMPLICATIONS
Fat transplantation is a relatively safe procedure with a low complication rate. Fat allows a patient to benefit from autologous tissue without risk of allergy, rejection or possible transmission of viral infection. The most common complication of fat transplantation is absorption of fat; this can be avoided by some overcorrection [Figures 5a and b] as per the author's preference. Unintentional overcorrection is also frequently encountered. This is particularly problematic in the infra orbital area where visible nodules may develop. Other complications are:
-
Superficial nodules can also result from the injection of an extremely large bolus of fat too superficially.[34]
-
Other common complications that may occur include post-operative erythema, oedema, bleeding and ecchymosis.
-
Infections following augmentation have been reported.[35] During the pre-operative period, the physician needs to screen for and if necessary, treat active, chronic or recurrent infections, particularly of adjacent facial areas such as sinus, dental or ocular regions. There are reports of the centrifuge serving as a source of infection with Pseudomonas. It is recommended that sterile centrifuge sleeves be utilized to decrease the chance of transmission.
-
Vascular occlusion or the development of emboli is the most serious complication associated with fat transplantation. A case of blindness following transplantation in the glabellar region was reported by Dreizen.[36] In addition, occlusion of the middle cerebral artery and ocular fat embolism has occurred following transplantation of fat in the face.[37] There was a report in the literature of an acute fatal stroke immediately following autologous fat transplantation in the face.[38] Vascular compromise is often indicated by dramatic blanching of the skin. In the rare event that this does occur, it is suggested that the patient be placed in the trendelenburg position, apply nitroglycerin paste and massage the area until blanching resolves.[39] It is believed that sharp instrumentation and 10-cc syringes with high injection pressures were involved in the majority of cases with vascular involvement. Coleman reports that the use of a blunt-tipped cannula with initial withdrawal prior to injection will decrease the risk of vascular penetration.[40] To safeguard against intravascular injections, it has also been suggested that physicians use1-cc syringes as well as epinephrine in the anaesthesia so as to promote vasoconstriction. Other recommendations postulated to decrease the risk of fat embolisation include slow injections of small aliquots of fat using low injection pressures. Furthermore, avoidance of fat injection into pre-traumatised tissue [41] is recommended.
-
Fat hypertrophy following weight gain may occur after augmentation. This may require surgical revision to correct.[42]
-
Depressions, asymmetries and bulges may occur.
-
Fat necrosis may lead to contour irregularities. A case, in which a growing liponecrotic pseudocyst developed in the submandibular area, 6 months following fat injection to the cheek and mandibular area was described.[43] In this scenario, it was suggested that the lesion should be excised as aspiration may cause leakage of contents and possible further granulomatous reactions.
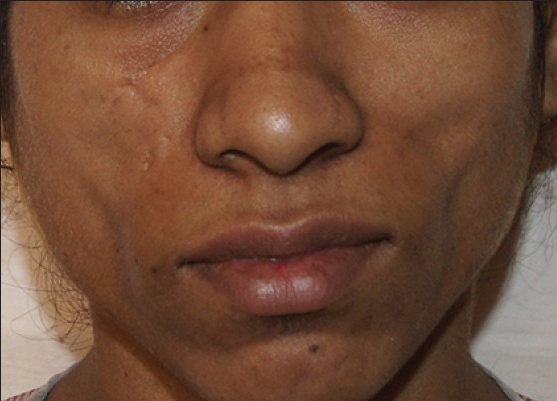
- Patient with bilateral loss of facial contour
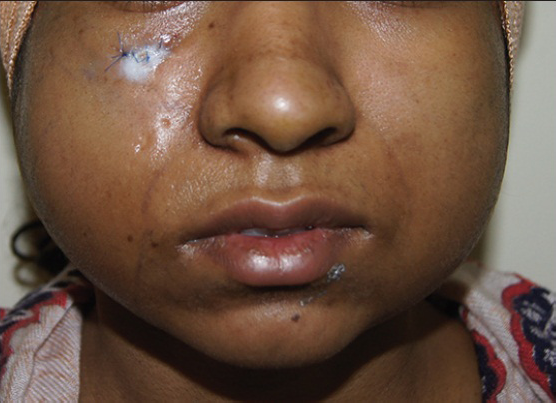
- Post-operative oedema and overcorrection seen
Graft survival
Since overall take rate of fat grafting by even more experienced surgeons ranges from about 50-90%,[44] additional procedures are always necessary to achieve an optimal result. However, there is no scientific study, which has addressed the timing of subsequent fat grafting. So far, only “expert” opinion has been mentioned in the literature regarding this specific issue.[45] In our experience, fat survival is for 6-8 months and a repeat procedure is required then.
CONCLUSION
This paper gives a brief review on autologous fat grafting, from evolution to current times. The often abundant supply of this autologous material in our patients requiring aesthetic correction following disease or intrinsic aging behoves us to refine our knowledge of this valuable technique. Furthermore with the new discovery of adipose-derived mesenchymal stem cells, there is added scientific interest now in this procedure. It is hoped that this article will help those new to the field of autologous fat grafting, like cutaneous surgeons, to better understand the procedure.
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Liposculpture in the superficial plane: Closed syringe system for improvements in fat removal and free fat transfer. Am J Cosmet Surg. 1992;11:127-34.
- [Google Scholar]
- Enhancement of autologous fat transplantation with platelet rich plasma. Am J Cosmet Surg. 2001;18:59-71.
- [Google Scholar]
- Contribution a l’etude desgreffes adipeueses. Bull Acad Roy Med Belgique. 1914;28:440.
- [Google Scholar]
- Loss of weight and volume in human fat grafts with postulation of “cell survival theory”. Plast Reconstr Surg. 1950;5:217.
- [Google Scholar]
- The fat cell “graft”: A new technique to fill depressions. Plast Reconstr Surg. 1986;78:122-3.
- [Google Scholar]
- Cervicofacial liposurgery. In: Bailey BJ, ed. Head and Neck Surgery- Otorhinology. Philadelphia: Lippincott- Williams and Wilkins; 2001. p. :2419-33.
- [Google Scholar]
- Free autogenous pearl fat grafts in the face – A preliminary report of a rediscovered technique. Ann Plast Surg. 1986;16:179-94.
- [Google Scholar]
- Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-63.
- [Google Scholar]
- Autologous collagen? Lipocytic dermal augmentation? A histopathologic study. J Dermatol Surg Oncol. 1993;19:1032-40.
- [Google Scholar]
- Current concepts of fat graft survival: Histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000;26:1159-66.
- [Google Scholar]
- Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17-26.
- [Google Scholar]
- Seeing the trees in the forest: Selective electroporation of adipocytes within adipose tissue. Am J Physiol Endocrinol Metab. 2004;287:E574-82.
- [Google Scholar]
- Fat transplantation for treatment of the senescent face. Dermatol Ther. 2006;19:169-76.
- [Google Scholar]
- In search of improved fat transfer viability: A quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg. 2004;113:391-5.
- [Google Scholar]
- Searching for the favorable donor site for fat injection: In vivo study using the nude mice model. Dermatol Surg. 2005;31:1304-7.
- [Google Scholar]
- Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211-28.
- [Google Scholar]
- Sources of processed lipoaspirate cells: Influence of donor site on cell concentration. Plast Reconstr Surg. 2008;122:614-8.
- [Google Scholar]
- Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222-8.
- [Google Scholar]
- Discussion: Sources of processed lipoaspirate cells: Influence of donor site on cell concentration. Plast Reconstr Surg. 2008;122:619-20.
- [Google Scholar]
- Autologous fat grafts harvested and refined by the Coleman technique: A comparative study. Plast Reconstr Surg. 2008;122:932-7.
- [Google Scholar]
- An alternative method for harvest and processing fat grafts: An in vitro study of cell viability and survival. Plast Reconstr Surg. 2007;120:285-94.
- [Google Scholar]
- Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: A comparative study. J Plast Reconstr Aesthet Surg. 2010;63:1375-81.
- [Google Scholar]
- Evaluation of centrifugation technique and effect of epinephrine on fat cell viability in autologous fat injection. Aesthet Surg J. 2009;29:35-9.
- [Google Scholar]
- Influences of centrifugation on cells and tissues in liposuction aspirates: Optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008;121:1033-41.
- [Google Scholar]
- A murine model for studying diffusely injected human fat. Plast Reconstr Surg. 2009;124:74-81.
- [Google Scholar]
- The role of recipient sites in fat-graft survival: Experimental study. Ann Plast Surg. 2005;55:63-8.
- [Google Scholar]
- Autologous fat augmentation and periorbital laser resurfacing complicated by abscess formation. Am J Cosmet Surg. 2003;20:155-7.
- [Google Scholar]
- Sudden unilateral visual loss after autologous fat injection into the glabellar area. Am J Ophthalmol. 1989;107:85-7.
- [Google Scholar]
- Middle cerebral artery occlusion and ocular fat embolism after autologous fat injection in the face. J Neurol. 1998;245:53-4.
- [Google Scholar]
- Acute fatal stroke immediately following autologous fat injection into the face. Neurology. 2003;61:1151-2.
- [Google Scholar]
- Tissue augmentation. In: Present and future. San Francisco, CA: American Academy of Dermatology Annual Meeting; 2000.
- [Google Scholar]
- Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-7.
- [Google Scholar]
- Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996;80:1026-7.
- [Google Scholar]
- Fat hypertrophy after autologous fat transfer. Ophthal Plast Reconstr Surg. 2002;18:228-31.
- [Google Scholar]
- Large liponecrotic pseudocyst formation following cheek augmentation by fat injection. Aesthetic Plast Surg. 1996;20:417-9.
- [Google Scholar]
- Autologous fat grafting to the reconstructed breast: The management of acquired contour deformities. Plast Reconstr Surg. 2009;124:409-18.
- [Google Scholar]
- Breast reconstruction and augmentation using pre-expansion and autologous fat transplantation. Clin Plast Surg. 2009;36:269-80. viii
- [Google Scholar]






