Translate this page into:
Linear Leucoderma Following Intralesional Steroid: A Report of Three Cases
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Dear Editor,
Intralesional steroid therapy is an effective treatment modality for various dermatological and orthopaedic disorders, but it is associated with a few peculiar side effects. Linear hypopigmentation and depigmentation is a side effect, which is rare and merits discussion. We report three cases of linear depigmentation following intralesional triamcinolone injection for orthopaedics and dermatological indications. Two patients received intralesional triamcinolone acetonide injections for ganglion [Figures 1 and 2] and one patient received it for hypertrophic lichen planus [Figure 3]. The details of their treatment, disease onset and final outcome till last follow-up visit have been summarized in Table 1. First two patients refused to get biopsy. In the last patient, histopathology examination (hematoxylin and eosin stain, 200×) from depigmented macule showed normal number of melanocytes with decreased melanin content [Figure 4]. All patients were counseled about the condition, avoidance of intralesional steroid treatment and possibility of partial or complete repigmentation. Injectable steroids offer many advantages over systemic or topical preparations; causing less irritation and minimal side effects. Moreover, potent insoluble steroid compounds are longer acting and more effective due to significantly reduced dissolution at the site of injection and are therefore, preferred for chronic conditions. Triamcinolone acetonide is a macrocrystalline molecule and is a potent steroid. It has been used effectively intralesionally for the treatment of hypertrophic scars, keloids, alopecia areata, hypertrophic lichen planus and various non-dermatological conditions such as ganglion, tenosynovitis and intra-articularly for arthritis.[1] The concentration of triamcinolone acetonide varies from 2.5 to 40 mg depending upon the indication.
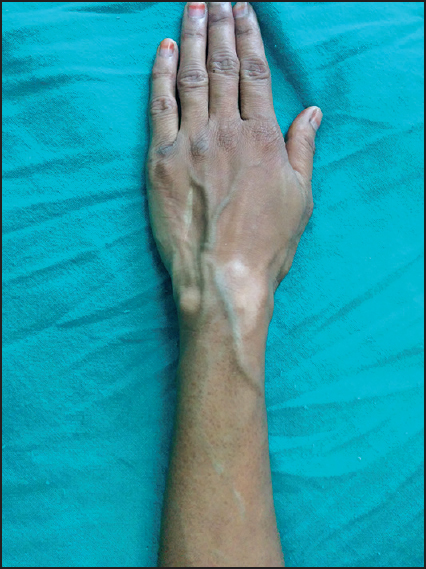
- Clinical photograph showing hypo- to depigmented macules associated with atrophy present over dorsal aspect of wrist and extending distally with a discrete lesion proximally over left forearm
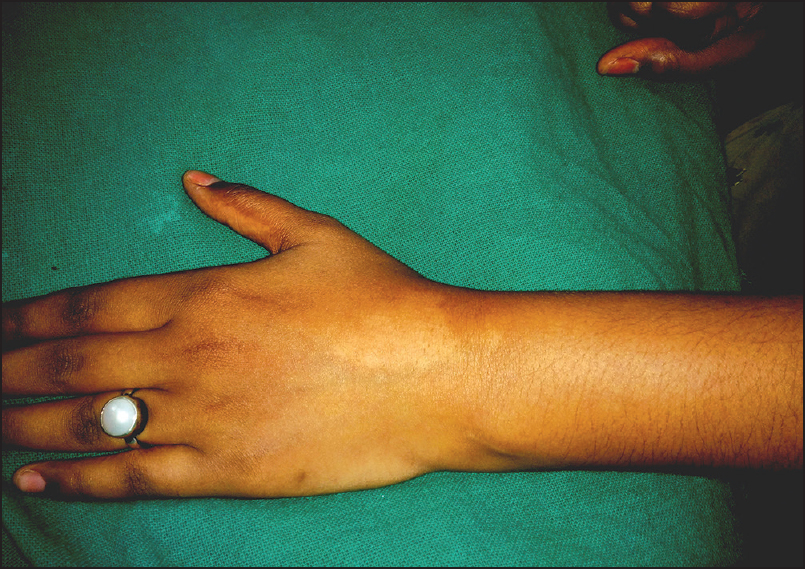
- Clinical photograph showing hypopigmented macules present over dorsal aspect of wrist and another discrete lesion proximally over left forearm
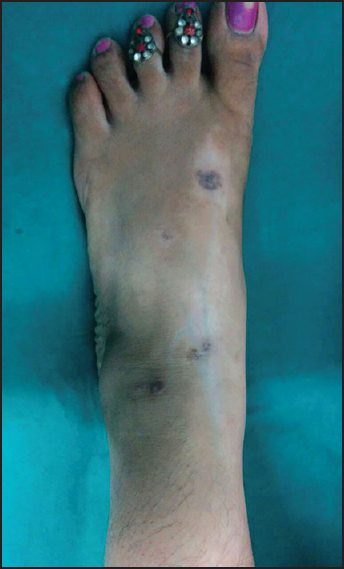
- Clinical photograph showing linear depigmentation extending from left foot to left lower leg along with flattened lesions of lichen planus
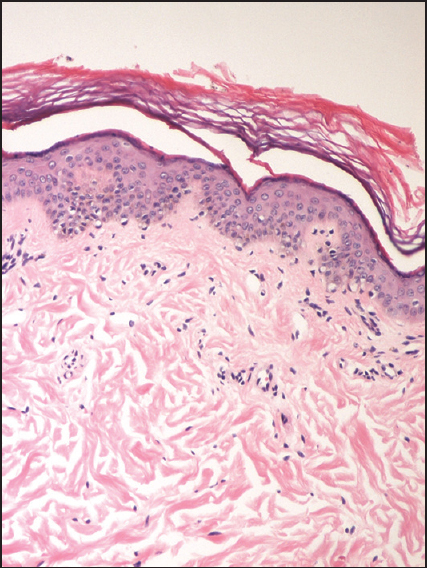
- Histopathological examination (hematoxylin and eosin stain) demonstrates the presence of normal number of melanocytes with decrease melanin content (200×)
Intralesional injections are an effective treatment modality; however, perilesional hypopigmentation, depigmentation, cutaneous atrophy, alopecia, infection, ulceration and localized dystrophic calcification are commonly observed local side effects.[1] Linear hypopigmentation or depigmentation following intralesional steroid is a rarely seen side effect with few reports in the literature and even fewer reports presenting in a linear distribution.[234] It may develop after a variable latency period ranging from a few weeks to months and may develop following a single, a few or even after several intralesional corticosteroid injections.[2]
The exact pathogenesis of corticosteroid-induced hypopigmentation is unknown. The hypothesized etiology relates to corticosteroids-induced inhibition of prostaglandin or cytokine production in various epidermal cells, thereby altering the melanocyte function by suppressing the secretory metabolic products from melanocytes without causing their destruction.[3] Friedman et al.[2] and Venkatesan et al.[4] demonstrated reduced melanin pigment and activity of melanocytes in the presence of normal melanocyte number, by histopathology added with histochemical staining. In addition, the exact pathogenesis of linear hypopigmentation or atrophy is also unknown. The most widely accepted mechanism is the lymphatic spread of the corticosteroid suspension along the lymphatics. Lymphatic vessels run in a unidirectional manner and are responsible for removal of macromolecules and proteins. Kikuchi et al. proved the relationship between these linear lesions with the lymphatic vessels after injecting Evans Blue Dye or Alphazurine 2 G (Patent Blue) into atrophic lesions.[5]
However, there are a few factors which predispose to this corticosteroid-induced hypopigmentation or depigmentaion. The concentration of the injected corticosteroid seems to be important. When the concentration increases, more of the steroid remains in the unbound state because the protein-binding capacity exceeds. This freely available steroid then enters cells and mediates effects. Moreover, depigmentation seems to be more likely with triamcinolone due to larger size, higher tendency to aggregate and higher density.[1]
There is no specific treatment for this condition and repigmentation, which may be partial in some cases might take several months. Further injections should be withheld and the patients should be kept under follow up.
To conclude, this case report highlights the potential side effect of depigmentation or hypopigmentation following injectable steroids and the related cosmetic distress to the patient. Therefore, it is emphasized that care should be taken to minimize this side effect. Appropriate concentration of steroid should be injected and excess drug and deeper injections into the underlying dermis and subcutaneous tissue should be avoided especially in hyperpigmented individuals.
REFERENCES
- Intralesional corticosteroid induced perilesional and perilymphatic hypopigmentation. Indian J Dermatol Venereol Leprol. 2002;68:356-7.
- [Google Scholar]
- Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy.Report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-41.
- [Google Scholar]
- Benefits and risks of intralesional corticosteroid injection in the treatment of dermatological diseases. Clin Exp Dermatol. 1995;20:363-70.
- [Google Scholar]
- Linear hypopigmentation and cutaneous atrophy following intra-articular steroid injections for de Quervain's tendonitis. J Drugs Dermatol. 2009;8:492-3.
- [Google Scholar]





