Translate this page into:
Preparation of Platelet-rich Fibrin Membrane over Scaffold of Collagen Sheet, its Advantages over Compression Method: A Novel and Simple Technique
Address for correspondence: Dr. Raju G. Chaudhary, Department of Dermatology, Room No. 9, Skin OPD, Sheth V. S. General Hospital, Ellis Bridge, Paldi Road, Ahmedabad, Gujarat, India. E-mail: jagatiashish@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction:
Platelet-rich fibrin (PRF) is compressed by using various tools to make platelet-rich fibrin membrane (PRFM). Preservation of platelets and plasma content of PRFM depends on the compression method used. To overcome limitations of compression method, we prepared PRFM over scaffold of collagen sheet without using any compression device.
Aims and Objective:
To prepare PRFM without using any compression device over a scaffold of collagen sheet and to evaluate its efficacy in chronic nonhealing ulcer.
Materials and Methods:
PRFM was prepared, with minor modification in Choukron’s protocol, over a collagen sheet without using compression device. To study its efficacy and reproducibility, total 15 patients over 18 years of age with chronic, nonhealing ulcers of more than 3 months of various causes were included and patients with active wound infection were excluded.
Results:
We were able to prepare and reproduce PRFM by our technique. It overcomes the limitations of compression method with comparable efficacy to compression method. Results obtained on comparison at week 0, 3, and 6, by paired t-test, were found to be statistically significant (P < 0.0001).
Conclusion:
Preparation of PRFM with the method described is easy and reproducible. Use of collagen sheet synergistically improved wound healing.
Keywords
Collagen sheet
compression tool
nonhealing ulcers
platelet-rich fibrin membrane
Introduction
Ross et al.,[1] in 1974, described that the growth-promoting activity of serum is derived from platelets and suggested the concept of platelets growth factor. In 2001 at France, Choukroun et al., developed protocol of platelet-rich fibrin (PRF) gel to accumulate platelets along with released cytokines in a fibrin clot.[2] Choukroun’s protocol is centrifuged natural blood without any anticoagulant.[3] To make a PRF membrane (PRFM), moist or dry gauze to compress the PRF clot was used. Cytokines from platelet, leukocytes, and supporting fibrin membrane are the factors responsible for the therapeutic effects of PRFM. Slow, prolonged, and controlled release of cytokines along with fibrin matrix acting as glue in PRFM is more efficacious then uncontrolled and fast release of cytokines of platelet-rich plasma (PRP).[4] Platelets are found in very less quantity in the acellular supernatant or in the red blood corpuscles base, according to various hematologic studies. Histologic analyses have determined that the platelets are most abundantly distributed in the lower part of the fibrin clot, at the junction between the red thrombus and the PRF clot itself, supporting the inclusion of the red clot more effectively than the higher part of the fibrin clot. The PRFM includes glycosaminoglycans (heparin and hyaluronic acid) from blood and platelets. Alcian blue staining showed that these glycemic links are combined within fibrin polymers. Platelet cytokines have strong affinity for the glycosaminoglycan and these glycosaminoglycans support cell migrations and healing processes.[5]
Materials and Methods
Method of PRFM preparation
As a part of the protocol, approximately 6–10mL of venous blood was collected from patient using 18 G needle in a sterile plastic vacutainer for the preparation of autologous PRFM. Without adding any anticoagulant, the original amount of blood was divided equally in two tubes, which were placed symmetrically around the rotor axis for proper balancing. The sample was then centrifuged between 2200 and 3000rpm for 3min depending on the volume of blood. A total of 6mL blood was centrifuged at 2200rpm, 8mL at 2600rpm, and 10mL at 3000rpm in laboratory centrifuge at 22°C room temperature, with a swing out rotor. Meanwhile, a sheet of paraffin-impregnated gauze (Jelonet Manufacturer, Smith & nephew healthcare Limited, Maharashtra, India) was spread on a sterile petri dish. Over this, a sterile collagen dry sheet (NeuSkin, Eucare pharmaceuticals private limited, Tamil Nadu, India) was spread. After centrifugation, the supernatant was separated. It was immediately and slowly poured over the prepared petri dish with the help of pipette and left over for approximately 20min. The sheet of fibrin membrane starts to form in approximately 10min and is complete by 20min in most cases. The immediate appearance after pouring the supernatant was transparent thin yellow fluid, as the membrane matures, it become more yellowish with gelatinous consistency and it settles on the Jelonet as such that it does not drip on tilting the petri dish. This membrane, along with Jelonet and collagen sheet, was directly placed as a dressing over the wound without any manual handling [Figure 1].

- Preparation of PRFM
Design of the study
To see the efficacy, we conducted an open, prospective, interventional study. Study was of 3 months. The sample size of the study was duration based, which was conducted at the dermatology outpatient department of tertiary care center.
Inclusion criteria: Patients over 18 years of age with chronic, nonhealing ulcers of more than 3 months were included in the study. Causes of chronic, nonhealing ulcer included in the study were patients of pemphigus vulgaris with extensive erosions unresponsive to conventional therapy for more than 2 weeks (with controlled disease activity by standard therapy), patients with evidence of any nonhealing ulcers over striae atrophicans, trophic ulcers in patients with Hansen’s disease, and nonhealing leg ulcers secondary to peripheral vascular disease.
Exclusion criteria: Active infection at the wound site, ulcers secondary to infectious cause, ulcer size <2-inch diameter, with severe uncontrolled systemic infection and uncontrolled disease activity (pemphigus vulgaris) as evidenced by a positive Nikolsky sign, and patients who were not willing to give their written informed consent were excluded from the study.
Patients were recruited in the study duration of 3 months and were followed up weekly till 6 weeks. The study began after the approval of institutional ethics committee. The patient’s demographic data such as name, age, and sex were collected along with the duration of the ulcer and approximate size and history of any medical comorbidity in the case record form. Local wound swab was sent for bacterial, mycobacterial, and fungal culture. Patients were screened at baseline for hepatitis B and serum human immunodeficiency virus (HIV).
Weekly dressing with PRFM in conjunction with collagen sheet and white soft paraffin-impregnated gauze was carried out under occlusion, with aseptic precautions. Strict surgical asepsis was followed at each and every step of the procedure. All other ongoing topical therapies were withdrawn. The effect on the wound was photographically assessed at every sitting, with chief parameters of healthy granulation tissue as well as decrease in the dimensions of the wound.
Results
A total of 15 patients aged between 25 and 60 years (mean age, 39 years) were included in this study. Male to female ratio was 3:2. Ten cases were due to leg ulcers secondary to venous, arterial, or diabetic microangiopathy followed by two cases because of nonhealing erosions of pemphigus vulgaris, two cases due to leprosy, and one case of nonhealing ulcers over striae atrophicans (corticosteroid abuse). Lower leg (around the malleoli and shin of tibia) was the most common site of ulcer in 66.7% of patient followed by dorsum of feet, great toe, heel, breast, and lower abdomen (6.66% each) [Table 1]. Initial mean size of ulcer at week 0 was 10.7cm2. After three sessions of weekly PRFM dressing, the mean size of the ulcer (week 3) was 4.7cm2. After 6 weekly PRFM dressing, the mean size of ulcer (week 6) was 0.66 cm2 [Table 2] [Figures 2 and 3]. A result between week 0, 3, and 6 was compared by paired t-test. The two-tailed P value was less than 0.0001 between week 0 and 3. By conventional criteria, this difference is considered to be extremely statistically significant. Intermediate value used in calculation was t = 7.8572, degree of freedom (df) = 14, and standard error of difference = 0.761. The two-tailed P value was less than 0.0001 between week 0 and 6 at the end of the study. Here also, by conventional criteria, this difference is considered to be extremely statistically significant. Intermediate value used in calculation was t = 8.3769, df = 14, and standard error of difference = 1.199.
| Patient no. | Age/gender | Duration of ulcer in months | Site of ulcer | Initial measurement in cm2 | Measurement after 3 weeks cm2 | Measurement after 6 weeks cm2 |
|---|---|---|---|---|---|---|
| 1. | 56/M | 22 | Leg | 11 | 4.6 | 0 |
| 2. | 48/M | 15 | Leg | 5.5 | 2.2 | 0 |
| 3. | 42/M | 10 | Leg | 12.8 | 3.2 | 1.2 |
| 4. | 35/M | 8 | Lower abdomen (over striae) | 6 | 2.6 | 0 |
| 5. | 28/M | 11 | Leg | 22.5 | 12.2 | 2.8 |
| 6. | 60/M | 15 | Leg | 9 | 2.4 | 0.3 |
| 7. | 32/M | 12 | Leg | 18 | 8.6 | 1.4 |
| 8. | 25/M | 9 | Feet (head of great toe) | 8 | 1.8 | 0.02 |
| 9. | 28/F | 13 | Leg | 8.4 | 3.5 | 0 |
| 10. | 35/F | 9 | Breast | 18 | 6.5 | 0 |
| 11. | 30/F | 8 | Leg | 6.5 | 2.8 | 0 |
| 12. | 38/F | 13 | Feet | 12 | 8 | 1.8 |
| 13. | 45/F | 7 | Leg | 9.3 | 4.3 | 0 |
| 14. | 52/M | 10 | Feet (heel) | 7 | 3.8 | 0.8 |
| 15. | 32/F | 9 | Leg | 6.6 | 4.4 | 1.6 |
| No. of patient | Enrolled 15 |
|---|---|
| Dropouts 0 | |
| Age group | 25–60 years |
| Mean age, 39.067 years | |
| Duration of ulcer (in months) | Min/Max, 8/22 |
| Mean duration, 11.4 months | |
| Week 0 measurement (in cm2) | Min/Max, 5.5/22.5 |
| Mean measurement, 10.707cm2 | |
| Week 3 measurement (in cm2) | Min/Max, 1.8/12.2 |
| Mean measurement, 4.727cm2 | |
| Week 6 measurement (in cm2) | Min/Max, 0/2.8 |
| Mean measurement, 0.6613cm2 |
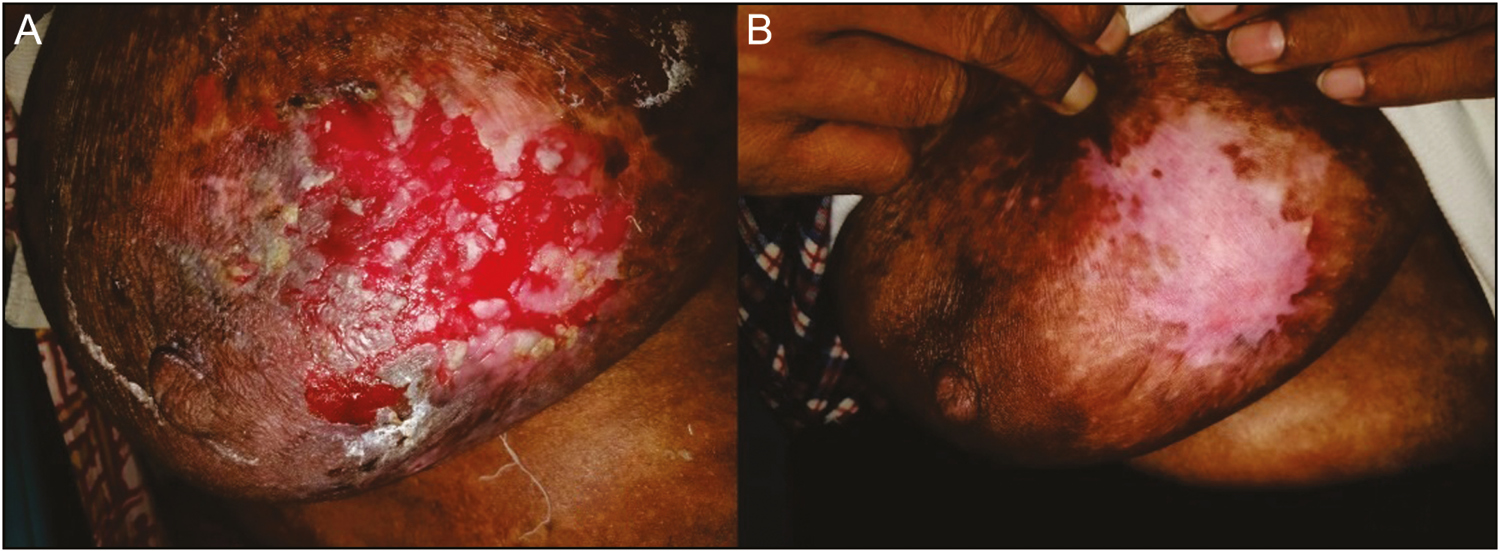
- Ulcer of pemphigus vulgaris. (A) At week 0. (B) At week 6
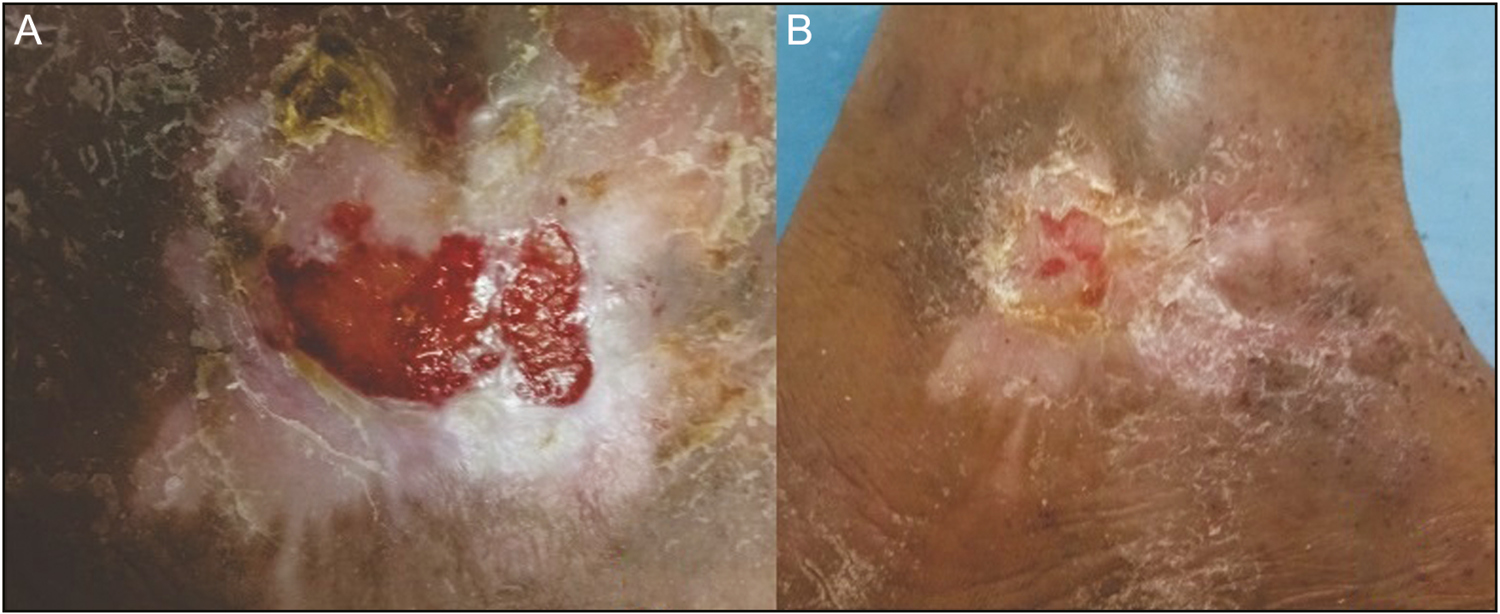
- Leg ulcer. (A) At week 0. (B) At week 6
Discussion
Depending on leukocyte and fibrin content, platelet concentrate is classified into the following four categories: (1) leukocyte-rich PRP, (2) pure PRP, (3) leukocyte PRF, and (4) pure PRF.[67] On the basis of addition of anticoagulant, PRP preparations are classified into two generations. First-generation platelet concentrate includes PRP and second-generation platelet concentrate includes PRF.[89]
Choukroun et al., in 2001, first described the PRF to combine platelets and released cytokines in a fibrin clot.[2] PRP has a higher platelet concentration in comparison to PRF.[10] Still, PRF has superior efficacy as compared to PRP. This can be attributed to the presence of fibrin which increases the mean concentration of growth factors many times in comparison to conventional PRP.[11] A study by Dohan et al.[12] proved that as compared to PRP, slower release of growth factor and cytokines in PRF gave better healing. On the basis of these findings, recently PRF is being used in chronic nonhealing leg ulcers.[1314]
Traditionally, to make a PRF membrane, moist or dry gauze was used to compress the PRF clot. However, this compression damaged the platelets and exuded significant quantities of valuable growth factors. This concern has been validated in the studies by Su et al.[15] and Burnouf et al.,[16] who showed that substantial amounts of growth factors, involved in tissue regeneration, are removed by squeezing. This squeezing process influenced the quality and clinical effectiveness of the PRF preparations.[151617]
PRP, PRF prepared by compression method, and PRFM prepared by our technique was compared [Table 3]. The conventional PRP method has several pitfalls such as the need of a bovine thrombin or anticoagulant such as sodium citrate, double-spin method for preparation, which is slightly more time-consuming and effortful, and a relatively increased risk of coagulopathy as compared to the other two methods. Moreover, anticoagulant use also modifies the characteristics of the PRP. Most of these pitfalls were dealt with effectively by the PRF matrix prepared by the compression technique, the chief drawback being the production of limited quantity.
| Variables/Parameters/Attributes | Platelet-rich plasma | Platelet-rich fibrin matrix (compression method) | Platelet-rich fibrin membrane (our technique) |
|---|---|---|---|
| Use of anticoagulant | Yes (bovine thrombin/sodium citrate/ACDA | Not required | Not required |
| No. of spin | Double spin | Single spin | Single spin |
| Time required for preparation | 1st spin, 10 min | 10min only | For spin 3 and 20min waiting period to form membrane |
| 2nd spin, 10 min | |||
| Parameter required | 1st spin, 1300–1500rpm for 10 min | 3000rpm for 10 min | 2200–3000rpm (depending on the amount of blood) for 3 and 20min waiting period to form membrane |
| 2nd spin, 3000rpm for 10 min | |||
| Handling | More | Less compared to PRP | Minimum compared to PRP/PRF |
| Growth factor release time | Immediate release of growth factor | Slow release of growth factor | Slow release of growth factor |
| Quantity of end product | Sufficient quantity but in liquid form | Less quantity | Sufficient quantity can be produce |
| Therapeutic concern | Use of bovine thrombin, may have factor Va, may cross-react with human factor Va and may produce coagulopathies and bleeding episode. Recent use of sodium citrate/ACDA reduces these chances | No coagulopathy no bleeding episode | No coagulopathy, no bleeding episode |
| Biochemical changes | Use of anticoagulant can change biochemical property | No biochemical modification | No biochemical modification |
| Advantages | Sufficient quantity of therapeutic platelet concentrate | Can be used as dressing | - No compression device required |
| - Sufficient quantity can be produced compared to that in conventional method of PRFM preparation | |||
| - Use of collagen sheet synergistically acts as a biological dressing | |||
| Disadvantages | Invasive and painful | - Need of compression device | |
| - Large amount of blood required to cover large erosion/ulcer |
ACDA = Acid citrate Dextrose formula A; Va = Factor V a (5 a)
PRF has been used in different forms and prepared by different techniques as described in the literature. Preservation of platelets and leukocyte content also depends on the compression methods used. To overcome the limitations of compression method, collagen sheet was used for the preparation of PRFM in this study. We have successfully prepared PRFM in all our patients using collagen sheet. Advantages of our technique over compression technique are as follows: no need to compress PRF gel, covering the larger erosions and usage of collagen sheet for PRF preparation improves the platelet and growth factor yield, thus providing a more suitable biological dressing for wound healing. It is low-cost method. Though being operator dependent, fairly uniform PRFM can be reproducibly performed in most patients.
PRFM prepared by our technique showed comparable results similar to various other studies.[181920]
Limitation of the study
A histological analysis of the prepared PRFM was not carried out.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to express our gratitude to Dr. Devang Rana for his guidance on statistical analysis.
References
- A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci. 1974;71:1207-10.
- [Google Scholar]
- Introducing Choukroun’s platelet rich fibrin (PRF) to the reconstructive surgery milieu. J Implant Clin Adv Dent. 2009;1:21-30.
- [Google Scholar]
- Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638-46.
- [Google Scholar]
- Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-44.
- [Google Scholar]
- Platelet rich plasma: a short overview of certain bioactive components. Open Med (Wars). 2016;11:242-7.
- [Google Scholar]
- The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol. 2012;13:1153-62.
- [Google Scholar]
- Platelet-rich fibrin: evolution of a second-generation platelet concentrate. Indian J Dent Res. 2008;19:42.
- [Google Scholar]
- Platelet concentrates: past, present and future. J Maxillofac Oral Surg. 2011;10:45-9.
- [Google Scholar]
- Platelet concentration in platelet concentrates and periodontal regeneration-unscrambling the ambiguity. Contemp Clin Dent. 2015;6:510.
- [Google Scholar]
- Basic studies on the clinical applications of platelet-rich plasma. Cell Transplant. 2003;12:509-18.
- [Google Scholar]
- Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45-50.
- [Google Scholar]
- Cascade® autologous system platelet-rich fibrin matrix in the treatment of chronic leg ulcers. Adv Wound Care (New Rochelle). 2012;1:52-5.
- [Google Scholar]
- Use of autologous platelet-rich fibrin on hard-to-heal wounds. J Wound Care. 2008;17:60-3.
- [Google Scholar]
- In vitro release of growth factors from platelet-rich fibrin (PRF): a proposal to optimize the clinical applications of PRF. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:56-61.
- [Google Scholar]
- Human blood-derived fibrin releasates: composition and use for the culture of cell lines and human primary cells. Biologicals. 2012;40:21-30.
- [Google Scholar]
- A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals. 2012;40:323-9.
- [Google Scholar]
- Randomized prospective double-blind trial in healing chronic diabetic foot ulcers: CT-102 activated platelet supernatant, topical versus placebo. Diabetes Care. 1992;15:1598-604.
- [Google Scholar]
- The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44:1013-8.
- [Google Scholar]
- Autologous platelet-rich fibrin matrix as cell therapy in the healing of chronic lower-extremity ulcers. Wound Repair Regen. 2008;16:749-56.
- [Google Scholar]






