Translate this page into:
Reconstruction following Excision of Malignant Scalp Tumors with Split Thickness Skin Graft with and without Acellular Dermal Matrix: A Comparative Study
Address for correspondence: Dr. Omid Etemad, Department of Plastic and Reconstructive Surgery, Tehran University of Medical Sciences, Imam Khomeini Hospital Complex, Tehran, Iran. E-mail: omid.etemad@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the most common malignancies, which occur on the scalp. There are various therapeutic methods for managing these malignancies in which the standard treatment method of both is surgical excision with a good margin. Sometimes, the patients need full-thickness excision due to the deep invasion, so exposing the underlying calvarium may be a challenge for surgeons.
Objectives:
We evaluated the outcomes of using the combined therapy of acellular dermal matrix and split-thickness skin graft (STSG) in comparison with using only STSG in the treatment of defects caused by the excision of scalp malignant tumors among the patients who attended Imam Khomeini Hospital Complex and Razi Hospital of Tehran, Iran. We also evaluated the satisfaction of both surgeons and patients among these two methods of treatment.
Materials and Methods:
All the candidates were divided into the two groups, that is, of case and control, randomly. The case group underwent the treatment using acellular dermal matrix and STSG, whereas the control group underwent the treatment by only STSG on the wound. In both groups, BCC and SCC were excised with a margin of 6 and 10 mm, respectively, on the skull bone. Then, a layer of bone was removed by osteotomy in order to reach the bleeding points. All patients were followed up for 7, 30, and 90 days after the surgery, and the results were recorded.
Results:
A significant difference in Manchester Scar Scale, wound contour formation, the mobility of the repair site, and patients and surgeon satisfaction was observed among both groups based on visual analog scale. We found better outcomes in the case group, especially in wound contour formation during 90 days of follow-up. However, the satisfaction of both surgeons and patients was achieved in the case group. Satisfaction of surgeons was achieved in the case group with a relative superiority to the control group according to the Manchester Scar Scale.
Keywords
Acellular dermal matrix
scalp malignant tumor
skin graft
wound repair
Introduction
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the most common malignancies that occur on the scalp.[12] Most of the causes are exposure to sunlight, radiation, and chronic burn ulcer (Marjolin’s ulcer). One of the clinical differences between BCC and SCC is the malodor existence in SCC caused by keratin maceration and infectious necrosis in SCC. SCC may cause distant metastasis although it is rare in BCC; however, BCC is malignant because it may lead to the local invasion.
There are different methods for the treatment of BCC and SCC malignancies, but the standard treatment method is the surgical excision with a safe margin for both cases.[3] Sometimes, because of deep invasion, galea and periosteum removal is needed; this full-thickness scalp defect poses challenges to cover this area.
Scalp reconstruction after the tumor excision is possible performed by one of the following methods: primary closure,[4] granulation and secondary restoration,[5] using local and regional flaps,[678] tissue expansion,[9] free flaps,[101112] and using skin graft.[13]
Using one of the aforementioned methods depends on the anatomical and patients risk factors. Anatomical factors include wound bed depth, laxity, and loosening of the tissue surrounding the defect. The patient-related factors include the mental and physical conditions, comorbidities, age, expectations, and the ability to care for the reconstructed tissue.
Primary repair is used for small defects in which there is no tension but it is not practical in large defects.[5] The secondary repair is effective by creation of granulation in smaller superficial defects, wherein there is no bone exposure.[14]
Because of non-resilient scalp in large defects, local flaps may lead to tension; in this case, covering the defect is also impossible and it is difficult to tolerate for the elderly patients.[151617] Tissue expansion is also associated with some complications such as expander infection and exposure, pressure and deformation of underlying tissues, and the rejection of the deformity during expansion with serum injection, which is especially observed in patients who underwent radiotherapy because the radiated skin has no good resiliency.[9]
Random pattern flaps have a limited use in the scalp, and locoregional and free flaps are better choices, but most of the patients with scalp tumor do not tolerate the more complex operations because of their age and comorbidities, which may lead to many complications.[13] In these cases, there are no suitable recipient vessels around the defect and the surrounding skin also has no good texture, so we can use skin graft. However, the grafts are prone to contraction and distortion in the face and head and lead to contour defect deformity in the scalp.
For such defects, using dermal alternatives are recommended.[18] One of these alternatives is acellular dermal matrix (ADM), which is a dermal allograft. The allograft will be screened in order to evaluate not containing human immunodeficiency virus and hepatitis to avoid virus and infection transmission. Then, the cells will be removed in order to prevent the immune response and graft rejection.
Fibroblasts, macrophages, lymphocytes, and endothelial cells infiltrate the surrounding tissues toward the replaced dermis.[1920] It creates granulation and a vascularized layer, which is a reason for better graft taking on the exposed bone.
In this study, we evaluated the effects of using split-thickness skin graft (STSG) and in the other hands, ADM and STSG in the defect area and compared the cosmetic results and examined the satisfaction of both groups of surgeons and patients from the results of procedures.
The ethics code number of IR.TUMS.IKHC.REC.1397.226 was adopted by this study.
Materials and Methods
Twenty patients, who participated in this study, include the ones who attended Imam Khomeini Hospital Complex and Razi Hospital of Tehran, Iran. The occurrence of SCC and BCC of scalp and the necessity for full-thickness removal in order to reach the minus margin were the inclusion criteria, and the necessity for craniotomy and deeply bone involvement and diabetes were the exclusion criteria in this study. The patient satisfaction form was received from each patient. All patients were divided into two groups, that is, of case and control, randomly, after explaining both methods to the patients and obtaining informed consent. They underwent the treatment by using ADM and STSG in the case group and only STSG in the control group.
In both groups, BCC and SCC were excised from the skull bone with a margin of 6 and 10 mm, respectively. Osteotomy was performed by removing a layer of calvarium in order to reach the bleeding points; then the skin graft was harvested with a thickness of 0.4–0.6 mm. The defect area was fixed and tied over using chromic 4/0 and silk 2/0 sutures, respectively, and the tie-over was removed after five days.
In the case group, ADM was prepared with a thickness of 0.6–1.2 mm with the same size with defect area after the tumor excision and the osteotomy by a steotom to reach the bleeding points. It was fixed and tied over in the same way as we did in the control group. Two or three weeks were given for granulation formation; during this period, wet to dry dressing was performed for the patients after the tie-over was removed after five days. Then, the second step included the placement of STSG with a thickness of 0.4–0.6 mm, which was performed under sedation. It was also fixed and tied over using chromic 4/0 and silk 2/0, respectively, and the tie-over was removed after five days.
Both groups were followed up in 7, 30, and 90 days after the surgery for surveying the graft take, and the visual analog scale (VAS) and Manchester Scar Scale (MSS) were filled for each patient. The data were analyzed using the Statistical Package for the Social Sciences (SPSS, version 23) software at the end of this study.
Data collection was performed by a researcher-made questionnaire form. Regarding qualitative variables, frequency, mean, and standard deviation were calculated; t test, chi-square test, and Fisher exact test were used in case of need.
Results
Gender: Among 20 patients who were selected for this study, three patients were female (two women in the case group and one in the control group) and 17 patients were male (eight men in the case group and nine men in the control group), and no significant difference was observed between the two groups in terms of gender distribution (P = 0.9).
Age: In this study, the mean age of patients was 71.8 (standard deviation [SD] = 9.636) and 65.2 (SD = 3.938) years in the case and control groups, respectively, and no significant difference was observed between the two groups (P = 0.06).
MSS: The mean MSS in the case and control groups was 7.2 (SD = 0.833) and 10 (SD = 1.333), respectively, and we found a significant difference between the two groups in surgeon satisfaction based on MSS (P < 0.0001).
The skin color in comparison with the surrounding area: According to Table 1, no significant difference was observed in skin color among the two groups, that is, case group and control group.
Skin texture (matte/shiny): Table 2 shows that there is no significant difference in skin texture between the two groups.
Scalp defect contour: In the case group, the scalp defect height in 66.7% of patients was equal to the surrounding area, and in 33.3% of them, a mild depression was seen. On the contrary, a mild depression, moderate depression, and severe depression were seen in 40%, 50%, and 10% of the control group patients, respectively, and a significant difference was achieved (P = 0.003), which are recorded in Table 3.
Distortion: No distortion was reported in our study.
Mobility of the repair area: As shown in Table 4, we observed a significant difference between the two groups regarding the mobility of repair site, which was in favor of better mobility among the case group.
Tumor type: No difference was observed between the two groups regarding the tumor type. More information is tabulated in Table 5.
Graft take: Seventy percent of patients in the case group experienced a successful graft take; however, one patient rejected the ADM graft because of inadequate wet dressing in three weeks, he underwent a split-thickness graft treatment. In the control group, full graft take was observed in 80% of patients, and no rejection was reported. We observed two patients with relative graft take in both groups. Regarding the graft take, we found no significant difference between the two groups (P = 1).
Surgeon and patient satisfaction: According to the VAS questionnaire form, a score of 3.22 and 6.4 was achieved in the case group (SD = 1.093) and the control (SD = 1.43) group, respectively, and a significant difference was observed in favor of more satisfaction in the case group (P = 0.001). On the contrary, surgeon’s satisfaction score was achieved as 2.44 and 5.6, respectively, in the case (SD = 1.333) and control (SD = 1.647) groups (according to VAS questionnaire form), which showed a significant difference between two groups in favor of more satisfaction in the case group from the surgeon’s point of view (P < 0.0001). These data are tabulated in Table 6.
| Groups | P value | ||||
|---|---|---|---|---|---|
| ADM + STSG | STSG | ||||
| The skin color compared with the surrounding area | Normal or homogenous | Numbers | 0+4 | 0+2 | 0.3 |
| Percentage | 44.4% | 20% | |||
| Heterogeneous | Numbers | 4+1 | 5+3 | ||
| Percentage | 55.5% | 80% | |||
| Groups | P value | ||||
|---|---|---|---|---|---|
| ADM + STSG | STSG | ||||
| Skin transparency | Matte | Numbers | 8 | 7 | 0.6 |
| Percentage | 88.9% | 70% | |||
| Shiny | Numbers | 1 | 3 | ||
| Percentage | 11.1% | 30% | |||
| Groups | P value | ||||
|---|---|---|---|---|---|
| ADM + STSG | STSG | ||||
| Scalp contour | Mild and moderate depression | Numbers | 9 | 4 | 0.003 |
| Percentage | 100% | 40% | |||
| Severe depression | Numbers | 0 | 6 | ||
| Percentage | 0% | 60% | |||
| Groups | P value | ||||
|---|---|---|---|---|---|
| ADM + STSG | STSG | ||||
| Repair site mobility | Mobility | Numbers | 8 | 3 | 0.0198 |
| Percentage | 88.9% | 30% | |||
| Difficult to move and impenetrable | Numbers | 1 | 7 | ||
| Percentage | 11.1% | 70% | |||
| Groups | P value | ||||
|---|---|---|---|---|---|
| ADM + STSG | STSG | ||||
| Tumor type | BCC | Numbers | 7 | 6 | 0.9 |
| Percentage | 70% | 60% | |||
| SCC | Numbers | 3 | 4 | ||
| Percentage | 30% | 40% | |||
| Groups | ||||
|---|---|---|---|---|
| ADM + STSG | STSG | |||
| Satisfaction | Patients | Mean | 3.22 | 6.4 |
| Standard deviation | 1.093 | 1.43 | ||
| Surgeons | Mean | 2.44 | 5.6 | |
| Standard deviation | 1.333 | 1.647 | ||
| P value | 0.001 | <0.0001 | ||
Figures 1–5 show five patients who were evaluated in the case and control groups. These images show the process of healing among these patients. In Figure 6, we used both techniques on one patient, and the results showed a better cosmetic outcome in the defect, which was reconstructed by using ADM and STSG.

- (A) SCC scalp tumor in the case group (patient 1). (B) Marginal SCC excision (patient 1). (C) ADM placing (patient 1). (D) Removing ADM tie-over and STSG placing (patient 1). (E) One month after the STSG placing (patient 1)
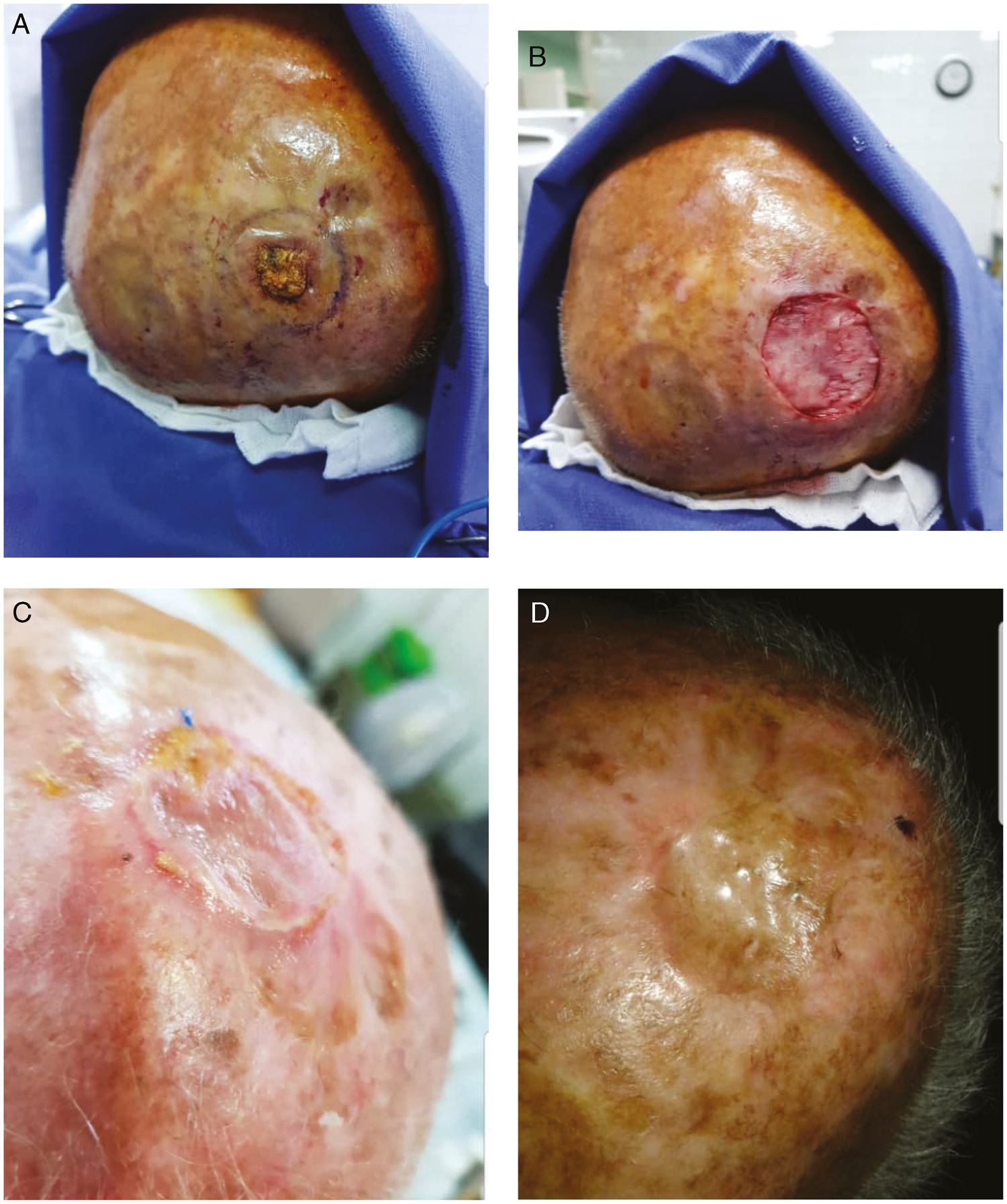
- (A) SCC scalp tumor in the case group (patient 2). (B) ADM placing (patient 2). (C) Three weeks after STSG placing (patient 2). (D) Three months after STSG placing (patient 2)
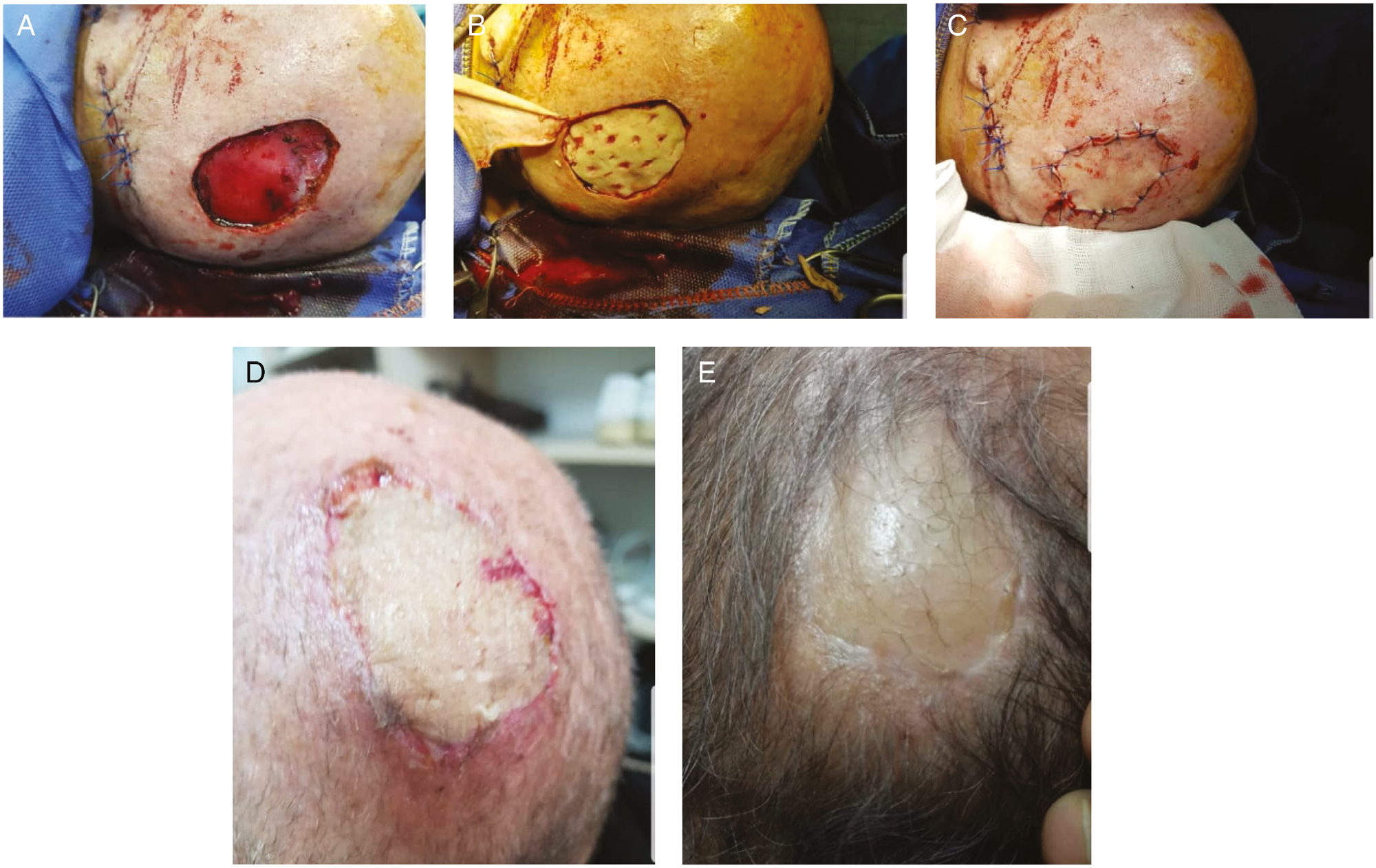
- (A) BCC scalp tumor in the case group (patient 3). (B) ADM placing (patient 3). (C) STSG and ADM placing (patient 3). (D) Ten days after removing tie-over (patient 3). (E) Three months after the surgery in the case group (patient 3)
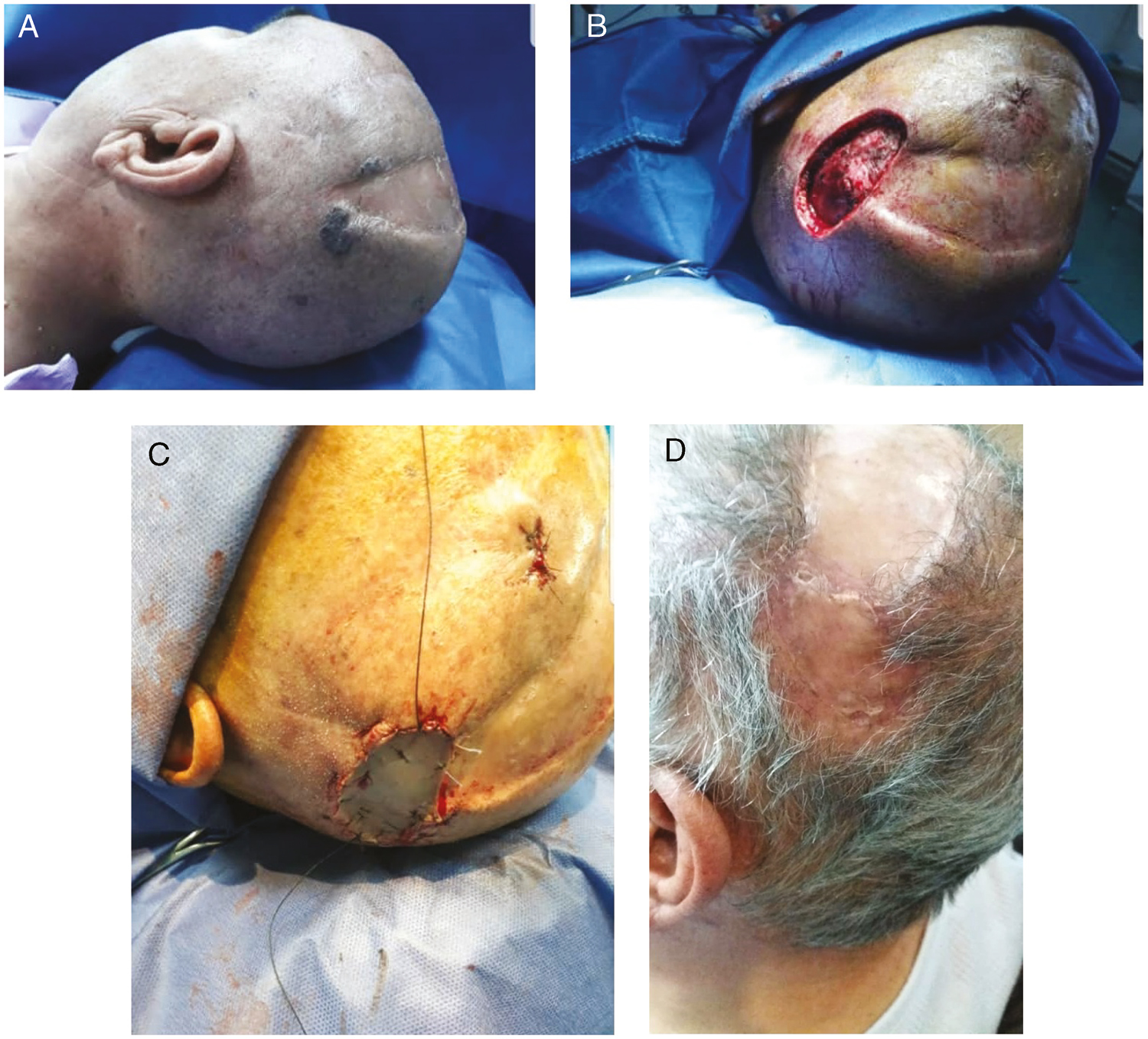
- (A) BCC scalp tumor in the case group (patient 4). (B) Preparing for ADM and STSG placing (patient 4). (C) ADM and STSG placing (patient 4). (D) After three months (patient 4)
- (A) SCC scalp in the control group (patient 5). (B) STSG placing without ADM (patient 5). (C) After three months without ADM (patient 5)
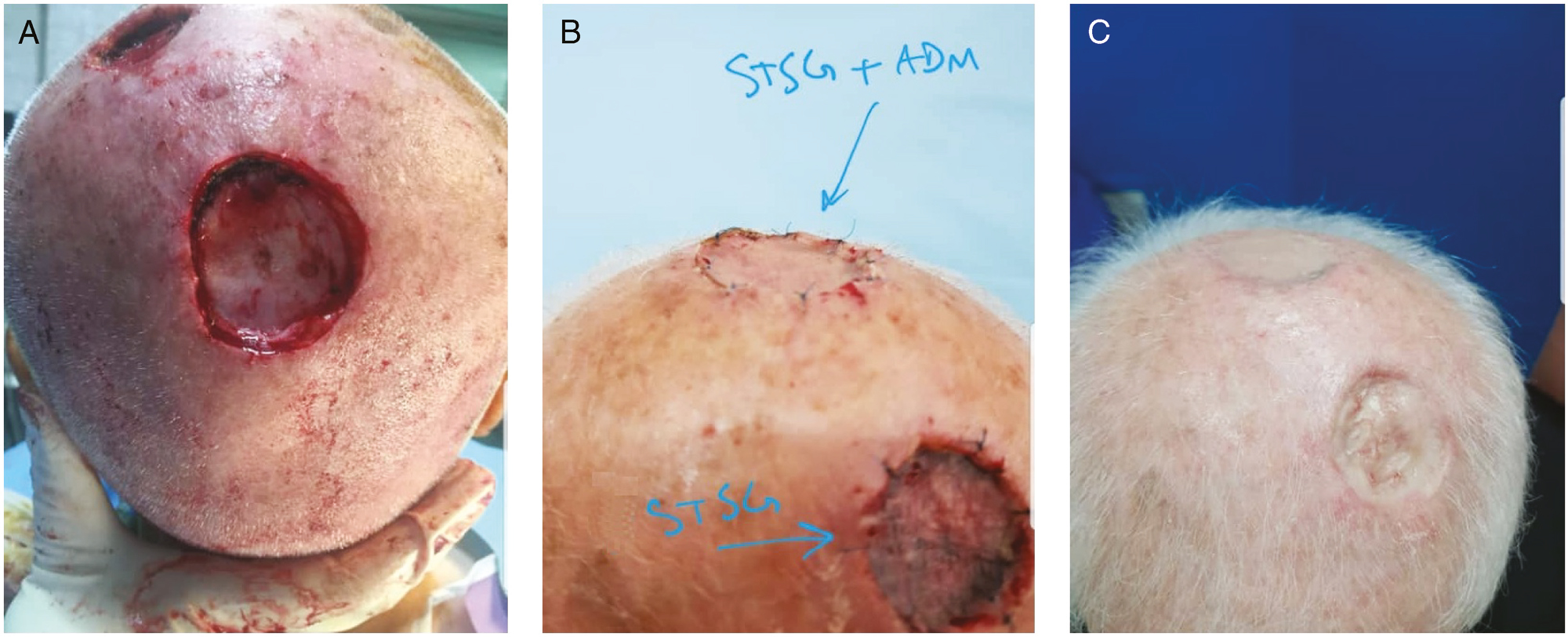
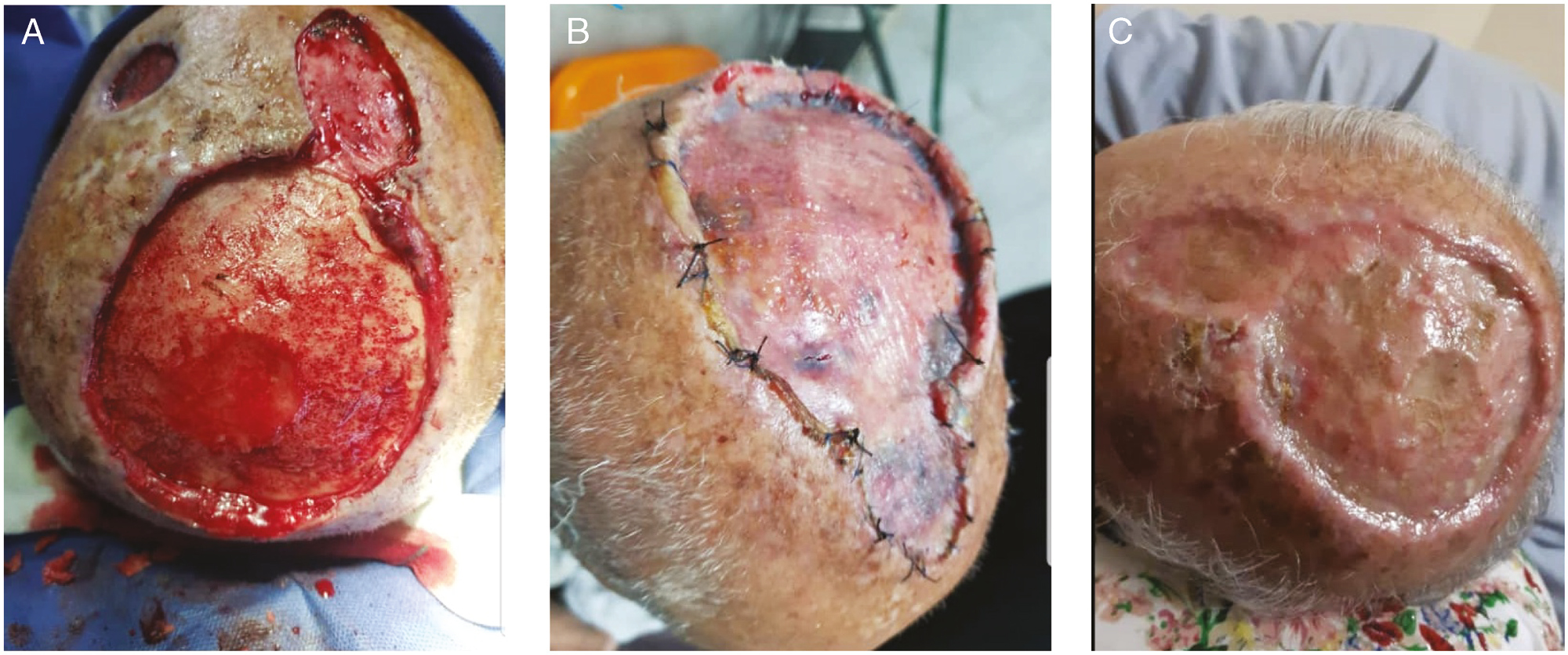
- (A) Case and control group outcomes in one patient with SCC scalp tumor. (B) Tie-over removing. (C) Outcomes of case and control group in one patient after three months. We can see a better cosmetic outcome in upper reconstruction with ADM and STSG in comparison with lower repair with only STSG
Discussion
BCC and SCC are the most common scalp malignancy with the treatment plan including the full-thickness excision of the defect, which sometimes may cause the calvarial exposure.
In a study by Chun and Verma[21] on an 82-year-old woman with a large scalp defect and calvarial exposure, a single-stage ADM and STSG reconstruction were used. Shorter period of treatment and excellent clinical outcome were observed in their study. In most of the cases, scalp tumor excision leads to the exposure of skull bone, so this full-thickness defect is always a big challenge for surgeons. Because of secondary repair, scar and distortion are formed, we are faced with unwanted cosmetic results. So they found ADM and STSG reconstruction technique as a practical method for the management of scalp defect, especially in the elderly patients with comorbidities.
Another study on eight patients with primary or secondary scalp tumor was performed by Corradino et al.[22] All of them underwent the tumor removal operation, and the defects were covered by a dermal substitute STSG. There was a full graft take for all cases; two patients were found with early tumor recurrence two months after the surgery; however, all patients were satisfied from the results of the surgery. In our study, one patient in the case group experienced ADM rejection because of inadequate wet dressing and two patients experienced relative STSG take, which healed spontaneously with time. There were also two patients with more than 50% STSG take problem in the control group and approximately 75% of their defect stayed without coverage and the bones were exposed.
In 2011, Brunetti et al.[23] reported a 77-year-old case, who underwent the treatment of BCC, which was multifocal recurrent in the right temporoparietal region. This case experienced several recurrences and resection during 10 years, and the last resection was wider with minus margin and led to a 1.2 × 5.7 cm defect. The defect was covered by ADM for 20 days, and a decrease in diameter and defect depth caused by granulation let the surgeon to place a STSG on the area. The result showed a full graft take and suitable vascularization in the defect area.
Thirty patients with various scalp tumors were studied by Koenen et al.[24] in the dermatology department of Mannheim Medical University. For all patients, the scalp tumors were excised with a good margin up to the skull bone and then external table of skull bone was shaved to reach the bleeding point. Then, the ADM was fixed in the defect area for three weeks, and finally, STSG was placed. In none of them, hematoma, necrosis, and cerumen occurred, and full graft take was observed in all of them. Cosmetic results were achieved in all patients, and no failure was reported in them.
The purpose of this study was to evaluate the satisfaction of surgeons and patients from the results of using ADM and STSG as a reconstruction method in the management of scalp defects in the patients with scalp tumors. No significant difference was observed in the two groups of case and control regarding the graft take (P = 0.9). There was a full graft take in 70% and 80% of patients in the case and control group, respectively. One patient in the case group experienced ADM necrosis due to an inadequate dressing, and two patients with partial graft take in the control group whose calvarium stayed uncovered and exposed.
According to the treatment results, we observed more satisfaction among the patients in the case group; this parameter was determined using VAS form. The score range of satisfaction was between 0 and 10; the score near to 0 showed more satisfaction, and for this factor, there was also a significant difference between the two groups. The same results were obtained during the evaluation of surgeons’ satisfaction.
We showed that using ADM in defect area helps to fill the defect area with a granulated tissue that lets the surgeon to place a STSG on this site. These approaches lead to the full graft take and appropriate homogeneity in the restored and surrounding tissues in terms of quality and thickness of the tissue. As can be seen in the figures, a homogenous reconstruction was obtained in patients in the case group. Figure 6 shows a better view for comparing the cosmetic outcomes of both techniques in reconstructing the scalp defect.
Conclusion
This study has shown that using ADM and STSG reconstruction has some advantages because of ADM availability and easier application in comparison with local, regional, or free flaps. It is also a better choice for the elderly patients with comorbidities, and it helps us in the early diagnosis of tumor recurrence in the undertreatment area. ADM is more expensive than autologous graft, but it is a simple procedure.
Using local or regional flaps may cause deformity and some complications such as flap loss or necrosis in the donor site and longer operating time; however, ADM is more acceptable for patients with minimal complications and is an easier procedure. More studies are needed with larger sample size and longer follow-up periods in order to further evaluate the benefits of this procedure as compared to other techniques.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Epidemiology of basal cell carcinomas and squamous cell carcinomas in a Department of Dermatology—a 5-year review. 87:212-19.
- [Google Scholar]
- Surgical treatment of basal cell carcinoma and squamous cell carcinoma. G Ital Dermatol Venereol. 2015;150:435-47.
- [Google Scholar]
- One stage reconstruction of scalp after full-thickness oncologic defects using dermal regeneration template. Biomed Res Int. 2015;2015:1-11.
- [Google Scholar]
- Double purse-string closure for scalp and extremity wounds. Dermatol Surg. 2007;33:369-73.
- [Google Scholar]
- Repair of scalp defect using a superficial temporal fascia pedicle VY advancement scalp flap. Br J Plast Surg. 2005;58:676-80.
- [Google Scholar]
- Repair of a large, full-thickness scalp defect with exposed bone using a thin transposition flap. Dermatol Surg. 2013;39:646-8.
- [Google Scholar]
- Scalp reconstruction with island hair-bearing flap. Plast Reconstr Microsurg. 2005;115:1366-71.
- [Google Scholar]
- Free abdominal flaps: variations in design and application to soft tissue defects of the head. J Reconstr Microsurg. 1989;5:193-201.
- [Google Scholar]
- Reconstruction of extensive scalp defects with rectus free flaps. Ann Plast Surg. 1995;34:281-5.:285-5.
- [Google Scholar]
- Rationale for reconstruction of large scalp defects using the anterolateral thigh flap: structural and aesthetic outcomes. J Reconstr Microsurg. 2005;21:539-45.
- [Google Scholar]
- Rapid wound healing of scalp wounds devoid of periosteum with milling of the outer table and split-thickness skin grafting. Br J Dermatol. 2012;167:343-7.
- [Google Scholar]
- Second-intention healing of exposed facial-scalp bone after Mohs Surgery for skin cancer. J Am Academy Dermatol. 1994;31:450-4.
- [Google Scholar]
- Reconstruction of nasal skin cancer defects with local flaps. J Skin Cancer. 2011;2011:181093.
- [Google Scholar]
- One stage reconstruction of skull exposed by burn injury using a tissue expansion technique. Arch Plast Surg. 2012;39:118-23.
- [Google Scholar]
- Technical aspects of prolonged scalp expansion. Arch Otolaryngol Head Neck Surg. 1994;120:431-6.
- [Google Scholar]
- The use of a bovine collagen construct for reconstruction of full-thickness scalp defects in the elderly patient with cutaneous malignancy. Ann Plast Surg. 2005;54:297-301.
- [Google Scholar]
- Two-stage reconstruction of head and neck defects after tumor resection with a dermal regeneration template. J Cutan Med Surg. 2011;15:259-65.
- [Google Scholar]
- Single-stage full-thickness scalp reconstruction using acellular dermal matrix and skin graft. Eplasty 2011:11(e4).
- [Google Scholar]
- Reconstruction of full thickness scalp defects after tumour excision in elderly patients: our experience with Integra dermal regeneration template. J Plast Reconstr Aesthet Surg. 2010;63:e245-7.
- [Google Scholar]
- The use of acellular dermal matrix in reconstruction of complex scalp defect. Dermatol Surg. 2011;37:527-9.
- [Google Scholar]
- Removal of the outer table of the skull for reconstruction of full-thickness scalp defects with a dermal regeneration template. Dermatol Surg. 2008;34:357-63.
- [Google Scholar]






