Translate this page into:
Extensive Abdominal Wall and Genital Pyoderma Gangrenosum: Combination Therapy in Unusual Presentations
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Dear Editor,
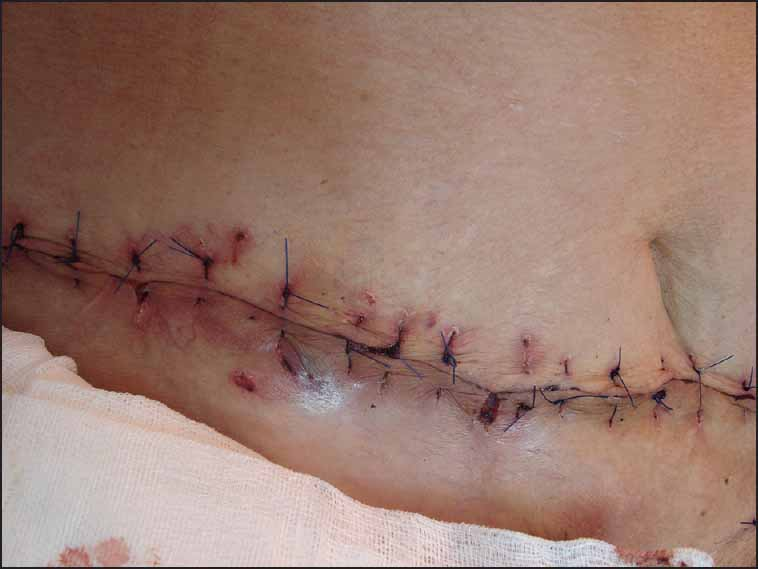
Pyoderma gangrenosum (PG) is a rare inflammatory disease of unknown aetiology characterised by neutrophilic infiltration of the dermis and destruction of the related tissue.[1] The precise aetiopathogenesis of PG is not well understood. However, immunological factors can be considered relevant in this respect.[2] There is no effective therapy for PG. The curative strategy is influenced by the number, size and depth of the wounds, the rate of extension and appearance of new wounds, the medical condition of the patient, the related diseases, and patient tolerance of prolonged therapy. The therapeutic aim is to decrease the inflammation in order to promote healing and decrease the pain and to control the associated under-lying disorders with the minimum adverse side effects.[3] A 73-year-old female patient [Figure 1] was referred to the dermatology department at Isfahan University of Medical Sciences, Isfahan, Iran, with 5-day old symptoms of severe sepsis and ulcers on the lower abdomen, and upper thigh that extended to the genitalia and perineum. The patient had first noted papules and vesicles on the lower abdominal wall a month before. These vesicles then ruptured and gradually developed erythematous wounds with no improvement forming a large ulcer. The ulcer then extended inferiorly to the external genitalia and groin. She was admitted with the impression of necrotising fasciitis. Despite treatment with broad spectrum systemic antibiotics, the lesions enlarged and gradually extended to the subcutis. On clinical examination one ulcer was seen on the lower abdomen. The surrounding area of the ulcer was red and inflamed. The ulcer was tender on palpation [Figure 1]. There were similar ulcers on her genitalia and upper thigh. Her past medical history was unremarkable except for arterial hypertension, controlled with treatment with captopril. HBs Ag, anti-hepatitis C virus antibody and ELISA tests for HIV were negative. No evidence of malignancy status was revealed. Based on these clinical findings, histology, and microbiology, a diagnosis of PG was made. Treatment included a high dose of prednisone 60 mg/day (0.9 mg/kg) with tapering to 25 mg/day after 2 months, and local treatment with topical clobetasol propionate and cromolyn sodium. To achieve a clean wound, the patient was referred to a surgeon and underwent one session of debridement treatment, and then the ulcers were sutured [Figures 2 and 3].

- Pyoderma gangrenosum. Before treatment: Clinical appearance of the lower abdomen (ulceronecrotic variant)

- Pyoderma gangrenosum. After surgical debridement on lower abdomen
- Pyoderma gangrenosum. After treatment: Healing lesion after surgical treatment and 1 month of prednisone therapy
No specific therapy is effective for patients with PG. Topical therapies contain gentle local wound care, topical corticosteroids, cromolyn sodium 2% solution, nitrogen mustard and 5-aminosalicylic acid. The topical immune modifiers such as tacrolimus and pimecrolimus may have some advantage in some cases. Systemic therapies contain corticosteroids, cyclosporine, mycophenolate mofetil, azathioprine, dapsone, tacrolimus, cyclophosphamide, chlorambucil, thalidomide, tumour necrosis factor-alpha inhibitors and nicotine. Intravenous therapies include pulsed methylprednisolone, pulsed cyclophosphamide and infliximab.[45] Surgical treatment can be considered in some cases but aggressive surgical debridement or skin grafting is discouraged because of the risk of a pathergic response.[56] Reported cases of surgical debridement and split skin grafts for PG lesions generally have poor outcomes. Perhaps these poor outcomes result from the pathergy phenomenon, a key feature in the disease process in which any traumatised skin (debridement sites or skin graft donor sites) develops additional necrosis and ulceration.[78] But our patient responded well to surgery without showing further progression of the disease; this may be due to the positive pathergy test which is positive in about 25% of all patients (others do not manifest the pathegy phenomenon).[9] According to literature, surgical therapy should be given in conjunction with systemic therapy. Removing necrotic tissue in certain cases may be helpful to prevent bacterial infections. In addition, skin grafting of wounds might decrease morbidity, the duration of wound care, and the period of the hospitalisation.[4] In conclusions, although surgical intervention is not recommended as standard practice because pathergy in the lesion is positive in 25% of the patients, surgical treatment combined with systemic treatment can be considered in some cases.
REFERENCES
- Postoperative pyoderma gangrenosum: A rare complication after appendectomy. J Postgrad Med. 2015;61:42-3.
- [Google Scholar]
- Treatment recommendations for pyoderma gangrenosum: An evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-83.
- [Google Scholar]
- Pyoderma gangrenosum: A review and update on new therapies. J Am Acad Dermatol. 2010;62:646-54.
- [Google Scholar]
- Management of pyoderma gangrenosum - an update. Indian J Dermatol Venereol Leprol. 2004;70:329-35.
- [Google Scholar]
- A rationale for adjuvant surgical intervention in pyoderma gangrenosum. Ann Plast Surg. 2001;46:23-8.
- [Google Scholar]
- Minimizing the risk of post-operative pyoderma gangrenosum. Br J Dermatol. 1992;127:45-8.
- [Google Scholar]





