Translate this page into:
Autologous Dermal Filler Derived from Cultured Dermal Fibroblasts and Plasma Gel (Fibrogel): One-year Follow-up of a Case
Address for correspondence: Dr. Yasemin Oram, Department of Dermatology, Liv Hospital, Kelaynak Sok 9/10, Deniz Apt, Ulus, Besiktas 34340, Istanbul, Turkey. E-mail: yaseminoram65@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
In recent years, autologous fibroblast injections or plasma gel filler applications have been used in the treatment of facial contour deformities. In this case report, we describe a new method of autologous filler material derived from cultured dermal fibroblast and plasma gel. The plasma gel, which is the bioskeleton of the filler, is prepared from the patient’s plasma, which provides a dense environment for a three-dimensional configuration of dermal fibroblasts. Although the plasma gel provides immediate volume effect, the fibroblasts synthesize extracelluar matrix proteins to promote skin rejuvenation. The filler effect occurs immediately after the first injection and persists 12 months after the third injection, without any complication. Long-term result of the presented case is promising for the concept of autologous filler development.
Keywords
Autologous filler
dermal filler
fibroblast culture
platelet-rich gel
skin rejuvenation
Introduction
In recent years, autologous dermal fibroblast injections have been used to treat facial contour deformities including wrinkles, acne scars, and periorbital skin flaccidity. Unfortunately, the onset of the effect is slow and gradual. Moreover, particularly in large volume defects, it might be insufficient to achieve an acceptable result.[123] Another approach for autologous rejuvenation is to use an injectable gel based on autologous platelet-rich plasma (PRP) or platelet-poor plasma (PPP), which provides significant aesthetic correction of wrinkles and volume defects.[45]
In this case report, we describe a novel method for autologous soft-tissue augmentation. This novel autologous filler, Fibrogel, is composed of the patient’s own cultured fibroblasts and plasma gel, which is prepared from the patient’s own plasma. It provides an immediate filler effect because of its plasma gel component, especially in deep volume losses, and consequently cultured fibroblasts lead to skin rejuvenation. Here we describe the one-year follow-up of a patient who was treated with Fibrogel for elbow wrinkles.
Case Report
A 55-year-old woman was admitted to our clinic for the treatment of elbow wrinkles by using fibroblast injection [Figure 1]. Because the wrinkles and volume loss were prominent, we recommended Fibrogel filler, which is mainly a combination of cultured fibroblasts and plasma gel. The patient underwent the informed consent process and signed informed consent was obtained before the procedure.

- Elbow wrinkles before Fibrogel injection
A skin biopsy sample (3-mm punch) was taken from the postauricular area and immediately sent to the laboratory (GENKORD Health and Biotechnology Services, Istanbul, Turkey) in a sterile cell transport medium with 100mL of the patient’s blood for fibroblast culture. The fibroblasts were cultured using PRP and PPP prepared from the blood sample. After six weeks, approximately 40 million cultured fibroblasts were obtained, which is a sufficient number for injection. The quality control of the cells consisted of determination of the cell count, viability of the cells, morphologic analysis, immunophenotyping, deoxyribonucleic acid-polyploidy analysis, differentiation capacity analysis, and aerobic and anaerobic cultures. Second or third passage cultures were used to prepare the filler.
When the cultured fibroblasts were ready, two tubes of 10mL of blood sample were obtained from the patient, and the PRP and plasma were separated just before the injection. The PRP and plasma were then gradually heated to form the bioskeleton of the filler (plasma gel). The cultured fibroblasts were homogenously mixed with the plasma gel to obtain the 3D filler material (Fibrogel). The temperature of the plasma gel should be less than 37°C while mixing it with the fibroblasts. In each treatment, 10mL of the filler containing 4 × 106/mL cells were used.
After preparing the skin around the injection site with antiseptics, Fibrogel was injected into the intradermal and subdermal layers by using a 30-gauge needle. The injections were repeated every month for three months. The patient was evaluated before each treatment and 3, 6, and 12 months after the last treatment. The outcome of the procedure was evaluated based on the clinical improvement and controlled using photographs obtained at each visit.
Wrinkle improvement was evaluated as poor (0%–25%), moderate (26%–50%), good (51%–75%), and excellent (76%–100%) by two physicians independently. The patient showed excellent and instantaneous improvement after the injection, and was satisfied with the outcome throughout the following year, in which we conducted periodic examinations at intervals of 3–6 months (months 3, 6, and 12) without any complication. The physicians also considered the improvement satisfactory [Figures 2–4].
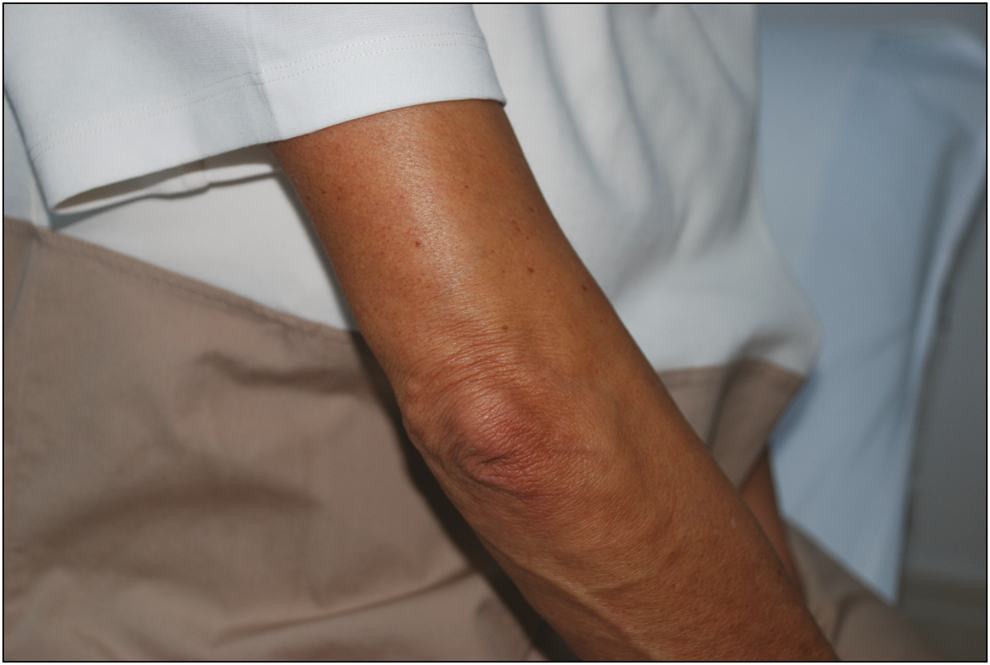
- One month after the first injection
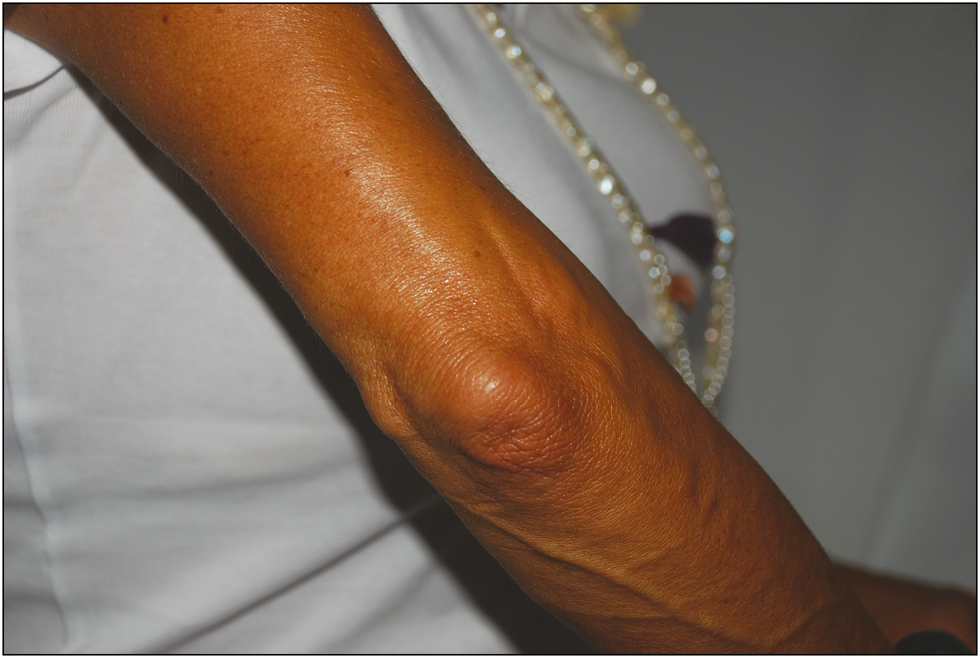
- Six months after the third injection
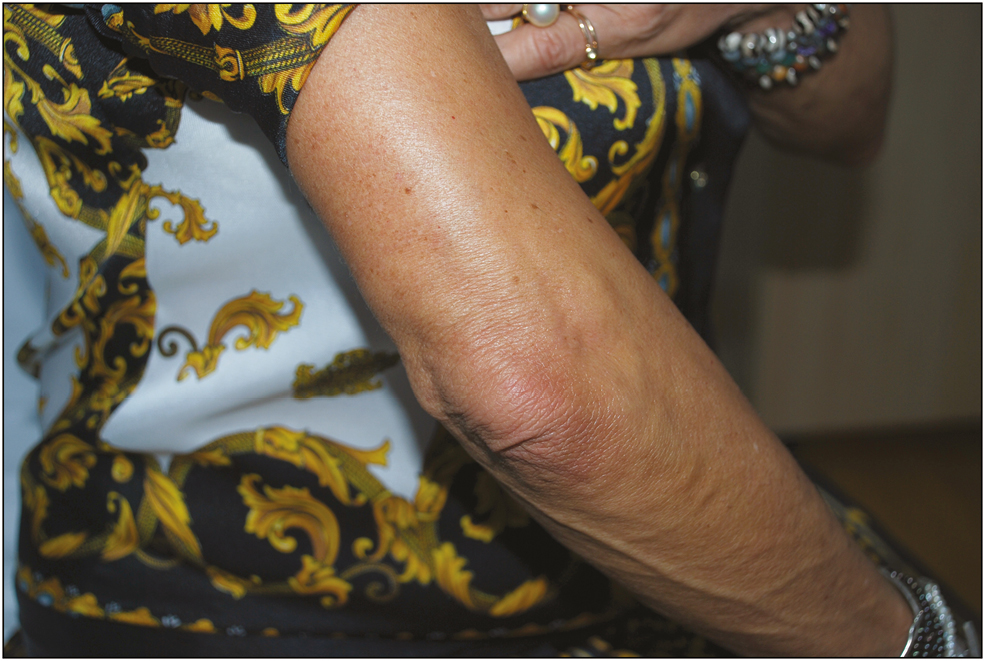
- Twelve months after the third injection
Discussion
Cultured autologous dermal fibroblasts are believed to promote skin regeneration and rejuvenation. Autologous fibroblasts are natural fillers and generate matrix proteins. It was shown that autologous fibroblast injection for facial rhytides led to dermal collagen increase along with the clinical improvement of the rhytides.[3] Smith et al.[1] reported successful results in a placebo-controlled study using autologous fibroblast injection therapy for nasolabial fold wrinkles. However, they emphasized that unlike other dermal fillers, the onset of the effect is gradual, and it does not replace the direct volume. In their study, a significant effect was observed as early as two months after the first treatment and it was persistent at the sixth month without any sign of degradation.[1]
The use of plasma gel is a new filler concept for skin rejuvenation. Plasma gel is an autologous gelatinous material prepared from the patient’s own PPP or PRP. Gomez et al.[4] have reported that autologous platelet rich in growth factor technology has desirable mechanical and bioactive properties and allows moderate wrinkle reduction and facial volume loss, which maintain for 16 weeks. Another clinical study evaluating the effect of PPP-derived plasma gel has shown that two sessions of plasma gel injections at two-week intervals lead to an immediate and significant clinical improvement, which continued for three months.[5]
Fibrogel is mainly the combination of cultured fibroblasts and plasma gel. Its use was associated with clinically impressive improvement in elbow wrinkles and volume loss in the presented case. The effect was prominent immediately after the first treatment and lasted over 12 months after the last injection. The plasma-originated bioskeleton of the filler holds the autologous fibroblasts in a dense nontoxic environment, allowing the fibroblasts to arrange in a three-dimensional configuration. The dense plasma gel bioskeleton of Fibrogel also leads to a volume effect in the subdermis. Therefore, as with other dermal fillers, the effect appears immediately after the injection, which increases patient satisfaction. Moreover, the plasma gel component enhances fibroblast proliferation because it is rich in growth factors. As Fibrogel is merely autologous, severe complications observed in other fillers including vascular occlusion, hypersensitivity reactions, nodule formation, or migration of the filler are not expected. Erythema, swelling, and needle puncture purpura resolving in two days were the only local side effects of Fibrogel injection, which is compatible with previous reports of fibroblast and plasma gel injections.[145]
Thus, Fibrogel can be considered as more sophisticated autologous fibroblast cell therapy, providing both the generation of matrix proteins and replacement of volume loss, which results in a sustained therapeutic effect.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Gürsel Turgut is the CEO and founder of GENKORD.
References
- A multicenter, double-blind, placebo-controlled trial of autologous fibroblast therapy for the treatment of nasolabial fold wrinkles. Dermatol Surg. 2012;38:1234-43.
- [Google Scholar]
- Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-36.
- [Google Scholar]
- Autologous fibroblasts for treatment of facial rhytids and dermal depressions: a pilot study. Arch Facial Plast Surg. 1999;1:165-70.
- [Google Scholar]
- Autologous platelet-rich gel for facial rejuvenation and wrinkle amelioration: a pilot study. J Cosmet Dermatol. 2019;18:762-72.
- [Google Scholar]
- Assessment of the efficacy and safety of platelet poor plasma gel as autologous dermal filler for facial dermal filler. J Cosmet Dermatol 2019:1-9.
- [Google Scholar]






