Translate this page into:
A Basic Algorithmic Surgical Approach for Nicolau Syndrome
Address for correspondence: Dr. Osman Kelahmetoğlu, Department of Plastic, Reconstructive and Aesthetic Surgery, Bezmialem Vakif University, Adnan Menderes Bulvarı Vatan Caddesi 34093 Fatih, Istanbul, Turkey. E-mail: osmankelahmetoglu@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Nicolau syndrome (NS) is a rare complication that develops after the administration of intramuscular diclofenac sodium. The etiology and surgical treatments of 11 patients with NS were evaluated and studies in the literature were examined. The aim of this study was to compose a basic algorithm for surgical approaches to treat NS.
Materials and Methods:
Eleven patients were evaluated for NS between December 2013 and January 2018. Two patients did not accept treatment, and nine patients underwent surgical debridement of necrotic tissues. The tissue defects of five patients were closed with a fasciocutaneous flap and, in four patients, the defects were repaired primarily.
Results:
No complications, such as wound infection, wound dehiscence, seroma, or flap necrosis, were encountered. Of the seven patients who received concurrent antibiotic therapy, no patient had any problems at their follow-up (2–30 months). The results were satisfactory from an aesthetic and functional point of view.
Conclusion:
NS was more frequent in women with a high body mass index and high fat in gluteal regions. We considered that any kind of medication could lead to NS. Different methods are discussed for treatment.
Keywords
Diclofenac sodium
injection
intramuscular
Nicolau syndrome
INTRODUCTION
Nicolau syndrome (NS), also known as Embolia cutis medicamentosa or livedoid dermatitis, is a complication that occurs subsequent to intramuscular (IM) injection of various drugs. The condition can often be overlooked.[1] It first presents as pain around the injection site and develops as inflammation in the skin, the underlying adipose tissue, and in the muscle.[2]
The condition has been associated with almost all drug classes, including nonsteroidal anti-inflammatory drugs (NSAIDs),[3] local anesthetic agents,[4] corticosteroids,[56] antibiotics,[789] vitamin B complex, vitamin K,[10] oxytocin,[11] antineoplastics,[121314] antihistamines, and vaccines.[15] The most acute form of the complication occurs after NSAID injections.[1617] In one case, a cold compress applied for local pain management was reported to have increased acute local effects, rapidly leading to necrosis.[18]
Although the etiopathogenesis of the condition remains unclear, the most prevalent explanation is accidental penetration of the drug into the intravascular area during IM injection, leading to erythema and a livedoid reticular and/or hemorrhagic wound secondary to arterial thrombosis. The reaction can lead to necrosis and ulceration of the skin, subcutaneous adipose tissue, and muscular tissue.[18]
IM injections are widely administered both in and outside of hospitals. Given that they are not performed by health-care professionals and performed under non-sterile conditions, those performed in nonhospital settings can lead to complications, such as NS. Therefore, it is important to be able to identify and manage NS.
In this study, we present the treatment results of nine patients who received basic surgical approaches to treat NS.
MATERIALS AND METHODS
We retrospectively evaluated 11 patients who were treated or referred to our department for wounds, and eventually diagnosed with NS between December 2013 and January 2018. Patient data on age, gender, height, and weight, etiology/drug and site of the IM injection, and reconstruction techniques for defect closure were recorded. Body mass index (BMI) was calculated, and concomitant diseases and medications were recorded.
RESULTS
Of the 11 patients (nine female and two males; mean age: 52.5, range: 24–78 years) who were diagnosed with NS, two did not accept treatment. NS had occurred after IM diclofenac sodium injection in eight patients, and after IM ceftriaxone injection in three patients. Seven patients presented with typical postinjection NS lesions in the right gluteal region, and three patients developed lesions in the left gluteal region [Figure 1]. One patient presented with a lesion in bilateral anterior thighs. Initial surgical debridement was performed in all nine of the treated patients. Culture samples were taken from four patients, of which one was reported contaminated. Extended-spectrum β-lactamase-producing Escherichia coli was seen in two wound cultures and Klebsiella pneumoniae was detected in one wound culture. Infected patients were started on antibiotics according to the antibiogram after consulting the infectious disease department. Of the 11 patients, five had diabetes mellitus, six had hypertension, and one had liver cirrhosis. The injections were administered by health-care professionals in eight patients and by their relatives in three patients. All patients reported a history of multiple injections. Negative pressure wound therapy was used for 6–19 days in all seven patients after debridement. Although granulation tissue developed in six patients, one patient required a second debridement procedure. Fasciocutaneous flaps were used in five patients and defects were primarily closed in four patients [Figures 2 and 3]. Patients were discharged on days 2–8 (mean, 3 days). No complications, such as wound infection, wound dehiscence, seroma, or flap necrosis, were encountered in any of the patients. No postoperative complications were observed in any of the nine patients throughout their mean 12-month follow-up period.
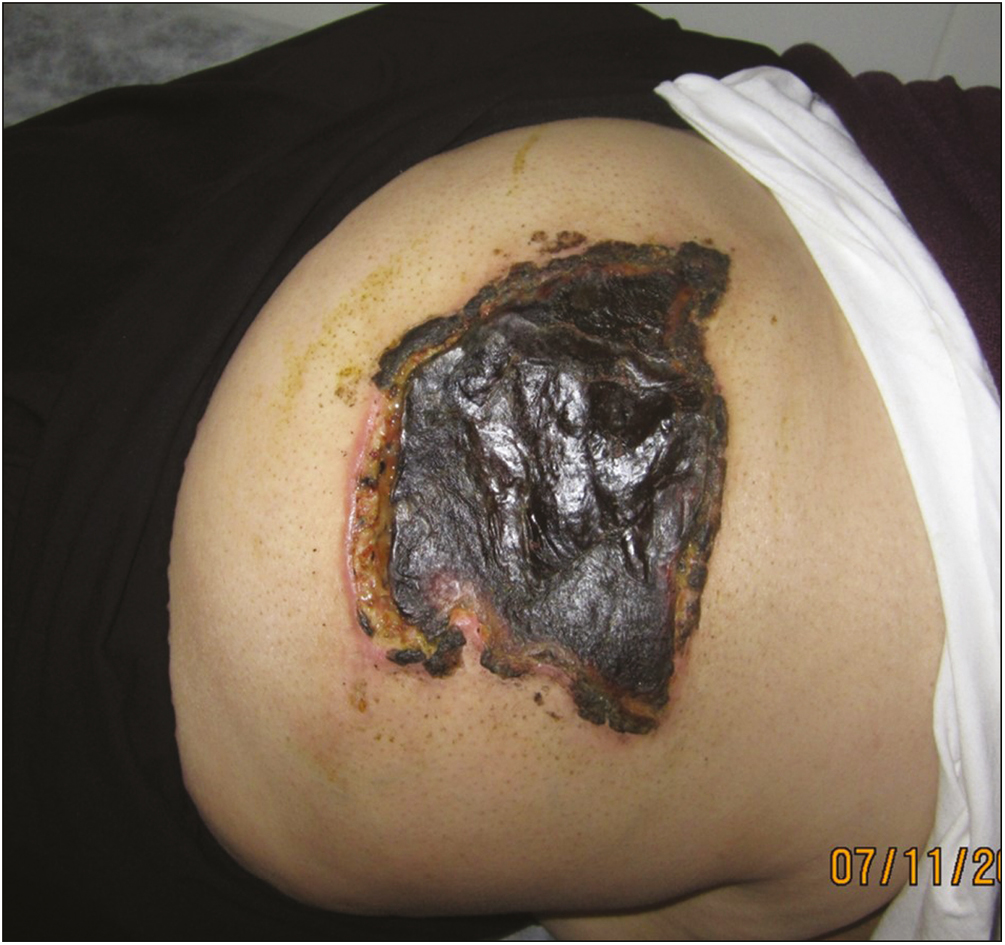
- Pathognomonic lesion for NS
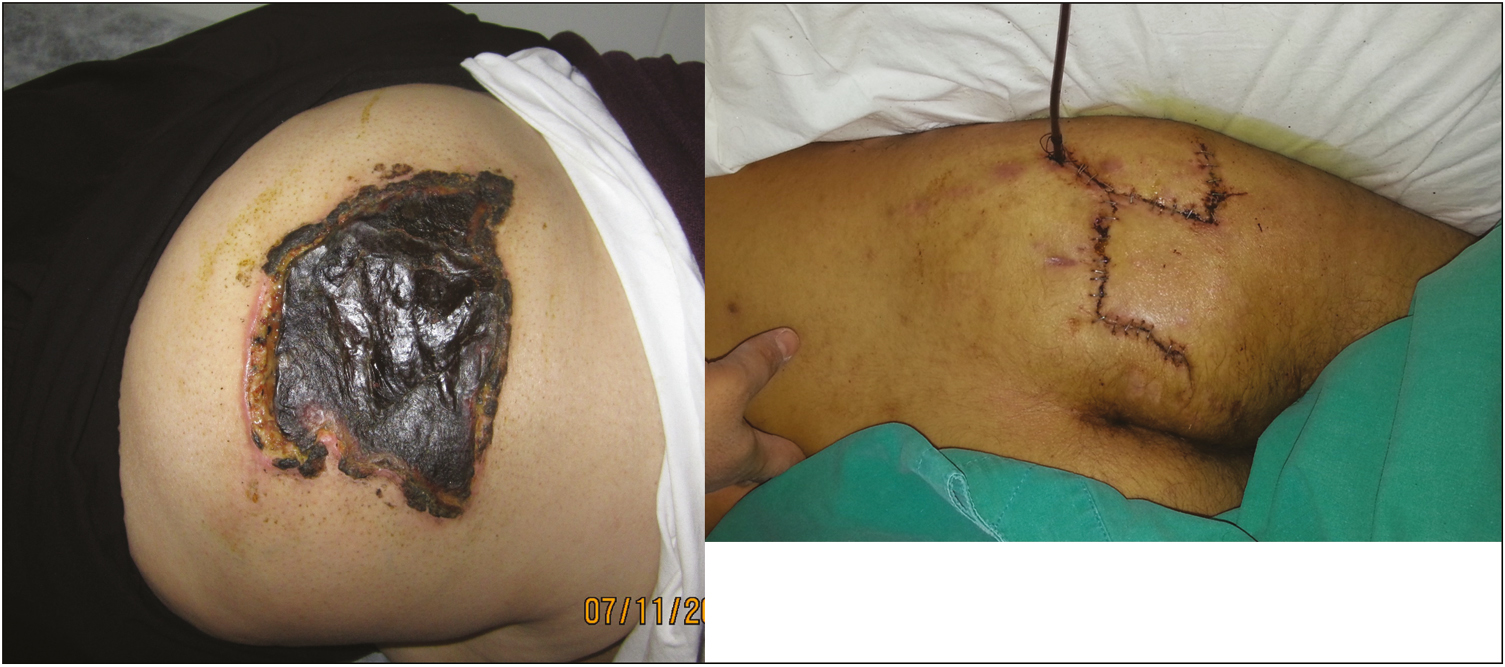
- Case 2. The 64-year-old female patient presented with an eschar wound of approximately 15cm × 15cm in the right gluteal region that developed after an IM diclofenac sodium injection given in an external clinic. Debridement, negative pressure wound therapy were applied, then the site was reconstructed with fasciocutaneous flap
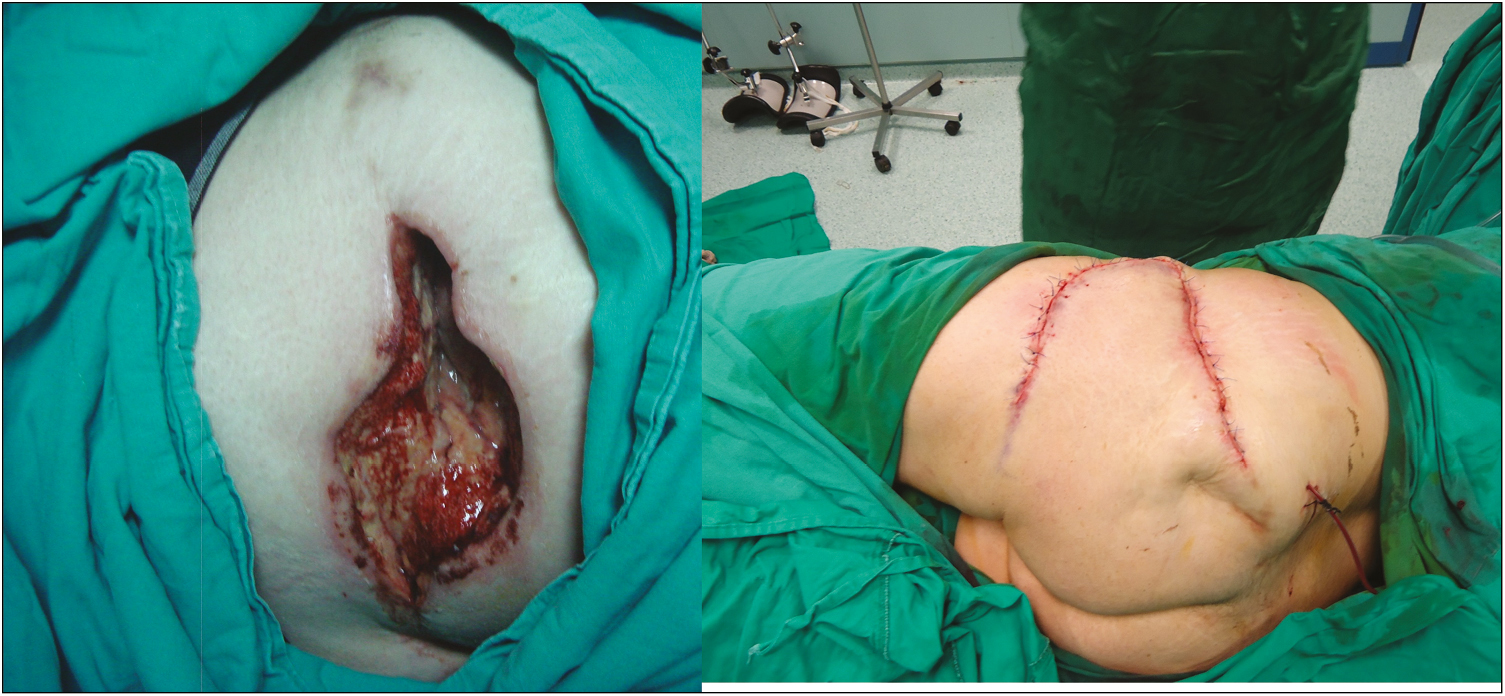
- Case 3. The 45-year-old female patient who was receiving IM diclofenac sodium injections for her migraine in her home presented with a partially necrose wound with discharge of approximately 15cm × 8cm in the left gluteal region. Debridement, negative pressure wound therapy were applied, then the site was reconstructed with fasciocutaneous flap
All nine female patients were obese (BMI 34–47, mean 40.44kg/m2). The BMIs of the two male patients were 30 and 40. The overweight female patients frequently received IM NSAID injections for pain. Demographic data, concomitant diseases, and treatment methods of the patients are given in Table 1.
| Age | Sex | Localization | Etiology | Treatment | BMI | Additional disease | |
|---|---|---|---|---|---|---|---|
| 1 | 46 | Female | Right gluteal | Diclofenac sodium | Fasciocutaneous flap | 35 | Hypertension diabetes |
| 2 | 64 | Female | Right gluteal | Diclofenac sodium | Fasciocutaneous flap | 41 | Hypertension |
| 3 | 40 | Female | Left gluteal | Diclofenac sodium | No treatment | 43 | – |
| 4 | 62 | Female | Left gluteal | Ceftriaxone | Primary repair | 34 | Hypertension liver cirrhosis |
| 5 | 24 | Male | Right gluteal | Diclofenac sodium | Fasciocutaneous flap | 30 | – |
| 6 | 62 | Female | Right gluteal | Diclofenac sodium | No treatment | 46 | Diabetes |
| 7 | 30 | Male | Right gluteal | Diclofenac sodium | Primary repair | 40 | – |
| 8 | 58 | Female | Right gluteal | Diclofenac sodium | Fasciocutaneous flap | 47 | Hypertension |
| 9 | 45 | Female | Left gluteal | Diclofenac sodium | Fasciocutaneous flap | 43 | Diabetes |
| 10 | 70 | Female | Right gluteal | Ceftriaxone | Primary repair | 35 | Hypertension diabetes |
| 11 | 78 | Female | Bilateral thigh anterior | Ampicillin/sulbactam | Primary repair | 40 | Hypertension diabetes |
BMI = body mass index
DISCUSSION
NS was first described by Nicolau in 1925 following IM injections of bismuth salt to treat syphilis.[19] Since then, many drugs have been reported to cause NS. As most of these drugs are routinely used in clinical practice, diagnosis and treatment of NS are important.
The etiopathogenesis of the condition remains unclear, but it is suggested to be the development of a livedo-like dermatitis secondary to arterial thrombosis as a result of the accidental penetration of the drug into the intravascular area during an IM injection.[617] Navrazhina et al.[20] reported performing a biopsy and tissue culture from a purpura-like NS plaque area that developed after a lumbar puncture. Although the tissue culture failed to grow, the biopsy result was reported as “pauci-inflammatory thrombogenic vasculopathy affecting the capillaries and venules throughout the dermis and subcutaneous fat with endothelial necrosis and sloughing into vascular lumens.” Another theory suggests that perineural, intra-arterial, and periarterial injections cause local pain, and vasospasm secondary to the local pain leads to sympathetic nerve stimulation followed by necrosis in cutaneous, subcutaneous, adipose, and/or muscle tissues.[18] Most cases in the literature are reported to have occurred after a diclofenac sodium injection.[118,21] An IM diclofenac sodium injection was the causative agent in 8 of our 11 patients. The development of NS after injecting NSAIDs suggests that the condition may be associated with the pharmacological characteristics of this drug group. NSAIDs inhibit prostaglandin synthesis by inhibiting cyclooxygenase. Ischemic necrosis develops once prostaglandin is suppressed by the drug and vasospasm is induced.[21] Some studies also indicate that NS has no allergic or immunological origins. There were no findings of NS after the treated patients received IM injections containing the same causative agent.[1822]
Although NS is mostly reported to be caused by IM-administered drugs, as compiled in the table given in Nischal et al.,[23] it is also caused by intravenous, intra-articular, and glucocorticoid injections, as well as by subcutaneously and subacromially injected drugs.
Although NS is a rare condition, IM injections increase the possibility of occurrence, particularly in obese patients.[22] A mean BMI of 41.28 (high-health risk level) in our female patients and 35 (overweight) in our male patients suggest that weight can be a significant risk factor in the occurrence of NS. Dadaci et al.[24] reported measuring the thickness of the adipose tissue on pelvic tomography images of gluteal injection sites, and concluded that green needles (3.8cm in depth) do not penetrate deep enough to pass the adipose tissue and reach the gluteal region.[24] Adipose tissue thickness was 5.4cm on magnetic resonance imaging. A needle depth of 3.8cm indicates that drugs that have toxic effects, disrupt circulation, and increase the risk of fatty necrosis are injected into the adipose tissue instead of the muscle. Furthermore, as a result of cumulative effects, greater inflammation and tissue necrosis were observed in patients receiving repeated injections. The depth of the subcutaneous tissue also varies depending on gender and weight of the patient. IM injections performed with a standard green needle reach the gluteal region in only 5% of women and 15% of men, and the remainder is injected into subcutaneous tissue.[17] Obesity in all of our female patients could have been a risk for NS. Another risk factor is frequent injections, which was present in all nine of our patients. Vascular problems, particularly in obese patients, have led to the increased administration of IM injections, suggesting that the increased rate of injections, both in hospital and nonhospital settings, could be a risk factor for NS.
IM injections should be duly administered by health-care professionals. Complications can be minimized with Z-track method, which is one of the injection methods.[25]
Treatments for NS remain controversial. Surgical debridement, local flaps, and partial-thickness skin grafts can be used to treat lesions with established necrosis. Analgesics, dressings, and antibiotics are useful as conservative treatment methods for pain management in many cases.[26] Some authors have reported using pentoxifylline to reduce blood viscosity, hyperbaric oxygen to increase oxygenation, fibroblast activity and angiogenesis in the wound, and heparin to reduce vascular occlusion.[520,26] There are also reports of using nifedipine, a calcium channel blocker, for its arterial vasospasm-reducing effects and systemic steroids for their inflammation-suppressing characteristics.[57] There is no standardized treatment for NS; the current aim was to prevent the condition from developing into soft tissue necrosis.[5] In many cases, surgical debridement should be considered to support wound healing and to reduce the risk of infection.[20] In their report of a 9-year-old patient who developed NS on the fingers after a penicillin injection into the deltoid muscle, Memarian et al.[7] described treating the patient with intravenous hydrocortisone, subcutaneous enoxaparin, and local trinitroglycerin. In addition, Alkan et al.[27] reported treating a 4-year-old patient with pentoxifylline and heparinization for NS caused by a penicillin injection, but the treatment was initiated immediately after the injection, before necrosis developed. Surgical debridement was primarily performed in all of our patients as all presented with the typical dark-colored lesion seen in NS. Although the best aesthetic outcomes can be achieved with primary closure, except in large wounds, partial thickness skin grafts will take time to heal if atrophic scarring and hyperpigmentation occur. Flaps are the best option for larger gluteal defects.[28] Kocman et al.[28] reported treating five NS patients with free-style perforator V-Y flaps designed vertically or horizontally according to the long axis of the wound. This provided reduced donor site morbidity and less tension on the recipient site.[28]
Pain around the site after injection, followed by erythematous, livedoid, and hemorrhagic formations and, ultimately, cutaneous, subcutaneous, adipose tissue, and muscular tissue necrosis were seen in the reported cases. Necrosis in related dermatomes is pathognomonic for NS.[29]
In our study, the wounds were primarily closed after surgical debridement in four patients, and a fasciocutaneous flap was used in five patients. In their case series of 17 patients with NS, Dadaci et al.[24] reported treating the wounds with primary closure in 11 patients and reconstructing with local flaps in six patients (four with Limberg flaps and two with V-Y advancement flaps). There are reports of NS cases reconstructed with partial-thickness skin grafts[2023,26] and full-thickness skin grafts[17] after surgical debridement. These cases healed with residual depressed scars. In their study of three patients, Seok-Kwun et al.[26] reported using a partial-thickness skin graft in one, primary closure in one, and bilateral advancement flap in the other case. We designed an algorithmic approach for surgical repair [Figure 4]. Surgical debridement and antibiotics should be given in cases of necrosis and infection. Vacuum treatment may be necessary to mature and granulate wounds that are not infected but are not ready for reconstruction. Skin grafts should be considered in cases where primary closure and faciokutan flap selection are not possible.
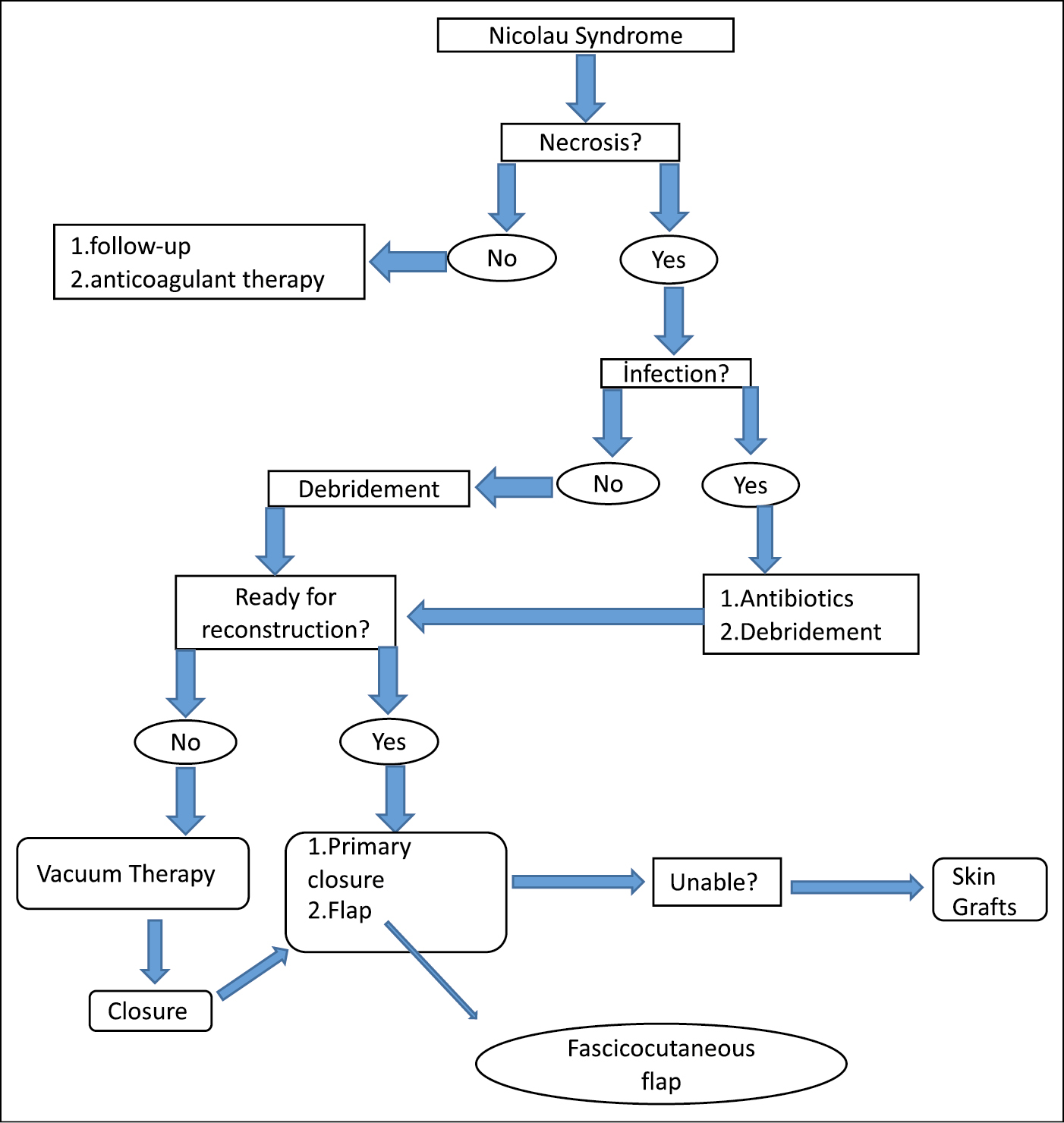
- An algorithmic approach for surgical repair
CONCLUSION
NS was more frequent in women with a high BMI and high fat in the gluteal region, particularly those with NS. We considered that any kind of medication could lead to this syndrome. Different treatment methods were discussed and we developed a basic, efficient surgical algorithm for NS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Sonographic findings in Nicolau syndrome following intramuscular diclofenac injection: a case report. J Clin Ultrasound. 2011;39:111-3.
- [Google Scholar]
- Nicolau syndrome in an athlete following intra-muscular diclofenac injection. Acta Orthop Belg. 2008;74:860-4.
- [Google Scholar]
- Nicolau syndrome due to diclofenac sodium (Voltaren®) injection: a case report. J Med Case Rep. 2014;8:404.
- [Google Scholar]
- Nicolau syndrome after lidocaine injection and cold application: a rare complication of breast core needle biopsy. Int J Dermatol. 2011;50:78-80.
- [Google Scholar]
- Livedoid dermatitis (Nicolau syndrome) following intra-articular glucocorticoid injection. J Clin Rheumatol. 2014;20:339-40.
- [Google Scholar]
- Nicolau’s syndrome after local glucocorticoid injection. Joint Bone Spine. 2003;70:390-2.
- [Google Scholar]
- Nicolau syndrome due to penicillin injection: a report of 3 cases without long-term complication. Case Rep Infect Dis. 2016;2016:9082158.
- [Google Scholar]
- Nicolau syndrome involving whole ipsilateral limb induced by intramuscular administration of gentamycin. Indian J Dermatol Venereol Leprol. 2014;80:96.
- [Google Scholar]
- Nicolau syndrome induced by intramuscular vitamin K in a premature newborn. Eur J Pediatr. 2009;168:1541-2.
- [Google Scholar]
- Delayed anaphylaxis with methimazole: Nicolau syndrome after oxytocin intramuscular administration anastrazole-induced autoimmune hepatitis amoxicillin- and cephalexin-induced eosinophilic colitis docetaxel-induced supravenous erythematous eruption. Hosp Pharm. 2016;51:520-3.
- [Google Scholar]
- [Nicolau syndrome after glatiramer acetate injection] Med Clin (Barc). 2015;145:e41.
- [Google Scholar]
- Nicolau syndrome secondary to subcutaneous bortezomib injection. J Eur Acad Dermatol Venereol. 2016;30:348-50.
- [Google Scholar]
- Nicolau syndrome following etanercept administration. Am J Clin Dermatol. 2010;11:51-2.
- [Google Scholar]
- Livedoid skin necrosis (Nicolau syndrome) due to triple vaccine (DTP) injection. Br J Dermatol. 1997;137:1030-1.
- [Google Scholar]
- Nicolau syndrome in patient following diclofenac administration: a case report. Ann Dermatol. 2011;23:501-3.
- [Google Scholar]
- Nicolau syndrome after lumbar puncture: a case report in a 22-month-old girl. JAAD Case Rep. 2017;3:33-5.
- [Google Scholar]
- Nicolau syndrome following intramuscular diclofenac administration: a case report. J Orthop Surg (Hong Kong). 2006;14:104-7.
- [Google Scholar]
- Nicolau syndrome after intramuscular benzathine penicillin injection. Iran J Med Sci. 2014;39:577-9.
- [Google Scholar]
- Nicolau syndrome: an iatrogenic cutaneous necrosis. J Cutan Aesthet Surg. 2009;2:92-5.
- [Google Scholar]
- Nicolau syndrome after intramuscular injection of non-steroidal anti-inflammatory drugs (NSAID) Bosn J Basic Med Sci. 2015;15:57-60.
- [Google Scholar]
- Nicolau Syndrome after Intramuscular Injection: 3 Cases. Arch Plast Surg. 2012;39:249-52.
- [Google Scholar]
- Anticoagulant and vasodilator therapy for Nicolau syndrome following intramuscular benzathine penicillin injection in a 4 year old boy. Arch Argent Pediatr. 2016;114:e184-6.
- [Google Scholar]
- Freestyle perforator-based fasciocutaneous flap reconstruction in Nicolau syndrome-related tissue necrosis. Indian J Surg. 2015;77:1187-90.
- [Google Scholar]
- Intramuscular gluteal injections in the increasingly obese population: retrospective study. BMJ. 2006;332:637-8.
- [Google Scholar]






