Translate this page into:
Fat Busters: Lipolysis for Face and Neck
Address for correspondence: Dr. Abhay Talathi, 19 Alankar, 2nd Floor, Goregaon West, Mumbai-400104. Ph: 9769585491. E-mail: skinspaceclinic@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Persistence and hypertrophy of fat pads particularly of the face and neck region disturb beauty proportions, thus demand treatments. Phosphatidylcholine and deoxycholic acid are the most commonly used solutions for injection lipolysis. As we stand today, sodium deoxycholate preparation is approved by the US Food and Drug Administration for the same. This article describes the correct use of solution to achieve fat reduction and ensure safety. Complete details of patient selection, assessment, dosing, and injection techniques are described in this article. A brief note on posttreatment care and complications is also provided.
Keywords
Fat busters
injection lipolysis
sodium deoxycholate
submental fat
INTRODUCTION
The demand for fat reduction injections in aesthetic therapies has always been on the rise. Persistence and hypertrophy of fat pads particularly of the face and neck region disturb beauty proportions, thus demand treatments. An effective reduction of this fat shall help individuals achieve better appearance and the desired contoured face and neck.
The history of use of fat-reducing injections goes way back in 1960s when phosphatidylcholine was first isolated in Ukraine[1] and subsequently used for dissolving fat. Initially, a formulation consisting mainly of lipid phosphatidylcholine dissolved in the bile salt deoxycholate was used as an intravenous medication to prevent or treat fat embolism.
HISTORY IN AESTHETIC USE[1]
In Italy, at the end of the 1980s, Dr. Sergio Maggiori began to use phosphatidylcholine in the infiltration of xanthelasma with satisfactory results. He presented this method at the Fifth International Mesotherapy Congress in Paris in 1988. In 1995, the Brazilian dermatologist, Dr. Patricia Rittes, treated her own lower eye pads by injecting phosphatidylcholine under her eyes. In 2003, “Network Lipolysis” was founded in Germany by Ulrich Bunzek and Dirk Brandl, and with this started the European investigation of the scientific background of this new aesthetic therapy. As we stand today, sodium deoxycholate preparation is approved by the US Food and Drug Administration (USFDA) for the reduction of persistent submental fat.
VARIOUS FAT-DISSOLVING SUBSTANCES
Phosphatidylcholine
Phosphatidylcholine[12] is the most important and essential phospholipid in the human body. It facilitates the emulsification of fat into the tiniest particles within the nanosphere, enabling the absorption and transportation of fat. After subcutaneous injections of phosphatidylcholine into fat tissue, the adipocytes burst and phosphatidylcholine increases the secretion of triaglycerol-rich lipoproteins.
Deoxycholic acid
Deoxycholic acid is a component of human bile acid, which plays a vital role in emulsification and digestion of fat in the intestine. Rotunda and colleagues[2] demonstrated that externally developed deoxycholic acid physically disrupts the cell membrane of adipocytes causing cell death and that relatively protein-poor tissue such as fat is more sensitive to the cytolytic effects of deoxycholic acid than relatively protein-rich tissues such as skin and muscle. Clinical efficacy and safety of deoxycholic acid have been established by multiple studies.
Deoxycholic acid injection[3] (Kybella in the United States) was approved in 2015 as a first-in-class injectable drug for improving the appearance of double chin associated with submental fat.
CONCENTRATION OF SUBSTANCES
On reviewing literature, one realizes that multiple variable combinations of both deoxycholic acid and phosphatidylcholine have been tried. Some standard combinations are as follows.
Lipostabil[4] contains phosphatidylcholine (50mg/mL) together with deoxycholic acid, sodium hydroxide, sodium chloride, α-tocopherol, benzyl alcohol, and ethanol.
Kybella contains deoxycholic acid (10mg/mL).[3]
GeoLysis contains deoxycholic acid (10mg/mL).
MECHANISM OF ACTION[5]
Following injection of lipolytic solution, the adipocyte cell wall is destroyed, resulting in the cascade of inflammatory necrosis of cell and reduction in the size of adipocytes. Inflammatory cascade also results in fibroblast migration and stimulation, resulting in buildup of more collagen [Figure 1].

- Mechanism of action of deoxycholic acid
INDICATIONS
Persistent submental fat: Clinically manifests as moderate-to-severe convexity or fullness inferior to jawline, also termed as a double chin appearance. Till date, the use of injection lipolysis is approved by the USFDA only in the reduction of persistent submental fat [Figure 2].
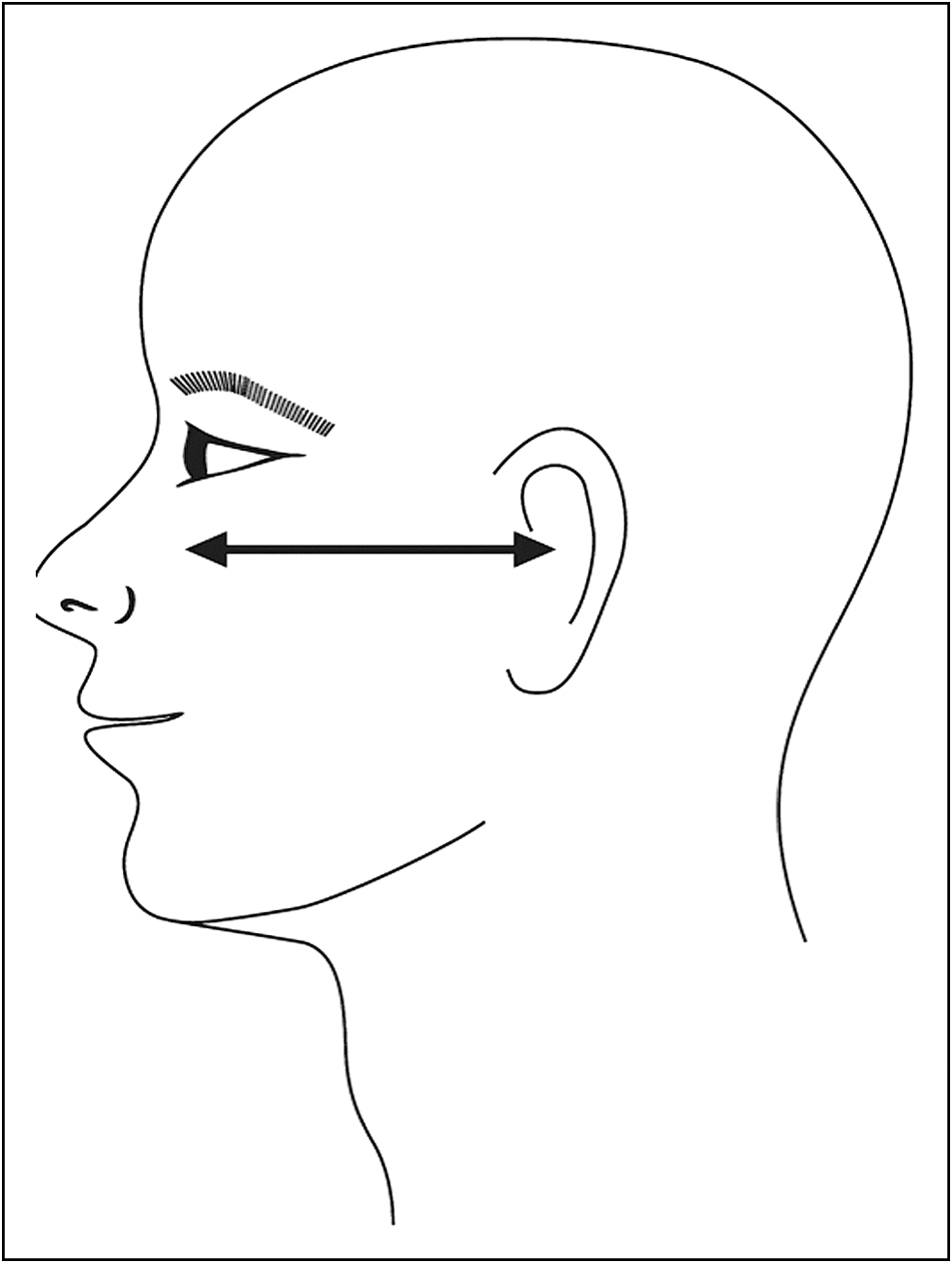
- Frankfort plane
Other face and neck fat pads: Facial fats like cheek, chin and jaw fat pads are injected by few physicians as off-label indications. The results of these indications are variable and techniques are not standardized as that of submental fat injection.
A few cases of injection of fat busters in lipoma and male breasts are also reported.
As the most published data exist on double chin fat reduction, we have explained the detailed technique for it in the following sections.
Contraindications: No Absolute Contraindications exist for procedure. It can be avoided if there is infection at the site of injection entry. It should not be done in patients not willing to accept the downtime of the procedure and having unrealistic expectations.
PATIENT ASSESSMENT
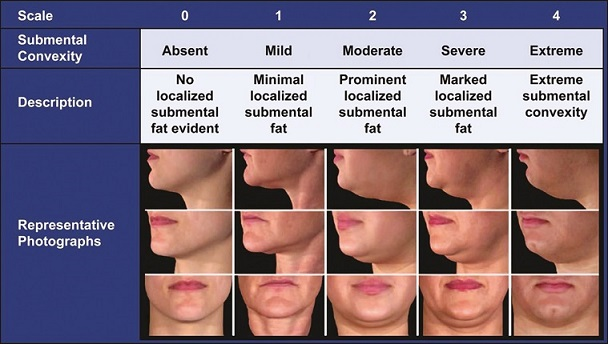
Patient assessment[6] is carried out by observing for submental fullness in frontal and profile view of face with Frankfort plane being parallel to the floor. Convexities are observed and graded as follows so as to decide dosing schedule for the patients and setting expectations right.
Double Chin Rating Scale (Adopted from Ref.[3])
PREPARATION AND MARKINGS
Double chin fat is located anterior to platysma muscle. Thus, before marking the injection site, the patient is asked to contract platysma muscle so that fat is palpated (platysma muscle is used when the patient moves jaw inferiorly while loudly saying “Eee”).
Submental fat compartment for deoxycholic acid treatment is delineated by marking the submental crease anteriorly, the hyoid bone posteriorly, and the lateral boundaries are judged on palpation usually corresponding with continuation of labiomental crease.
After marking the submental fat, multiple injection points are marked at a distance of 1cm away from each other in a gridlike manner [Figure 3].
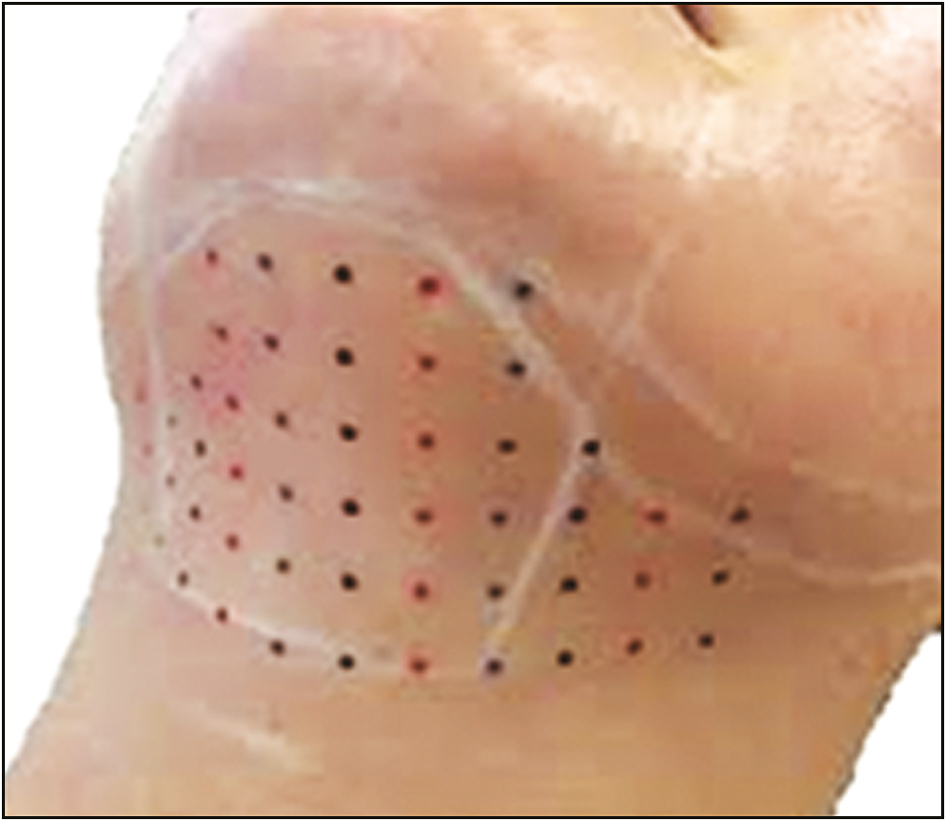
- Grid pattern of injection
Other important anatomic landmark is the inferior mandibular border with the antegonial notch (a bony landmark at the anterior masseter that approximates the location of the marginal mandibular nerve). Marginal mandibular nerve is located within 1 to 4 cm away from the inferior border of mandible and is in close proximity of antegonial notch. This area has to be cautiously injected.
INJECTION TECHNIQUE
Deoxycholic acid is injected into the submental fat with the help of a 1-mL tuberculin syringe and thin needle (usually 30 G and 13mm)[6].
The following steps are carried out:
-
After consent and accurate photography, the injection area is marked as explained previously.
-
Complete aseptic precautions are followed while injecting. In our opinion, topical anesthesia does not play any role in reducing the discomfort of injection as fat is placed deep; however, for sensitive patients, it may be used.
-
Typically 3–5mL of deoxycholic acid is injected per session divided in multiple pricks, and dose per injection prick is close to 0.2–0.3mL. This leads to delivery of 30–50mg of deoxycholic acid per session.
-
It is wise to pinch the submental fat with a nondominant hand so as to ensure the correct plane of injection. The depth of the prick has to be between 6 and 10mm as the fat is deep seated.
-
Typically three to five sessions are required with an interval of 4 weeks between two sessions. In our experience, the duration between two sessions can be increased to 8 weeks so as to reduce the number of sessions.
-
Topical antibiotics are applied after injections.
-
Patients shall experience some discomfort and pain after injection, lasting from 2 days to 2 weeks followed by reduction of fat.
POSTTREATMENT CARE
Patients can experience mild swelling, discomfort, and pain as an indicator of inflammation for the duration varying from 2 days to 2 weeks postinjection.
Patients can be aligned for the same. Cold compresses can be effectively used to alleviate discomfort. Oral anti-inflammatory medicines are rarely used.
Patients are instructed not to massage the treated area for the next 48h.
Usually, the patients can be followed up after 4 weeks to assess the results and also to plan further sessions as per their requirement.
CLINICAL RESULTS
-
Most of the patients treated with injection lipolysis for the reduction of double chin are extremely satisfied with the outcome as per our experience.
-
Reports published about the treatment results of other body sites have claimed to achieve reduction in upper belly by 3.7cm, lower belly by 3.9cm, thighs by 1.9cm, and upper arms by 1.6cm.
-
For safety reasons, lower eye pads have been eliminated from the list of lipolysis indications by the Network Lipolysis, and the treatment should only be performed by physicians who are able to make a releasing cut in case of retro-orbital bleeding [Figure 4].
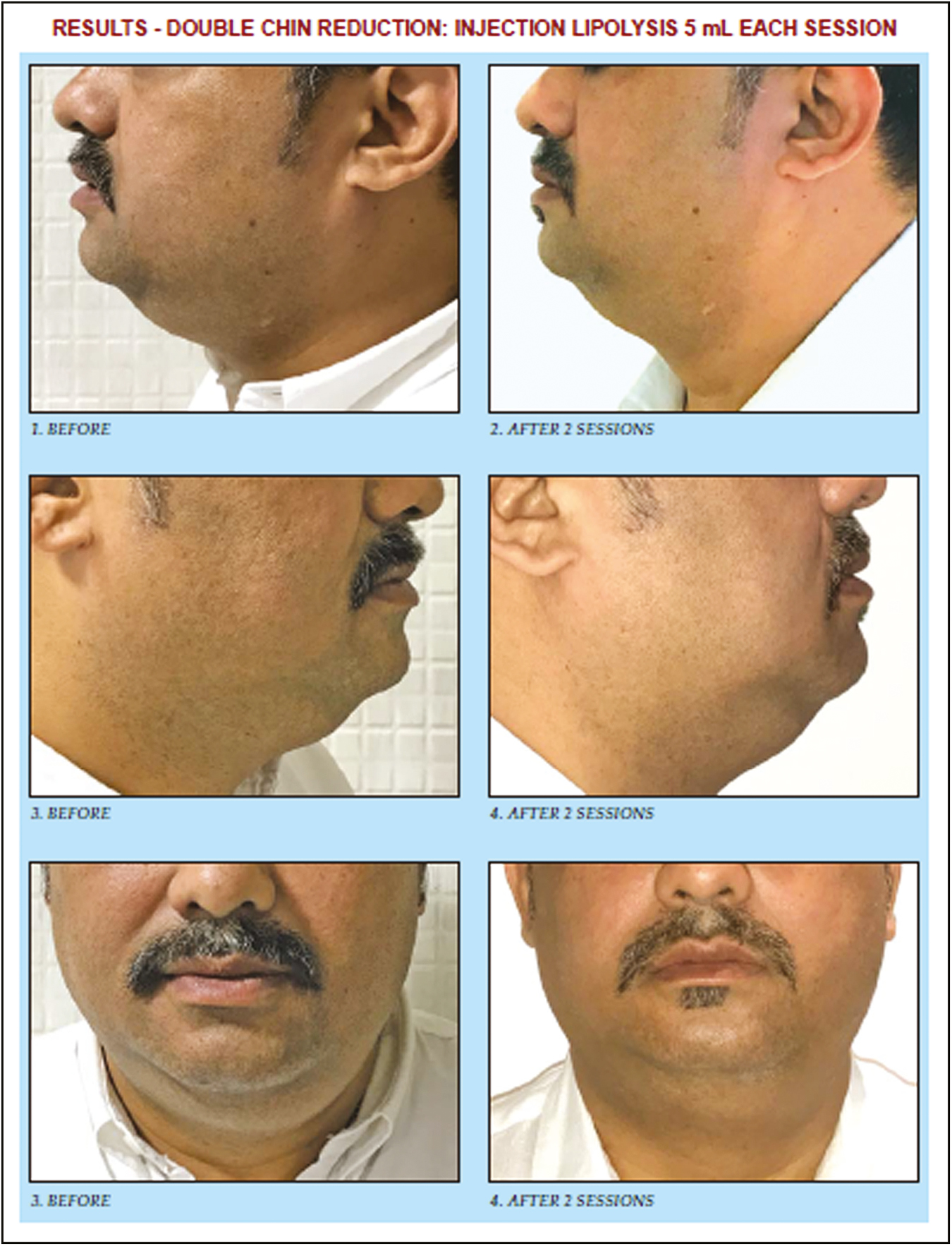
- Results of double chin reduction
COMPLICATIONS[17]
-
Discomfort, pain, and swelling: These are most common and shall subside with time. Swelling and pain after only deoxycholic acid injection may last up to 10–14 days, whereas the discomfort after injection of the combination of deoxycholic acid and phosphatidylcholine usually lasts for up to less than a week.
-
Bruise/ecchymosis: These are due to injury to local blood vessels, are very localized, and disappear in a few days.
-
Marginal mandibular nerve injury: This is due to injecting a lot of deoxycholic acid close to the nerve. Clinically, it may result in asymmetrical smile. It can be avoided with correct injection technique in most of the individuals.
-
Skin ulceration: Superficial injection may result in ulceration, hence should be avoided.
-
Rarely dysphagia can occur.
SUMMARY
Deoxycholic acid is a first-in-class injectable drug for the reduction of submental fat and represents a minimally invasive, customizable alternative to liposuction and surgery for the patients with moderate-to-severe submental fullness.
Phosphatidylcholine combination probably helps in the transportation of free fatty acids after breakdown. Injection lipolysis is extremely effective and safe in the treatment of submental fat, and usually without the need for adjunctive treatments to address skin laxity.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Phosphatidylcholine treatment to induce lipolysis. J Cosmet Dermatol. 2005;13:308-13.
- [Google Scholar]
- Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatol Surg. 2004;13:1001-8.
- [Google Scholar]
- Overview of ATX-101 (deoxycholic acid injection): a nonsurgical approach for reduction of submental fat. Dermatol Surg. 2016;13:S263-70.
- [Google Scholar]
- In vitro studies investigating the effect of subcutaneous phosphatidylcholine injections in the 3T3-L1 adipocyte model: lipolysis or lipid dissolution? Plast Reconstr Surg. 2009;13:419-27.
- [Google Scholar]
- Phosphatidylcholine and sodium deoxycholate in the treatment of localized fat: a double-blind, randomized study. Dermatol Surg. 2008;13:60-6.
- [Google Scholar]
- Proper technique for administration of ATX-101 (deoxycholic acid injection): insights from an injection practicum and roundtable discussion. Dermatol Surg. 2016;13:S275-81.
- [Google Scholar]
- Prevention and management of injection-related adverse effects in facial aesthetics: considerations for ATX-101 (deoxycholic acid injection) treatment. Dermatol Surg. 2016;13:S300-4.
- [Google Scholar]






