Translate this page into:
Periorbital Injectables: Understanding and Avoiding Complications
Address for correspondence: Dr. Catherine J Hwang, Cole Eye Institute, Cleveland Clinic Foundation, Cleveland OH, USA. E-mail: catherinehwangmd@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Periorbital rejuvenation with neurotoxins and dermal fillers address several aging changes. Safe and effective results require a thorough understanding of periorbital anatomy, proper injection techniques, and complications of these products. Prompt recognition and treatment of complications can minimize their adverse impacts. Complications can be divided into ischaemic and non-ischaemic effects. Hylauronidase, an enzyme that degrades hyaluronic acid, may improve outcomes after intravascular hyaluronic acid fillers.
Keywords
Periorbital
injectable
complication
blindness
fillers
hyaluronic acid gel filler
ischaemic complications
non-ischaemic complications
INTRODUCTION
Periorbital rejuvenation with botulinum toxin and dermal fillers is increasingly used to address the changes associated with aging. Understanding the periorbital anatomy, proper injection techniques and complications that can arise are important for every injector of this delicate area. However, even in the most skilled injectors, complications can arise and education, recognition and early treatment are of utmost importance.
PERIORBITAL ANATOMY
Understanding the underlying anatomy and aging changes of the periorbital presents a critical component to successful rejuvenation of the periorbital region.[1] A detailed description of anatomy is beyond the scope of this article, but understanding the soft tissue, vascular and bony anatomy is crucial for successful rejuvenation in the periorbital area. The periorbital region is one of the most dynamic and unforgiving in relation to volume enhancements. The eyelid skin is the thinnest in the body that lacks subcutaneous fat and is a dynamic structure. Irregularities are more readily apparent in this area than other areas of the face. Understanding the complex peri orbital anatomy is important to help avoid potential complications.
PERIORBITAL INJECTABLES
Both botulinum toxin and dermal fillers have been widely used to address periorbital aging. Complications arising from botulinum toxin injections are usually transient and related to its effect on the affected muscles. Most patients with complications arising from botulinum toxin injections can be treated supportively with ocular drops and referral to an ophthalmologist or oculoplastic surgeon may be considered.
Complications from dermal fillers are more varied and will be the main focus of this article. The classification of filler complications can be divided into early or late or into non-ischaemic and ischaemic complications.[23] In this article, the latter classification will be used.
Dermal fillers have been used effectively in the periorbital area for over 20 years.[45] Various dermal fillers are available; however, in the periorbital area, the reversible and temporary hyaluronic acid (HA) fillers are preferred [Table 1]. Proper selection and placement of product can help avoid some complications.[56] The use of permanent, non-reversible fillers are discouraged in the periorbital area as this area is constantly changing and complications with these fillers can be difficult to address. This article will mainly focus on non-ischaemic and ischaemic complications associated with HA fillers. Of note, when injecting HA fillers, hyaluronidase should be available.

NON-ISCHAEMIC COMPLICATIONS
Non-ischaemic complications include contour irregularities, bluish-discoloration or Tyndall effect, inflammatory reactions, and infection/biofilm formation [Figure 1]. Transient oedema and bruising are expected after any periorbital injection and can be minimised by avoiding blood thinners 2 weeks prior to injection, applying numbing cream with a vasoconstrictor such as epinephrine prior to injection and the use of cool compresses after injection. To help avoid contour irregularities, injections below the orbicularis or in the pre-periosteal plane will help to allow more coverage of the filler product. In addition, placement of small amounts of filler product, in a cross-hatched pattern or small amounts on the periosteum may be helpful.[5] To help avoid persistent oedema after HA fillers, it is important to note those patients that are prone to retaining fluid. Patients with fluid around their eyes prior to injection may not be ideal candidates for HA fillers and this should be discussed with the patient prior to treatment. If the contour irregularities or oedema does not resolve over time, dissolving the HA filler with hyaluronidase can be performed. In these cases, small amounts of hyaluronidase can be injected to the desired effect, usually around 15–50 IU.[78]
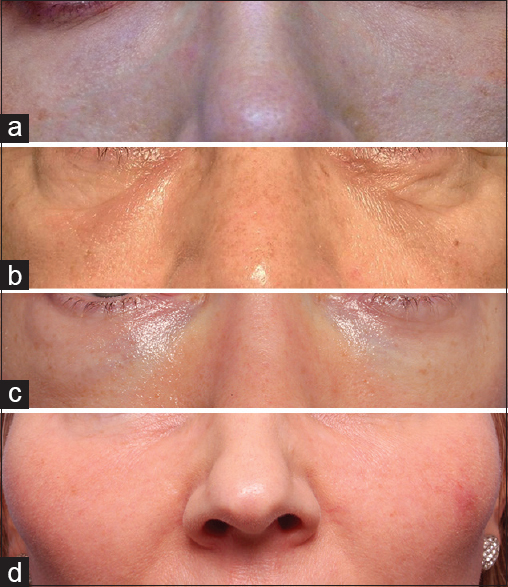
- (a) Tyndall effect, (b) contour irregularity, (c) persistent oedema, (d) inflammation/granuloma
The bluish-hue or Tyndall effect is commonly seen in lighter thin skinner patients. Sometimes this bluish-hue cannot be avoided and should be discussed with patients prior to injection. In addition, over time in some cases, there can be anterior migration of hyaluronic acid gel (HAG) filler product, causing more of a bluish hue. In these cases, the bluish-hue can be addressed with dissolving of the HA filler product with hyaluronidase.
In patients presenting with inflammatory signs after periorbital filler injection, infection should be highly suspected. True allergic reactions can occur but are less common. To minimise infection, thorough sterilisation of the treatment site should be performed prior to injection. Fillers should be treated as implants as that is what their use is approved as in the United States by the Food and Drug Administration. In those patients with possible infections, treatment with systemic anti-biotics should be initiated and close follow-up was performed. After the initial inflammation or infection is treated, hyaluronidase can be used to dissolve the HA filler product. Hyaluronidase is not recommended in cases of active infection because it could spread the infection.[9] In cases of suspected biofilm, more intense anti-biotic regimens should be considered as well as potential consultation with an infectious disease specialist. Biofilms tend to have more of an indolent inflammation/infection and the causative bacteria can be elusive. Eventually, the HAG filler product should be dissolved. In cases of granuloma formation, the HA filler product can also be dissolved to prevent further foreign body reaction. In patients with a history of inflammatory reactions, further injections with filler products should be made with caution.
Very rare complications including the inadvertent globe penetration with filler product has also been reported.[10] Using proper injection technique and being aware of the location of the needle tip at all times can help prevent globe penetration. Only injectors with the understanding of the periorbital anatomy and its complications should inject this complex region.
ISCHAEMIC COMPLICATIONS
Ischaemic complications related to filler injections, such as soft tissue necrosis and visual compromise, can occur and should be discussed with patients receiving any filler injection.[111213] The incidence of vascular occlusion has been reported to be up to 3 in 1000 injections.[9] For HA injections, the incidence of vascular occlusion may be a bit less at 3–9/10,000 injections.[12] The most high-risk areas include the glabellae and nasal area, but can also occur anywhere arteries run including the lip, nasolabial fold and temple.[1113] There are tips to help prevent accidental intra-arterial injection of product but no technique is 100% effective in avoiding ischaemic complications [Table 2]. Some techniques that can be useful are using local anaesthetics with epinephrine to vasoconstrict vessels prior to injection, injecting small volumes per pass, aspiration prior to injection, using low injection pressure, avoiding scarred areas and considering the use of blunt cannulas.[91113] However, remember cannulas can also act similarly to needles, especially those with smaller calibre and those used in scarred areas where the vessels are more likely to be tethered.

The mechanism of action of filler-associated ischaemia is due to direct intra-arterial injection of product.[1415] With injection, it is proposed that the filer product enters the artery initially in a retrograde fashion then once the plunger is released, anterograde embolisation of filler product occurs. Because of this, a larger area than the injection site along a vascular distribution is affected. In the periorbital area, the vascular structures to be aware of include the supraorbital, supratrochlear, infraorbital and angular arteries. In cases of filler induced ischaemia, early recognition and treatment is a key in treating patients.
SOFT TISSUE ISCHAEMIA
Signs of soft tissue ischaemia include whitening or blanching on injection, pain, mottling, blister formation, bluish discoloration and later tissue necrosis.[16] Not all of these signs may be present. The whitening or blanching may be transient and unnoticed and pain may not be present as anaesthetics are often concurrently present. Mottling in the area of a vascular distribution larger than the injected area is a clue that vascular ischaemia is occurring [Figure 2]. In cases of blister formation, ischaemia can be confused with a herpetic infection. In these cases, it is important to note the location of the vesicle formation and whether it respects a vascular distribution. The mottled appearance can then change into a bluish discoloration, which may appear like a large bruise [Figure 3]. With this, vascular dilation with increased artery pulsation occurs and can be seen on colour Doppler ultrasound [Figure 4]. Tissue necrosis or eschar formation appears even later. Once the deep dermal layers are affected, scarring will likely occur.
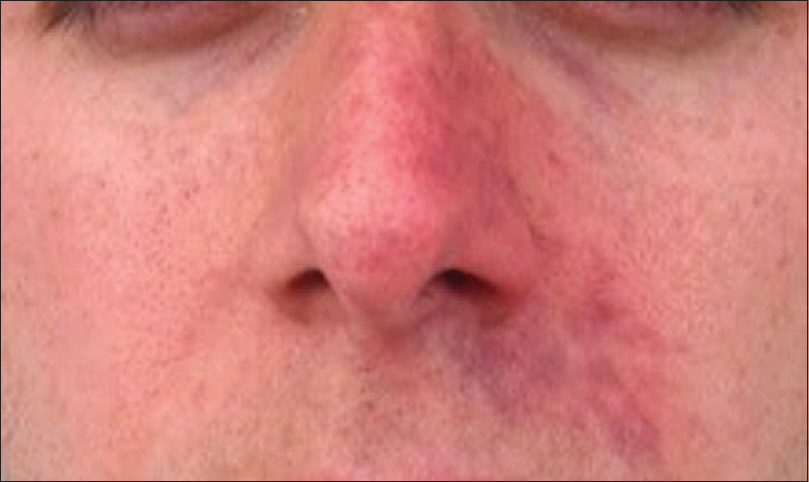
- Mottling in the area of a vascular distribution, sometimes respecting the midline, larger than the injected area
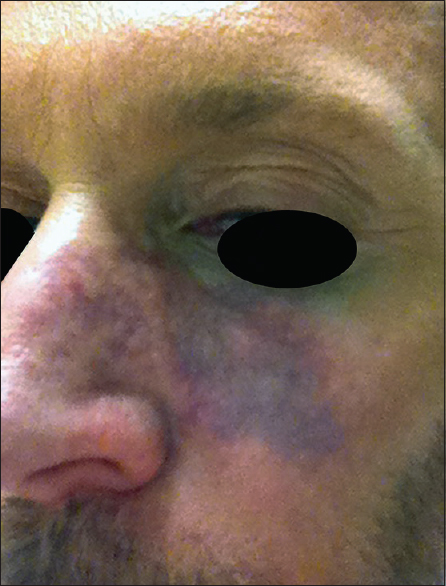
- Soft tissue ischaemia can become a bluish discoloration, which may appear like a large bruise
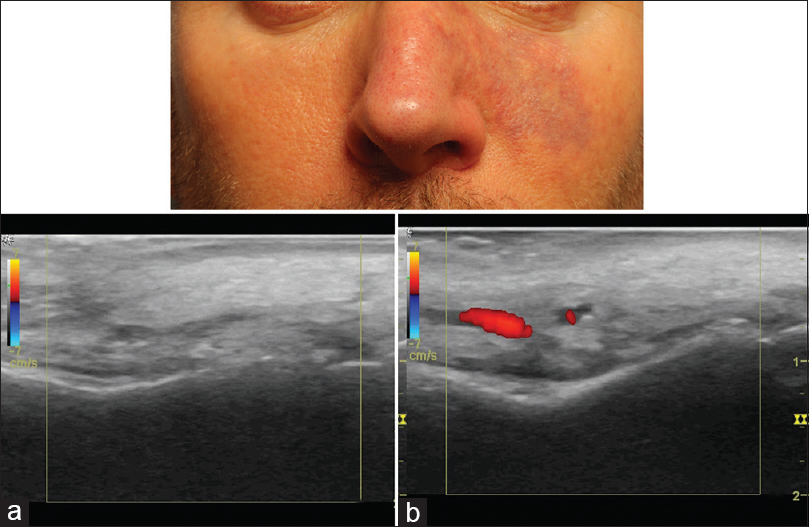
- Colour Doppler ultrasound: Vascular dilation (arterial) on affected side (b). (a) Affected side
Various treatment protocols have been suggested in cases of soft tissue ischaemia.[17] Treatments such as aspirin to prevent platelet aggregation, warm compresses to improve circulation, nitroglycerin paste, other vasodilators, hyaluronidase and hyperbaric oxygen have been reported to have been used.[1218] The use of nitroglycerinpaste is controversial in cases of filler induced ischaemia. Nitroglycerin paste has been used successfully in flap ischaemia; however, in filler-induced ischaemia, the vessels are occluded with particles and dilation may cause further worsening of ischaemia by further downstream embolisation of product.[1920] In addition, nitroglycerin paste is more of a venodilator.[21] The use of topical nitroglycerin in filler-induced ischaemia has little evidence and may have other unwanted systemic side effects; therefore, its use is not necessary in cases of filler induced ischaemia. The only proven treatment for soft tissue ischaemia in cases of HA filler is the use of early high-dose hyaluronidase. In a rabbit ear animal model, high-dose hyaluronidase (750 IU) given in the area of ischaemia within 4 h of ischaemia, resolved the filler-induced ischaemia; after 24 h, there was no benefit.[15] In our experience, patients who have presented within 24 h and treated with high-dose hyaluronidase until the resolution of ischaemia (in the range of 400–1500 IU; can be repeated every 24 h) in the entire area of the tissue ischaemia had no long-term scarring or sequlae. Even if patients present after 24 h, we still recommend treatment with high-dose hyaluronidase as we have had success in reducing ischaemia and scarring; however, the sooner the treatment the better the outcome. We have had also some success with the use of intra-arterial and subcutaneous hyaluronidase in the area of ischaemia with rapid resolution of ischaemia [Figure 5]. Intra-arterial hyaluronidase is not necessary with soft tissue ischaemia but can help resolve the ischaemia more rapidly and could be considered in instances of visual compromise, which we will discuss later. In the patients treated with intra-arterial hyaluronidase, we do discuss the risk of further embolisation of product if the filler product does not dissolve completely, so careful selection of patients is warranted. Intra-vascular hyaluronidase has been used safely in patients with myocardial infarctions and the half-life in the circulation is approximately 2–5 min.[2223] Other experts have suggested the use of high-dose subcutaneous hyaluronidase hourly starting around 400 IU until normalisation of capillary refill of the affected area (DeLorenzi C, presentation at American Society of Ophthalmic Plastic and Reconstructive Surgery Las Vegas November 2015).[24] The bottom line is early treatment with high-dose hyaluronidase in the entire area of ischaemia in cases of HA associated filler ischaemia is imperative.
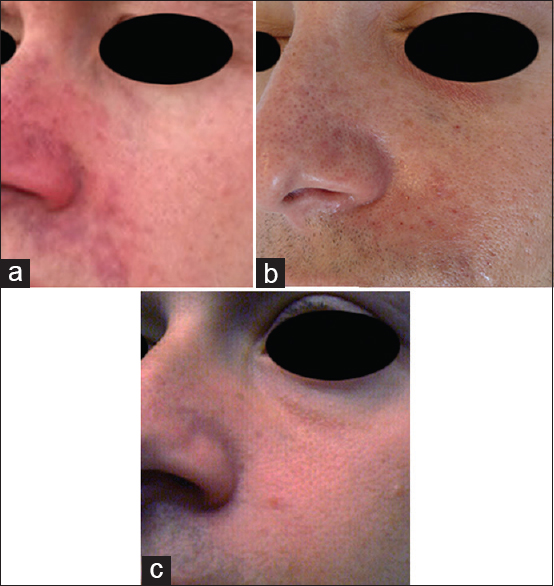
- Patient after treatment with intra-arterial and subcutaneous hyaluronidase with rapid resolution of ischaemia. (a) Initial presentation, (b) 12 h post-intra-arterial and subcutaneous hyaluronidase, (c) 72 h post-intra-arterial and subcutaneous hyaluronidase
VISUAL COMPROMISE
More cases of visual compromise including vision loss, decreased eye movements or ophthalmoplegia, droopy eyelid or ptosis and cerebral infarct are being reported with filler injections.[112526272829303132] When visual compromise occurs, it is immediate and usually associated with pain. It can also be associated with soft tissue ischaemia. Beleznay et al. compiled cases from 1906 to 2015 and found a total of 98 reported cases of dermal filler eye related complications, with most reported in the past 5 years. Twenty-three cases were found to be associated with HA filler injection. Most cases of visual compromise have been reported from South Korea, perhaps secondary due to their large volume of facial filling and/or location of facial augmentation.[11]
In 2014, our institution conducted a nationwide survey of retinal specialists with the American Society of Retina Specialists, similar to that performed by the Korean Retina Society.[26] The survey was carried out after the Institutional Review Board approval using the Survey Monkey system from October 15, 2014 to December 1, 2014. From this survey, 127 of the 720 retinal specialists responded. The survey captured a total of four cases; however, three had been reported previously.[30] The additional case was that of an unreported filler product injected in an unreported location of the face by a plastic surgeon. The patient had a branch retinal artery occlusion (BRAO) and their initial visual acuity was 20/20. Our survey suggests perhaps the incidence of visual compromise in the United States is less than in Korea, but could still be underreported. A method to anonymously report complications may help improve case reporting. With the increasing use of fillers worldwide, we are likely to see more cases of visual compromise.
The most high-risk injection areas associated with visual compromise include the glabellae, forehead, nasal region, nasolabial folds and temple.[11] The arteries in these high-risk areas have direct communication with the ophthalmic artery.[33] The mechanism of action of visual compromise is thought to be that product is pushed retrograde thru an artery with connection to the ophthalmic artery higher than arterial pressure. Once the plunger is released the circulation takes over and the filler particles travel anterograde and enter the ophthalmic artery and its various branches, causing vision loss, ophthalmoplegia, ptosis and/or ocular ischaemia. If the filler product is pushed further retrograde, it can enter the brain circulation and cause cerebral infarction via the internal carotid artery.[29]
Park et al. described six types of visual compromise based on the postulated level of occlusion of the ophthalmic artery and patient symptoms: Ophthalmic artery occlusion (OAO), generalised posterior ciliary artery occlusion (PCAO) with relative central retinal artery sparing, central retinal artery occlusion (CRAO), localised PCAO, BRAO and posterior ischaemic optic neuropathy (PION). They were also divided into diffuse occlusions (OAO, generalised PCAO, CRAO) and localised occlusions (localised PCAO, BRAO, PION). Most of the patients presenting with OAO, a more diffuse occlusion had undergone autologous fat injections. The occlusion of the ophthalmic artery, with an approximate diameter of 2 mm, would require a larger particle size such as autologous fat; however, they did report three cases of OAO with HA.[2634] In addition, a majority of the patients with OAO and fat injection had cerebral infarction, which may be due to the larger volume and injection pressure used during autologous fat injections. In contrast, the central retinal artery is approximately 160 µm in diameter.[35] In the study by Park, six of the eight CRAO had undergone fat injections, one with HA gel and one with collagen filler.[26] Still, with HAG filler products, care should be taken to inject slowly and under low injection pressure to reduce the potentiation of particles retrograde into the ophthalmic artery. The smaller HAG filler particles can potentially travel further anterograde for a more localised effect and occlude the central retinal artery, branch retinal artery, or posterior ciliary arteries.[26] Beleznay et al. also showed that HA filler injections have less serious ocular outcomes and central nervous changes, most likely related to the smaller particle size; however, if there was visual loss initially, there would likely be no improvement in visual outcome.[11]
Till date, there are no proven treatments for visual compromise secondary to filler injections. Many theories have been postulated but none proven.[11] For more diffuse occlusions, perhaps measures to restore ophthalmic artery flow can be tried. Injection of hyaluronidase either into the orbit (retrobulbar) has been proposed but to date has not been reported to be successful. With orbital or retrobulbar injection of hyaluronidase, if the ophthalmic circulation is not flowing, it will not likely reach distally into the retinal circulation to improve perfusion. Direct hyaluronidase injection into the vitreous cavity to dissolve product in the retinal circulation could potentially be another option; however, no cases have been reported of its use in humans. Hyaluronidase injection into the vitreous cavity for other indications has been used in the past safely.[363738] Direct intra-arterial injection to the ophthalmic artery, via interventional neuroradiology, or cannulating the supraorbital or supratrochlear arteries has also been proposed.[3940] We have had success in cases of soft tissue ischaemia and could be a potential avenue of treatment. Further investigation into potential uses of hyaluronidase in cases of visual compromise is needed and treatment may be a combination of the above.
Current treatments for filler-induced CRAOs attempt to dislodge the emboli from the retinal circulation. These are the same measures used in non-filler-associated CRAOs including lowering the intra-ocular pressure with drops and or systemic medications such as acetazolamide and mannitol, anterior chamber paracentesis and ocular massage.[26] Other treatments that have been reported but are unproven are corticosteroids, thrombolysis, vasodilatory agents and anti-coagulants.[1241] If visual compromise is encountered, patients should be referred promptly to an eye specialist, preferably a retina specialist, within 90 min.[31] Unfortunately, even with early recognition and treatment, the visual loss associated with filler injections is largely irreversible. Ophthalmoplegia and eyelid ptosis usually recover, likely due to the rich collateral circulation of the muscles and the lack of sensitivity to reduced blood flow.
When injecting filler products, it is important to remember to use low injection pressure as well as use small volumes per pass or area. Even though there are lower reports of ischaemic events in the periorbital area, care should be taken when injecting this vascular area. In addition, perhaps the lower reporting of vascular complication in the periorbital area is that only injectors who are comfortable with this region and understand the anatomy inject this area.
HYALURONIDASE
When injecting HA fillers, hyaluronidase is beneficial to aid with corrections as well as severe complications such as vascular compromise.[94243] There are two classes of hyaluronidase: Human recombinant or animal derived (bovine or ovine).[4445] The duration of action of hyaluronidase in soft tissue is approximately 24–48 h and intravascularly around 3–5 min.[23]
In patients with vascular compromise, skin testing is not needed but could be considered in non-urgent cases for allergic reaction in a non-urgent situation can be performed.[2343] In addition, there is no documented adverse effect of high-dose hyaluronidase on native tissue. We have treated patients with over 1500 IU in a single session without sequlae. Further investigations into the optimal dose, formulation and timing of hyaluronidase treatment for each complication, especially vascular compromise, needs to be performed. In addition, HA fillers have different degradation responses to hyaluronidase and should be considered when dissolving each individual product.[434647] We recommend having hyaluronidase available in any formulation available when injecting HA fillers.
Summary
Periorbital rejuvenation with injectables, primarily HA fillers, can be effective in addressing periorbital aging. Because of the increasing use of periorbital fillers, more complications, both non-ischaemic and ischaemic, will be encountered. Understanding the complex periorbital anatomy and proper injection techniques can help avoid complications, but are no guarantee. In addition, education of both patients and injectors of these potential complications are important as well as having hyaluronidase on hand.
Disclosure
Consultant for Merz Pharmaceuticals, Ischemic Complications Advisory Board for Allergan.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
I would like to acknowledge Jerome Comet Klein MD Foundation.
REFERENCES
- Comprehensive treatment of periorbital region with hyaluronic acid. J Clin Aesthet Dermatol. 2015;8:30-5.
- [Google Scholar]
- Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Plast Surg Nurs. 2015;35:13-32.
- [Google Scholar]
- Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008;34(Suppl 1):S92-9.
- [Google Scholar]
- Filling the periorbital hollows with hyaluronic acid gel: Initial experience with 244 injections. Ophthal Plast Reconstr Surg. 2006;22:335-41.
- [Google Scholar]
- Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4 Suppl 2):5S-21S.
- [Google Scholar]
- The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers. Aesthet Surg J. 2013;33:1167-74.
- [Google Scholar]
- Understanding, avoiding, and managing severe filler complications. Plast Reconstr Surg. 2015;136(5 Suppl):196S-203S.
- [Google Scholar]
- Temporary blindness after an anterior chamber cosmetic filler injection. Aesthetic Plast Surg. 2015;39:428-30.
- [Google Scholar]
- Avoiding and treating blindness from fillers: A review of the world literature. Dermatol Surg. 2015;41:1097-117.
- [Google Scholar]
- Vascular compromise from soft tissue augmentation: Experience with 12 cases and recommendations for optimal outcomes. J Clin Aesthet Dermatol. 2014;7:37-43.
- [Google Scholar]
- Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33:862-77.
- [Google Scholar]
- External compression versus intravascular injection: A mechanistic animal model of filler-induced tissue ischemia. Ophthal Plast Reconstr Surg 2015 2015 June 26 [Epub ahead of print]
- [Google Scholar]
- Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg. 2011;64:1590-5.
- [Google Scholar]
- Complications of injectable fillers, part 2: Vascular complications. Aesthet Surg J. 2014;34:584-600.
- [Google Scholar]
- Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: Consensus recommendations. Aesthet Surg J. 2015;35:844-9.
- [Google Scholar]
- Nitroglycerin: A review of its use in the treatment of vascular occlusion after soft tissue augmentation. Dermatol Surg. 2012;38:1889-97.
- [Google Scholar]
- Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: An animal model with ICG imaging. Ophthal Plast Reconstr Surg 2015 2015 Jun 26 [Epub ahead of print]
- [Google Scholar]
- Effects of nitroglycerin ointment on mastectomy flap necrosis in immediate breast reconstruction: A randomized controlled trial. Plast Reconstr Surg. 2015;135:1530-9.
- [Google Scholar]
- Glyceryl trinitrate (nitroglycerin) ointment and isosorbide dinitrate: A review of their pharmacological properties and therapeutic use. Drugs. 1982;23:165-94.
- [Google Scholar]
- Intravenous hyaluronidase therapy for myocardial infarction in man: Double-blind trial to assess infarct size limitation. Circulation. 1982;65:764-71.
- [Google Scholar]
- Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-41.
- [Google Scholar]
- Iatrogenic occlusion of the ophthalmic artery after cosmetic facial filler injections: A national survey by the Korean Retina Society. JAMA Ophthalmol. 2014;132:714-23.
- [Google Scholar]
- Clinical experience with hyaluronic acid-filler complications. J Plast Reconstr Aesthet Surg. 2011;64:892-6.
- [Google Scholar]
- Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol. 2012;154:653-62.e1.
- [Google Scholar]
- Blindness caused by cosmetic filler injection: A review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-201.
- [Google Scholar]
- Blindness following cosmetic injections of the face. Plast Reconstr Surg. 2012;129:995-1012.
- [Google Scholar]
- Panophthalmoplegia and vision loss after cosmetic nasal dorsum injection. J Clin Neurosci. 2014;21:890.
- [Google Scholar]
- Verification of embolic channel causing blindness following filler injection. Aesthetic Plast Surg. 2015;39:154-61.
- [Google Scholar]
- Calculation of the diameter of the central retinal artery from noninvasive measurements in humans. Curr Eye Res. 2002;25:341-5.
- [Google Scholar]
- Vitrase for Vitreous Hemorrhage Study Groups. Safety results of two phase III trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140:585-97.
- [Google Scholar]
- Vitrase for Vitreous Hemorrhage Study Groups. Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140:573-84.
- [Google Scholar]
- An anatomic basis for treatment of retinal artery occlusions caused by hyaluronic acid injections: A cadaveric study. Aesthetic Plast Surg. 2014;38:1131-7.
- [Google Scholar]
- A cadaveric feasibility study of the intraorbital cannula injections of hyaluronidase for initial salvation of the ophthalmic artery occlusion. Aesthetic Plast Surg. 2015;39:252-61.
- [Google Scholar]
- A treatment protocol for vascular occlusion from particulate soft tissue augmentation. J Clin Aesthet Dermatol. 2012;5:44-7.
- [Google Scholar]
- Changing role of hyaluronidase in plastic surgery. Plast Reconstr Surg. 2014;133:127e-32e.
- [Google Scholar]
- Hyaluronidase caveats in treating filler complications. Dermatol Surg. 2015;41(Suppl 1):S347-53.
- [Google Scholar]
- The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007;80:1921-43.
- [Google Scholar]
- Hyaluronidase in the correction of hyaluronic acid-based fillers: A review and a recommendation for use. J Cosmet Dermatol. 2009;8:317-23.
- [Google Scholar]
- Reversing facial fillers: Interactions between hyaluronidase and commercially available hyaluronic-acid based fillers. J Drugs Dermatol. 2014;13:1053-6.
- [Google Scholar]
- Molecular weight analyses and enzymatic degradation profiles of the soft-tissue fillers Belotero Balance, Restylane, and Juvéderm Ultra. Plast Reconstr Surg. 2013;132(4 Suppl 2):22S-32S.
- [Google Scholar]






