Translate this page into:
Clinical and Histologic Evaluation of Platelet-Rich Fibrin Accelerated Epithelization of Gingival Wound
Address for correspondence: Dr. Mansi Bansal, Department of Periodontology, Institute of Dental Studies and Technologies, Kadrabad, Modinagar - 201 201, Uttar Pradesh, India. E-mail: drmansibansal@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The foremost indication for gingival depigmentation is patient demand for improved aesthetics. In most cases after the removal of pigmented layer, the area is covered with periodontal packs. These dressings have no curative properties. They only minimise the likelihood of surface trauma during mastication. However, platelet-rich fibrin (PRF) accelerates wound healing by effective neovascularisation and promoting fast cicatricial tissue remodelling. In the present split mouth study, PRF membrane was applied in the first quadrant and non-eugenol dressing (Coe-Pack) in the second quadrant after depigmentation. Clinical evaluation of epithelization with toluidine blue revealed that PRF treated sites stained substantially less indicating better wound healing as compared to Coe-Pack sites, which appeared more erythematous after 5 days. The histologic evaluation also revealed greater inflammatory cell infiltrate on Coe-Pack sites as compared to PRF. Thus, PRF membrane as a periodontal dressing is a successful approach to protect the raw wound area of the depigmented site to reduce healing time and patient discomfort.
Keywords
Depigmentation
platelet-rich fibrin
wound healing
INTRODUCTION
The colour of the gingiva plays an important role in overall aesthetics. Ginwalla et al.[1] described the broad black zone of pigmentation on the gingiva as ‘unsightly’ and suggested its removal. The surgical depigmentation procedure essentially involves the removal of gingival epithelium along with a layer of the underlying connective tissue and allowing the denuded connective tissue to heal by secondary intention.
Coverage of exposed connective tissue minimises the likelihood of post-operative haemorrhage and facilitates healing by preventing surface trauma. Conventional non-eugenol periodontal dressings provide an inert mechanical barrier, thereby assisting healing by prevention of external influences on the wound area.[2]
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate and was first developed in France by Choukroun et al., in 2001.[3] It accelerates wound healing by effective neovascularisation and promoting fast cicatricial tissue remodelling and can be used to shield open wounds.[45] PRF membrane as a palatal bandage has been described as an efficacious approach to protect the raw wound area of the palatal donor site.[6] This technique has finally lead to the use of a fibrin and platelet concentrate for the topical and cutaneous application.[78] Therefore, a study was conducted to evaluate clinically and histologically wound healing with PRF membrane as periodontal dressing after depigmentation.
CLINICAL PROCEDURE
Five patients, within an age range of 15–35 years were selected for the study from the Department of Periodontology, who had aesthetic concerns regarding gingival hyperpigmentation [Figures 1a, 2a, 3a, 4a and 5a]. After taking the ethical clearance and patients consent, an infiltration anaesthesia with 2% lignocaine with adrenaline 1:200,000 was administered. The entire visible pigmentation was removed, exposing the underlying connective tissue [Figures 1b, 2b, 3b, 4b and 5b]. A split-mouth design was adopted and in all the patients the gingiva of 1st quadrant was covered with PRF membrane and sutured with 5-0 silk suture and that of 2nd quadrant was covered with a periodontal dressing (Coe-Pack) [Figures 1c, 2c, 3c, 4c and 5c]. The PRF was prepared according to the recommended protocol by Choukroun.[3]
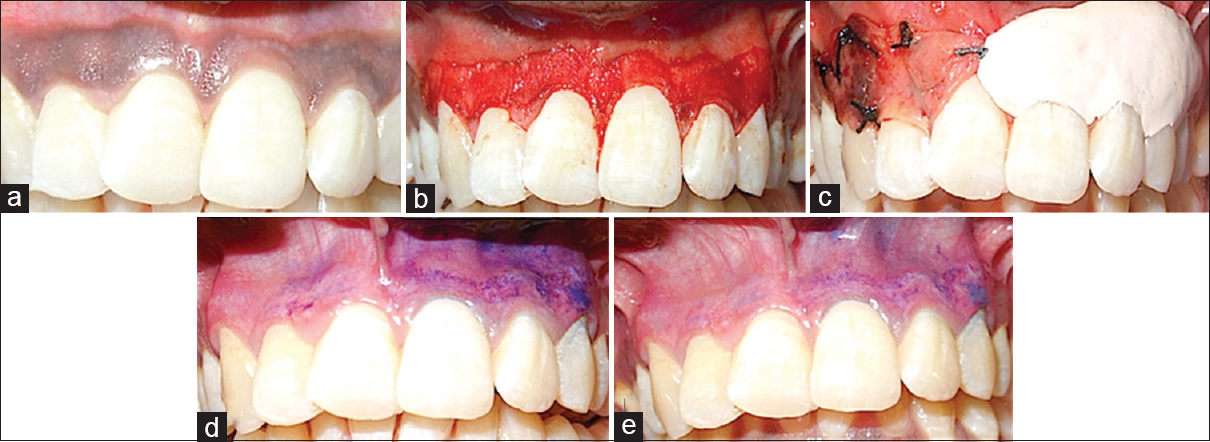
- (a) Dummett moderate gingival pigmentation at presentation in maxillary arch, (b) partial thickness flap on the facial surface of the gingiva of the maxillary arch, (c) the right half of the area (1st quadrant) was covered with platelet rich fibrin membrane and sutured with 5-0 suture and the left half (2nd quadrant) was covered with non-eugenol periodontal dressing (Coe-Pack), (d) toluidine blue test on 3rd day, (e) toluidine blue test on 5th day
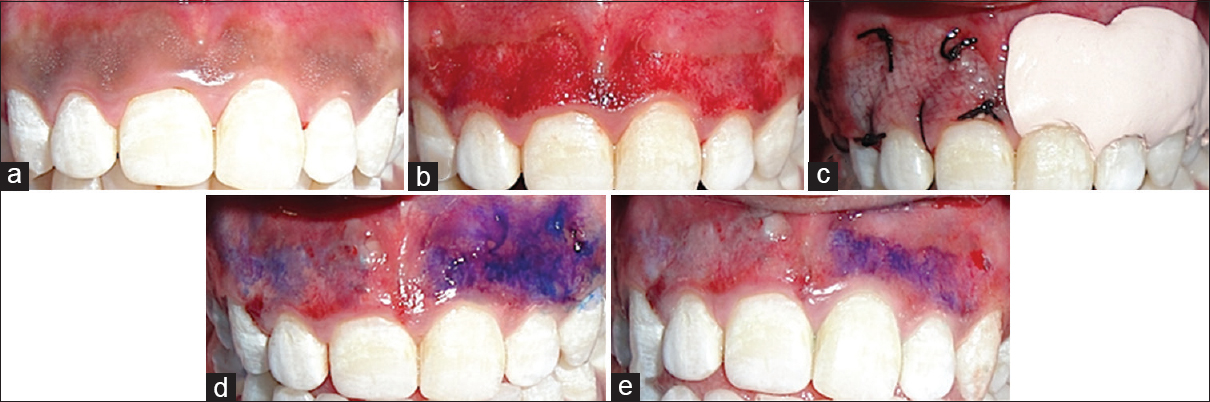
- (a) Dummett moderate gingival pigmentation at presentation in maxillary arch, (b) partial thickness flap on the facial surface of the gingiva of the maxillary arch, (c) the right half of the area (1st quadrant) was covered with platelet rich fibrin membrane and sutured with 5-0 suture and the left half (2nd quadrant) was covered with non-eugenol periodontal dressing (Coe-Pack), (d) toluidine blue test on 3rd day, (e) toluidine blue test on 5th day
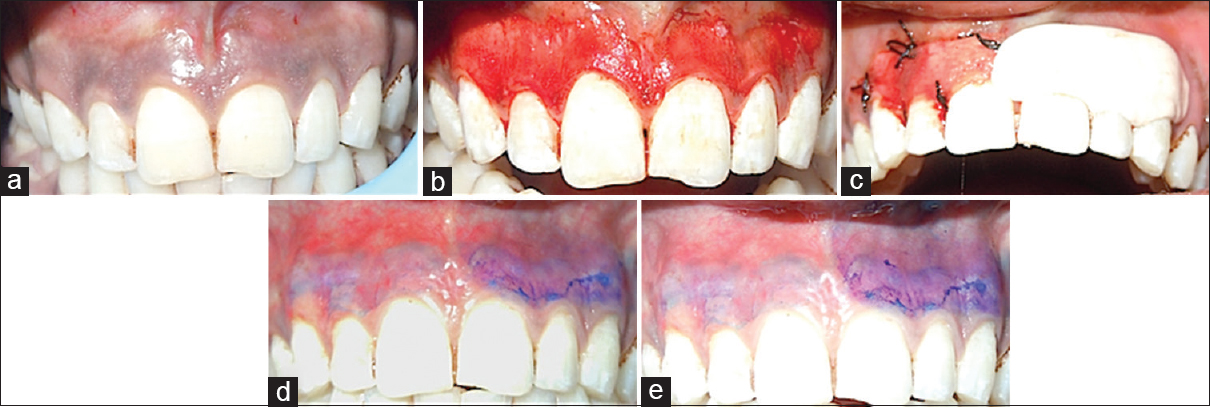
- (a) Dummett moderate gingival pigmentation at presentation in maxillary arch, (b) partial thickness flap on the facial surface of the gingiva of the maxillary arch, (c) the right half of the area (1st quadrant) was covered with platelet rich fibrin membrane and sutured with 5-0 suture and the left half (2nd quadrant) was covered with non-eugenol periodontal dressing (Coe-Pack), (d) toluidine blue test on 3rd day, (e) toluidine blue test on 5th day
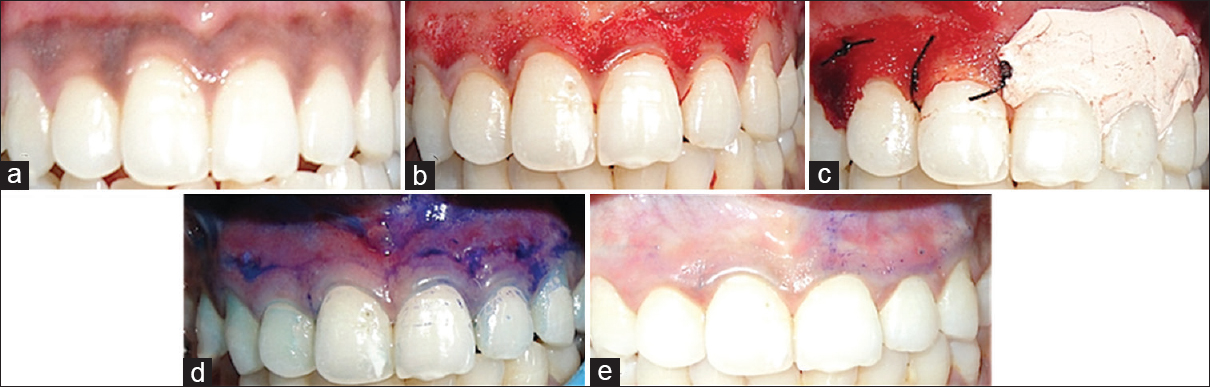
- (a) Dummett moderate gingival pigmentation at presentation in maxillary arch, (b) partial thickness flap on the facial surface of the gingiva of the maxillary arch, (c) the right half of the area (1st quadrant) was covered with platelet rich fibrin membrane and sutured with 5-0 suture and the left half (2nd quadrant) was covered with non-eugenol periodontal dressing (Coe-Pack), (d) toluidine blue test on 3rd day, (e) toluidine blue test on 5th day
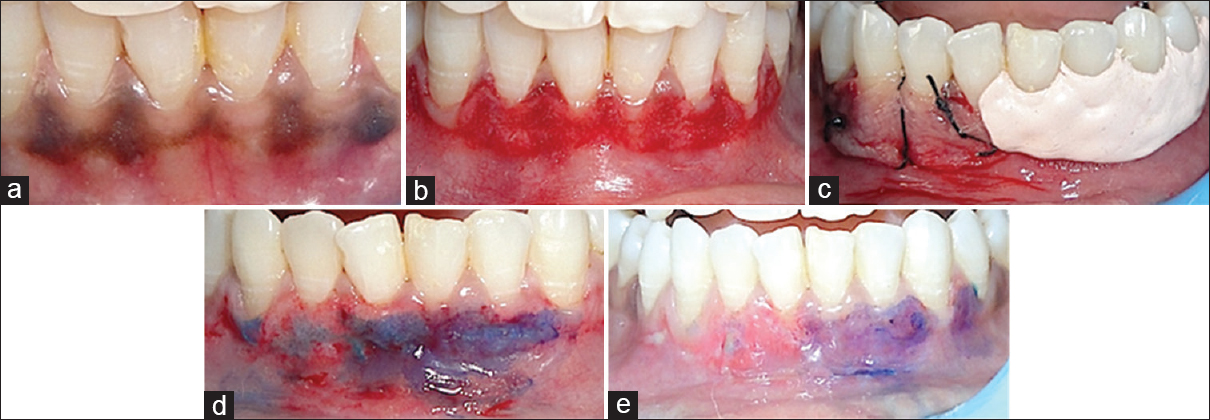
- (a) Dummett moderate gingival pigmentation at presentation in mandibular arch, (b) partial thickness flap on the facial surface of the gingiva of the mandibular gingiva, (c) the right half of the area (4th quadrant) was covered with platelet rich fibrin membrane and sutured with 5-0 suture and the left half (3rd quadrant) was covered with non-eugenol periodontal dressing (Coe-Pack), (d) toluidine blue test on 3rd day, (e) toluidine blue test on 5th day
Sutures were removed on the 3rd post-operative day. Wound healing was evaluated on the basis of healing index given by Landry et al.,[9] visual analogue scale scores,[10] epithelization test with toluidine blue and histological evaluation by punch biopsy on 5th post-operative day at mid of 11 and 21 region in both the quadrants.
RESULTS
Visual analogue scale scores and patient-centered outcomes
During the first 3 days, a small ulcer was seen in one patient on the Coe-Pack site. After 5 days, two patients complained of slight pain at Coe-Pack site whereas on PRF site none of the patient reported with pain. At 7 days (1 week), the patient had no complaint regarding pain.
Epithelization test
The amount of staining with toluidine blue was substantially less on PRF sites than the non-PRF site at 3rd day [Figures 1d, 2d, 3d, 4d and 5d]. Uneventful comprehensive healing was observed at all PRF sites by 5 days [Figures 1e, 2e, 3e, 4e and 5e].
Healing index (HI)
At both 3 and 5 days post-operatively [Graph 1], Coe-Pack sites had greater erythematous area as compared to PRF. At 7 days post-operatively, both the sites exhibited normal tissue architecture [Figure 6].
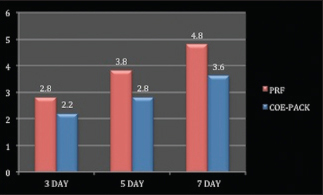
- Graph showing higher values of HI in platelet rich fibrin group as compared to Coe-Pack group
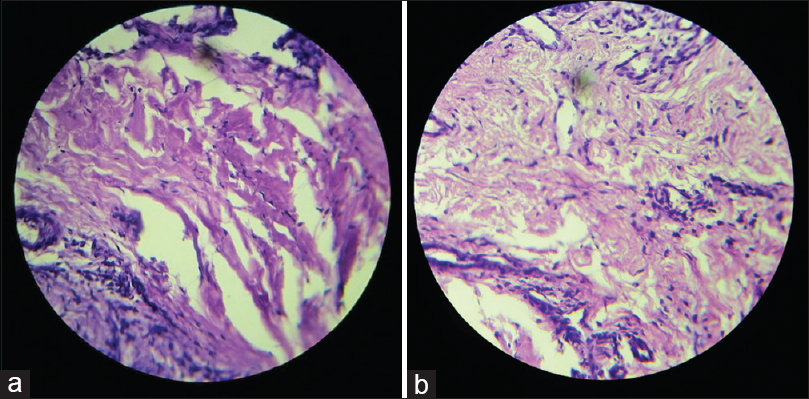
- (a) Histologic evaluation of platelet rich fibrin treated sites with negligible inflammatory cell infiltrate in the connective tissue, (b) histologic evaluation of Coe-Pack treated sites showing chronic inflammatory cell infiltrate in the connective tissue
Histologic evaluation
At 5 days after procedure, histologic examination of haematoxylin and eosin stained section of PRF site revealed parakeratinised stratified squamous epithelium with underlying fibrous connective tissue stroma and no inflammatory cell infiltrate [Figure 6a] whereas Coe-Pack site revealed inflamed juxtaepithelial connective tissue showing chronic inflammatory cells, whereas the deeper connective tissue was relatively fibrous [Figure 6b].
DISCUSSION
The scalpel surgery may cause unpleasant bleeding during and after the surgery, and it is necessary to cover the exposed lamina propria as healing occurs with secondary intention, which is delayed.[1112] The use of post-surgical dressings have no curative properties but protect the wound from mechanical trauma and provides patient comfort during tissue healing after surgery.[13] Coe-Pack is a dimensionally unstable material which shows contraction during the first minutes after completion of their setting, resulting in delayed healing.[14] However, PRF is the activated form of a plasmatic molecule called fibrinogen.[15] It is transformed into a kind of biologic glue capable of consolidating the initial platelet cluster, thus constituting a protective wall along vascular breaches during coagulation. It favours the sealing of wound borders and the facilitation of cutaneous reapplication in general and plastic surgery.[161718]
Coe-Pack although is biocompatible yet there are chances of foreign body reaction if the material becomes embedded in the tissues or underneath the flap.[8] PRF has the characteristic of polymerising naturally and slowly during centrifugation and the thrombin concentrations acting on the collected autologous fibrinogen are almost physiologic because there is no bovine thrombin addition.[4]
Utility of platelet-rich fibrin membrane in cutaneous surgery
Although platelet and leucocyte cytokines are important in the biology of this biomaterial, the fibrin matrix supporting them constitutes the determining element responsible for the real healing potential of PRF. The main angiogenesis soluble factors such as fibroblast growth factor basic, vascular endothelial growth factor, angiopoietin and platelet-derived growth factor are included in fibrin gel. Fibrin and fibrinogen degradation products increase the membrane's expression of CD11c/CD18 receptor which permits adhesion of the neutrophil to endothelium and fibrinogen as well as the transmigration of neutrophils. Fibrin matrix guides the coverage of injured tissues, affecting the metabolism of epithelial cells and fibroblasts. Around the wound's margins, epithelial cells lose their basal and apical polarity and produce basal and lateral extensions towards the wound side. PRF, as a physiologic fibrin matrix, serves as a net to stem cells and allows remodelling of fibrin in a more resistant connective tissue. Therefore, PRF membranes simultaneously provide angiogenesis, immunity and epithelial cover, which is indispensable for cutaneous healing.
CONCLUSION
With these fundamental considerations, PRF can be considered as a natural fibrin-based biomaterial favourable to the development of a microvasculariation and able to guide epithelial cell migration to its surface. The interest of such a membrane is evident, namely, to protect open wounds and accelerate healing. Thus, its utilisation seems to be of high interest in dermatologic and plastic surgical procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Surgical removal of gingival pigmentation.(A preliminary study) J Indian Dent Assoc. 1966;38:147-50.
- [Google Scholar]
- Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56-60.
- [Google Scholar]
- Platelet-rich plasma and platelet-rich fibrin in human cell culture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:48-55.
- [Google Scholar]
- Use of platelet rich fibrin (PRF) membrane as palatal bandage. Clin Adv Periodontics. 2013;4:1-6.
- [Google Scholar]
- In vitro effects of Choukroun's PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:341-52.
- [Google Scholar]
- Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-44.
- [Google Scholar]
- Effectiveness of benzydamine HCL in the treatment of periodontal post surgical patients. Res Clinic Forums. 1988;10:105-18.
- [Google Scholar]
- A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227-36.
- [Google Scholar]
- Melanin repigmentation after gingivectomy: A 5-year clinical and transmission electron microscopic study in humans. Int J Periodontics Restorative Dent. 1993;13:85-92.
- [Google Scholar]
- Significance of early healing events on periodontal repair: A review. J Periodontol. 1992;63:158-65.
- [Google Scholar]
- The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11-30.
- [Google Scholar]
- Influence of fibrin structure on the formation and maintenance of capillary-like tubules by human microvascular endothelial cells. Angiogenesis. 1998;2:153-65.
- [Google Scholar]
- Treatment of severe physiologic gingival pigmentation with free gingival autograft. Quintessence Int. 1996;27:555-8.
- [Google Scholar]






