A case of granulomatous dermatitis following dermaroller therapy for acne scars treated with methotrexate: A difficult-to-treat condition
*Corresponding author: Preema Sinha, Department of Dermatology, Base Hospital, Lucknow, Uttar Pradesh, India. sinhapreema@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sinha P, Dash M, Madakshira MG, Sharma J. A case of granulomatous dermatitis following dermaroller therapy for acne scars treated with methotrexate: A difficult-to-treat condition. J Cutan Aesthet Surg. 2025;18:127-30. doi: 10.4103/JCAS.JCAS_26_23
Dear Editor,
Microneedling is one of the vast multitudes of procedures available for the correction of wrinkles, scars, pigmentary changes, striae, and uneven skin texture.1 The benefits of microneedling are achieved via collagen induction in the treated skin. Microneedling is a simple procedure, which can be performed on an outpatient basis. Due to ease of performance, it is one of the commonest modalities practiced for the management of acne scars. Sometimes, microneedling results in unwanted adverse effects such as persistent erythema, hyperpigmentation or hypopigmentation, hypertrophic scarring or keloid formation.1,2 We herein discuss a patient with acne scars who developed granulomatous reaction following dermaroller.
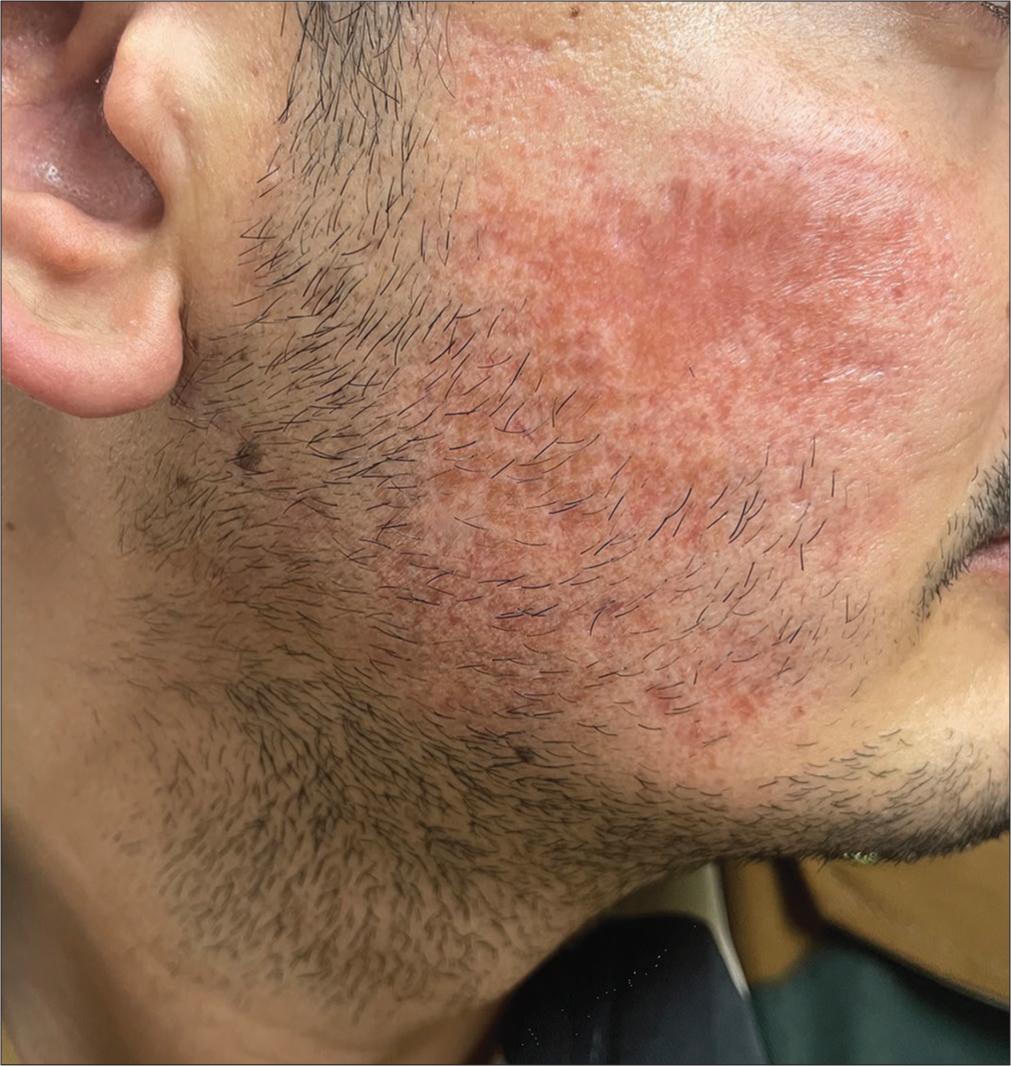
A 26-year-old male presented to us with the complaint of persistent erythematous plaques over bilateral cheeks of 16 months duration [Figures 1 and 2]. The patient had undergone three sessions of dermaroller for postacne scars over a period of 4 months at 4 weeks intervals before the onset of his lesions. He gave a history of sensitivity to sunlight in the form of a burning sensation in the lesions and an increase in the erythema. The eruption had persisted for the last 16 months despite numerous treatments, including topical and oral corticosteroids, metronidazole cream, intralesional corticosteroids, sunscreens, doxycycline (for 3 months), and dapsone (for 4 months) with minimal relief.
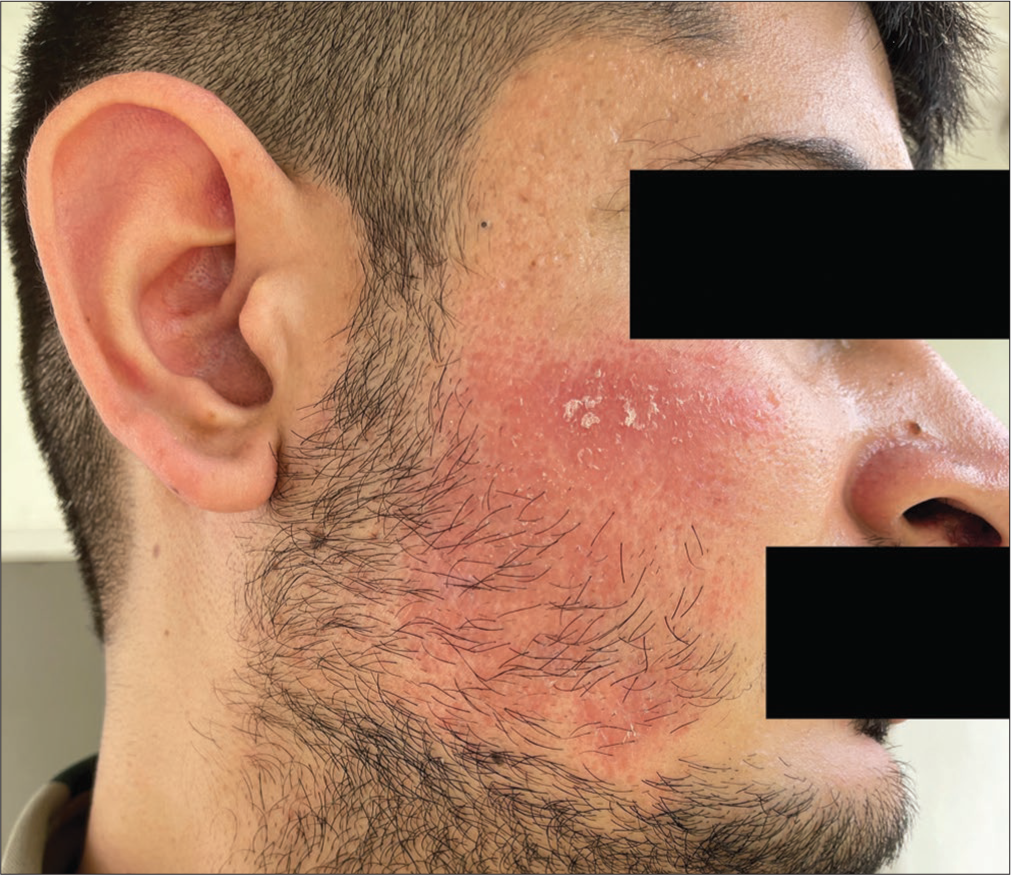
- Persistent erythematous plaque post dermaroller over the right cheek.
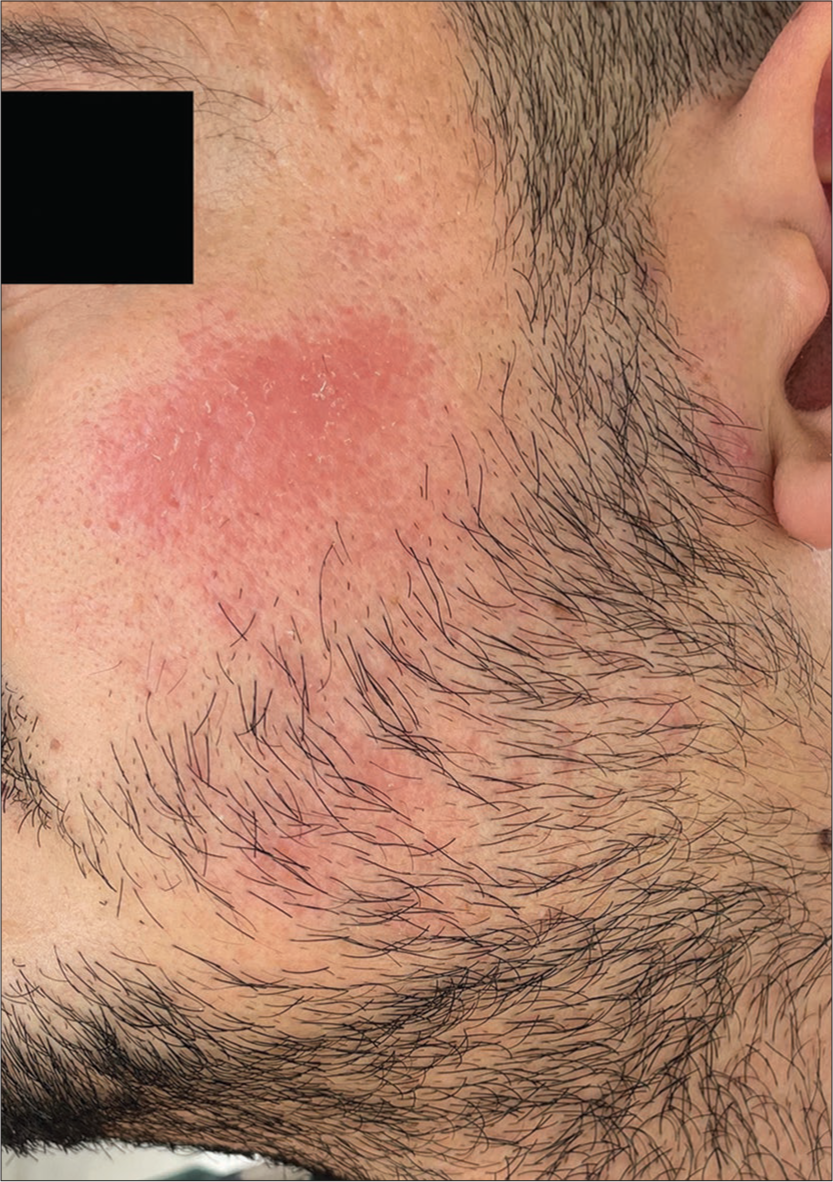
- Persistent erythematous plaque post dermaroller over the left cheek.
Histopathology revealed multiple ill-formed granulomas with aggregates of foamy histiocytes in the dermis. Multiple foreign-body giant cells were visible with two foreign-body giant cells showing intracytoplasmic refractile brownish foreign body [Figures 3 and 4]. Polarized microscopy showed that the foreign body was not polarized [Figure 5]. Periodic acid-Schiff, Gomori, and Ziehl-Neelsen stains did not highlight any organism.
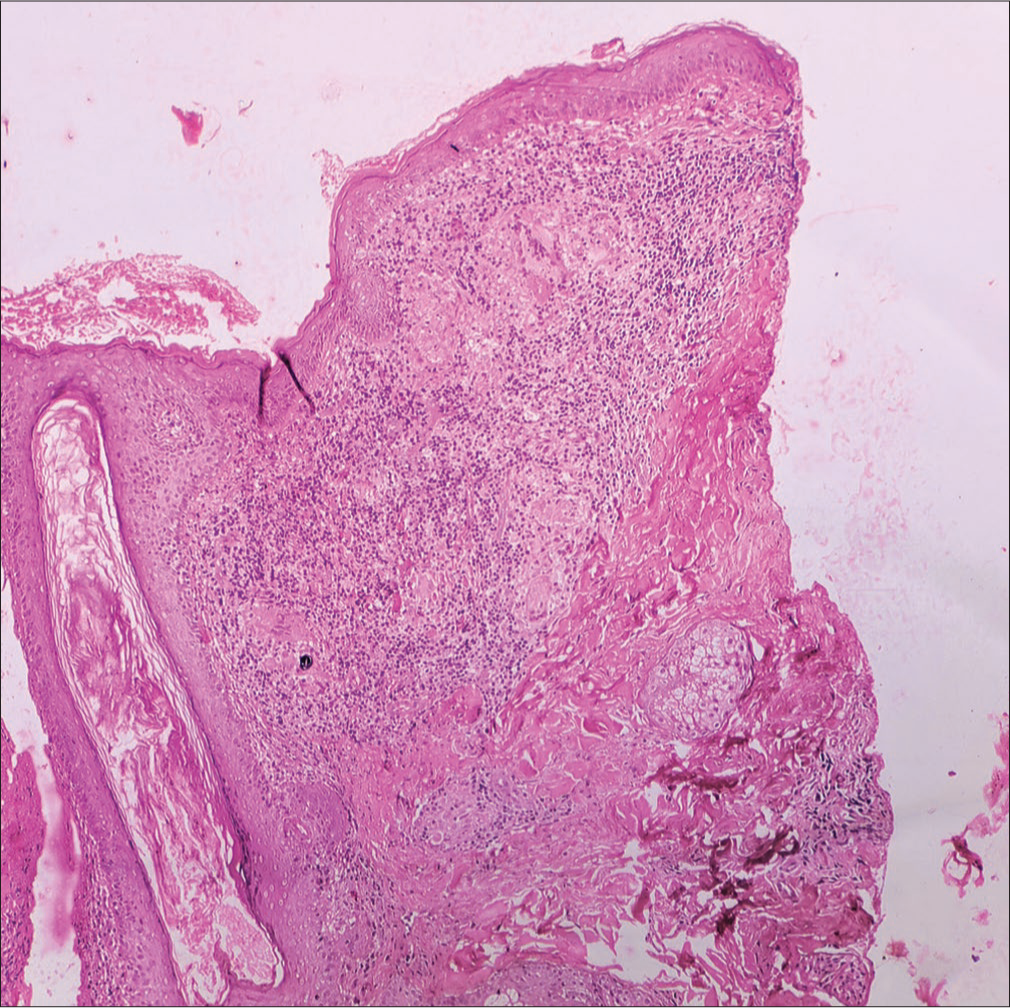
- Histopathology revealed multiple ill-formed granulomas with aggregates of foamy histiocytes in the dermis (H & E 100×). H & E = hematoxylin and eosin.
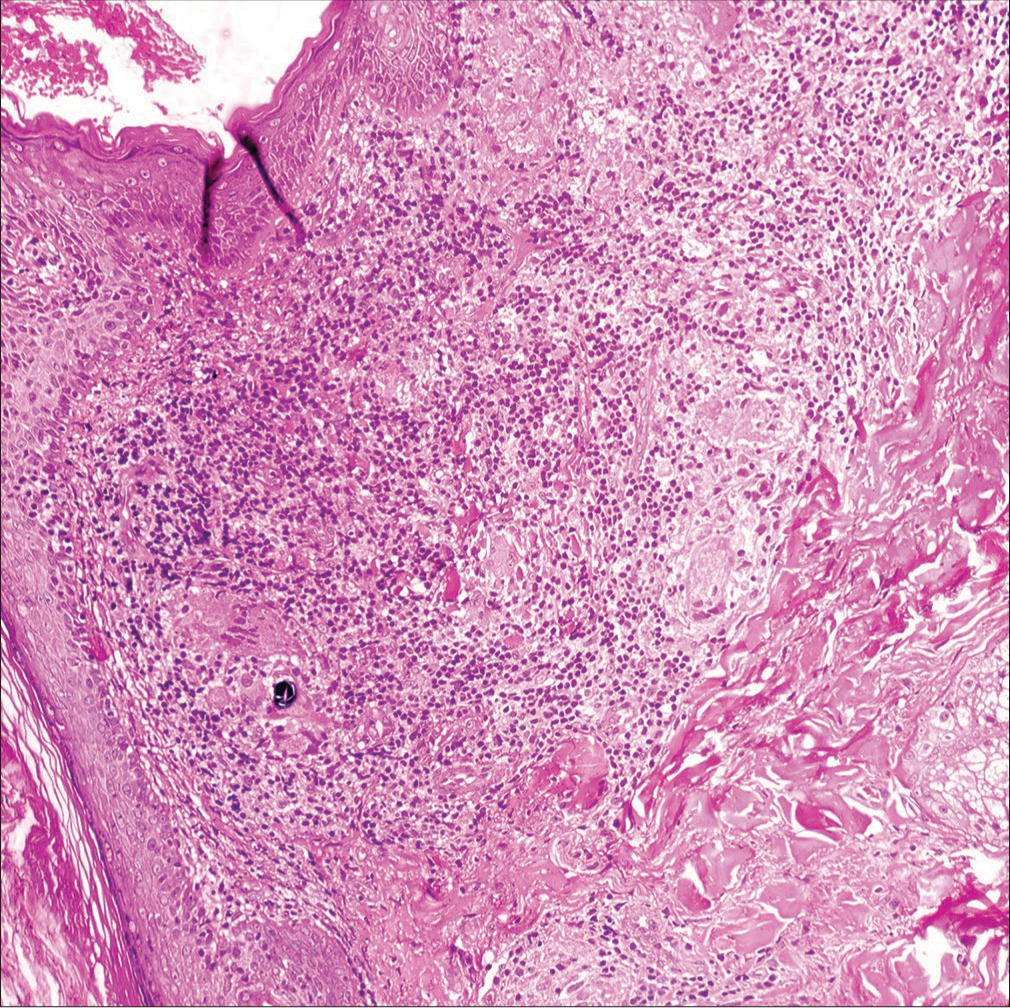
- Histopathology revealed multiple ill-formed granulomas with aggregates of foamy histiocytes in the dermis. Multiple foreign-body giant cells were visible with two foreign-body giant cells showing intracytoplasmic refractile brownish foreign bodies (H & E 200×). H & E = hematoxylin and eosin.

- Polarized microscopy showed that the foreign body was not polarized.
Our patient was finally diagnosed with a case of granulomatous dermatitis, which was in all probability induced by hypersensitivity to implantation of surface debris during the dermaroller sessions. He was managed with methotrexate at a dose of 15 mg/week and ointment of 0.1% tacrolimus at night and has shown significant improvement [Figures 6 and 7] over the last 3 months. He continues to be under monthly follow-up. The plan is to taper off methotrexate and maintain topical calcineurin inhibitors.
- Marked regression in induration of erythematous plaque over the right cheek after 12 weeks of treatment.
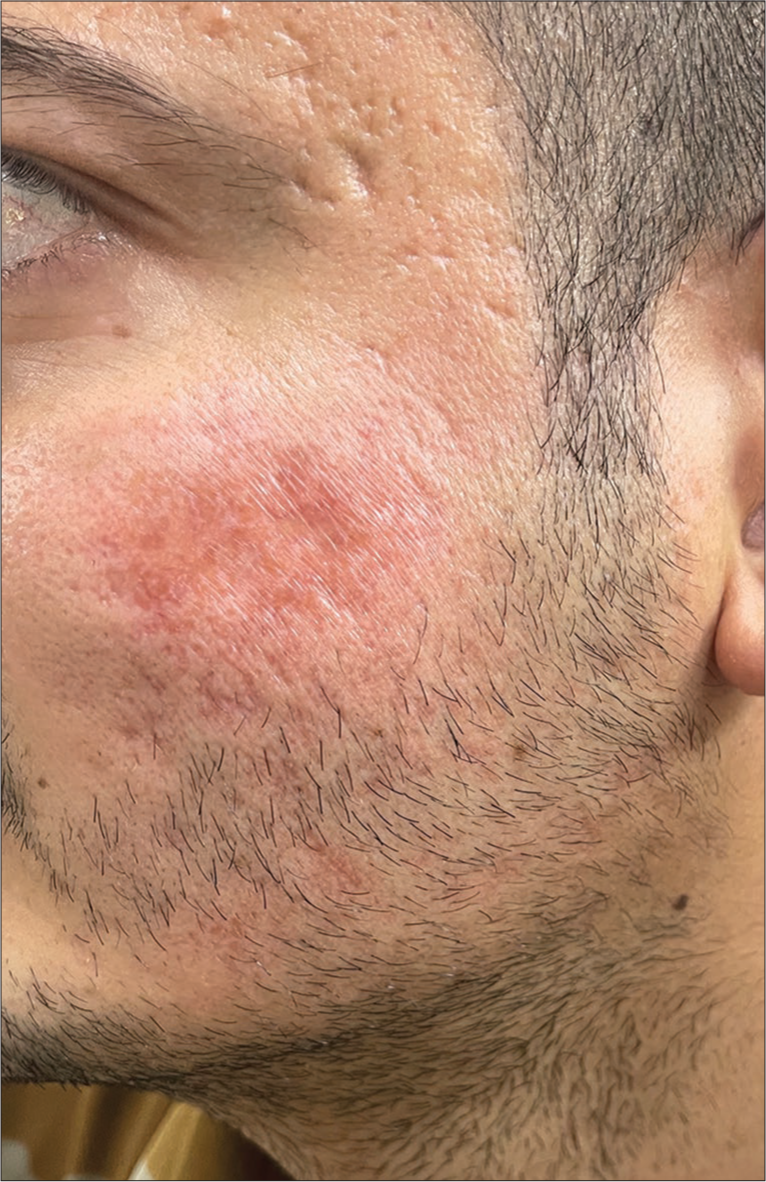
- Marked regression in induration of erythematous plaque over the left cheek after 12 weeks of treatment.
Postacne scars are a common presentation of patients attending any dermatology clinic. It causes major psychosocial morbidity to the affected patients. Microneedling is a common modality employed for the management of postacne scars due to its ease of performance. Though considered a safe and effective treatment for a myriad of dermatologic conditions, microneedling has been reported rarely to cause facial granulomatous reactions.2-4 These reactions are secondary to skin penetration of an antigenic topical product or needle material.3
Granulomatous reaction comprises focal aggregates of histiocytes in response to persistent inflammatory stimuli like foreign bodies or as a result of hypersensitivity. A review of literature revealed only a few cases including hypersensitivity to titanium where titanium-coated dermaroller was used and foreign body reaction to the application of nonstandardized topical cosmeceuticals.3,4
Methotrexate as a treatment modality for granulomatous inflammation finds a mention in reports by Martin et al.5 and Styperek et al.6 Styperek et al.6 reported improvement of granulomatous reactions secondary to dermal filler, the more common cause of such reactions in cosmetic dermatology.
We also found a good response to methotrexate in a patient who had been treated earlier with multiple modalities with minimal relief. This case emphasizes the adverse event of granulomatous dermatitis in the setting of microneedling and suggests a novel therapeutic option for such events.
Authors’ Contributions
Preema Sinha and Mahashweta Dash: Concepts, design, definition of intellectual content, literature search, manuscript preparation, manuscript editing, manuscript review. Manoj G. Madakshira and Juhi Sharma: Concepts, design, definition of intellectual content, manuscript editing, manuscript review.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72.
- [CrossRef] [PubMed] [Google Scholar]
- A cutaneous reaction to microneedling for postacne scarring caused by nickel hypersensitivity. Aesthet Surg J. 2016;36:NP168-70.
- [CrossRef] [PubMed] [Google Scholar]
- A case of a delayed granulomatous reaction on the face following microneedling: A case report. SAGE Open Med Case Rep. 2022;10:2050313X-221102489.
- [CrossRef] [PubMed] [Google Scholar]
- Resolution of microneedling-associated granulomatous dermatitis with oral methotrexate. JAAD Case Rep. 2021;10:96-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nonmedical-grade injections of permanent fillers: Medical and medicolegal considerations. J Clin Aesthet Dermatol. 2013;6:22-9.
- [Google Scholar]





