Translate this page into:
Epidermal Growth Factor in Aesthetics and Regenerative Medicine: Systematic Review
Address for correspondence: Dr. Dubraska V Suárez-Vega, 23rd Street between avenues 2 and 3, Research Department, University of Los Andes (ULA), Mérida, Venezuela. E-mail: dubraskasuarez.ula@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction:
Epidermal Growth Factor (rhEGF) is a promising skin antiaging agent that successfully promotes skin wound repair, and it has been investigated in the past decade for these purposes. However, there are no updated systematic reviews, in English or English, that support the efficacy of rhEGF as a regenerative skin treatment or systematic reviews that compile the uses of rhEGF as facial aesthetic therapy and regenerative medicine.
Aim:
To describe the current state of facial aesthetic and regenerative medicine treatments in which rhEGF has been effectively used.
Materials and Methods:
An exhaustive search was carried out in “Medline” (via “PubMed”), “Cochrane,” “Bireme” through the Virtual Health Library (VHL), “Elsevier” via “Science Direct,” “Springer,” “SciELo,” “ResearchGate,” and Google Scholar. Studies related to the use of rhEGF in addressing skin disorders or skin aging are included.
Results:
Overall, 49 articles were found, which described the use of rhEGF for skin regeneration and restructuring. Efficacy in the regeneration of skin wounds was verified through the intradermal and topical application of formulations with rhEGF. Most clinical trials in aesthetics point to an effective inversion of skin aging. However, uncontrolled or randomized trials abound, so that does not represent enough evidence to establish its efficiency. There are transient adverse effects for both cases.
Conclusion:
The rhEGF considers an effective therapeutic alternative for patients with recalcitrant skin wounds and skin aging, as it is a potent and specific mitogenic factor for the skin.
Keywords
Epidermal growth factor
facial aesthetics
regenerative medicine
skin aging
skin ulcers
✓ Similarities exist between the aging skin and wound healing, and mostly they involve the same oxidation and repair mechanisms
✓ In both cases, it is necessary to biostimulate fibroblasts and keratinocytes, inducing dermal restructuring.
✓ The EFG is more effectively applied by intradermal injections and using transdermal patches, thereby reducing the rhytids, folds, and hyperpigmentation and accelerating wound healing.

INTRODUCTION
In recent years, Epidermal Growth Factor (EGF) has been an important innovation.[1] It is a mitogenic polypeptide that is responsible for the maintenance and protection of the epithelia.[2] Thanks to genetic recombination, effective and clinically safe recombinant EGF (rhEGF) is available.
On the other hand, there are striking similarities between oxidative events that promote skin aging and the oxidation seen in delayed wound healing. In both cases, the skin’s repair mechanisms are overwhelmed, as the production of growth factors slows down, including the production of EGF.[3]
In both cases, it is necessary to biostimulate fibroblasts and keratinocytes, inducing the replacement of collagen and elastin and the extracellular matrix.[3] The success of the use of growth factors as a strategy to reverse photoaging lies in understanding its role in wound healing.[4] Therefore, the new cosmetic assets with recombinant growth factors as the rhEGF pursue this restructuring.[567]
The rhEGF has been investigated in the past decade as a treatment of facial photoaging.[8] However, the literature review revealed that there are no systematic reviews, in Spanish or English, about the efficacy of rhEFG as facial and regenerative therapy.
Given the lack of this information and the clinical potential of this factor, this systematic review aims at describing the current state of facial aesthetic and regenerative medicine treatments in which rhEGF has been effectively used with an emphasis on cutaneous restructuring.
MATERIALS AND METHODS
Search strategy
The search was started in health databases: Medline (via PubMed), Lilacs (via Bireme), Science Direct, Cochrane Library Virtual Health Library (VHL), SciELO, Medigraphic, and Google Scholar. To delimit the number of papers to select, the search was filtered by preferred languages (English and Portuguese) and the period 2001–2019 (since the information published in the past five years was not enough).
The logical AND operator was used to combine the following descriptors for the search:
— English (MeSH): facial antiaging therapeutics; skin rejuvenation; EGF recombinant; epidermal growth factor; tissue regeneration; EGF, rhEGF.
— Portuguese: Fator crescimento ou recombinant epidermal carcinoma; rejuvenescimento gives peel; redensificação dermal; com faciais therapies fatores of crescimento; EGF, rhEGF.
Selection of clinical studies
The following inclusion criteria were considered:
— Preclinical and clinical trials related to the use of the rhEGF in skin disorders or skin aging
— Texts that undergo a rigorous evaluation process (peer-reviewed publications and texts from renowned publishers)
Duplicated publications were removed. Literature reviews, case reports, incomplete publications or those did not comply with methodological rigor criteria (e.g. biases selection of participants or biases analysis of information), clinical treatment guidelines, and other irrelevant studies were excluded.
By utilizing skimming and scanning as analysis strategies for information, irrelevant items were discarded by title and abstract. Once the exhaustivity of the search had been confirmed (by depletion of publications of each source consulted), the veracity of the information was confirmed by each author by methodological analysis and the content analysis of each publication.
RESULTS
Seven sources of information were consulted, resulting in 75,986 publications that included the descriptors; of these, only 133 articles were directly related to the subject and they were examined by their title and abstract, and 73 articles were excluded because they did not meet the selection criteria. After reading the full text of the remaining 60 articles, taking into consideration the analysis criteria, 11 articles were removed, leaving a total of 49 articles [Figure 1] with a total sample of 821 patients.
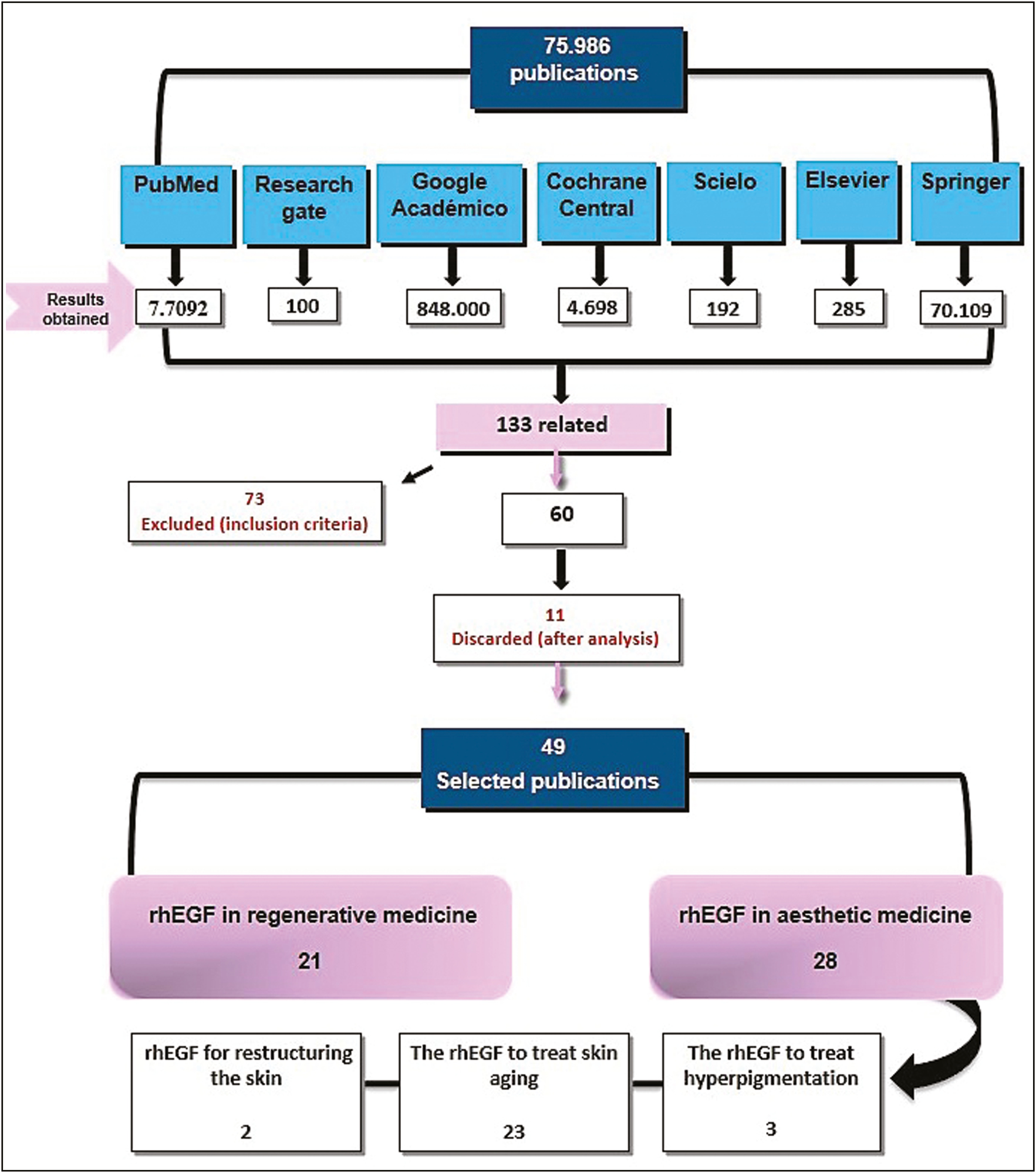
- Flowchart of literature search. Source: Prepared by the authors from the study results
The results indicate that most of the publications on rhEGF applications were led by Korea, followed by the United States and Japan. The years in which it was most published in this regard were 2015 and the period between 2017 and 2019.
Publications were classified according to the information source where they were located [Table 1]. Medline via Pubmed was the source that hosted major related scientific evidence.
| Information source | Number of articles | Reference |
|---|---|---|
| PubMed | 28 | [1112,1415,1819202129,3335,373839], [1027,30313240414243444546,4859] |
| Research Gate | 3 | [1726,36] |
| Google Scholar | 7 | [416,3447,5052,53] |
| Cochrane Central | 1 | [9] |
| Springer | 2 | [2251] |
| SciELO | 4 | [28,2349] |
| Elsevier | 4 | [124,2561] |
| Total | 49 |
Source: Prepared by the authors from the study results.
Likewise, 46 studies corresponded to original research articles, and they are predominantly clinical trials (24 articles) over preclinical trials and case series; also, 13 articles are review articles, 9 are traditional reviews, 2 are systematic reviews, and 2 are meta-analyses [Table 2].
| Type of study | Number of articles | Reference |
|---|---|---|
| Randomized and controlled clinical trials | 15 | [1821,2226,2729303132,3536373840,41] |
| Quasi-experimental or not controlled clinical trials | 9 | [1617,23242533,3439,42] |
| Preclinical trials | 11 | [10111214,15,19,20,44454647] |
| Meta-analysis | 2 | [4961] |
| Systematic review | 2 | [19] |
| Traditional review | 9 | [24,848,5051525359] |
| Case series | 1 | [43] |
| Total | 49 |
Source: Prepared by the authors from the study results.
Epidermal growth factor has been used basically in two major areas: regenerative medicine and aesthetic medicine. In aesthetics, there are three emerging categories: its application in hyperpigmentation, skin restructuring, and facial rejuvenation.
The rhEGF in regenerative medicine
Growth factor promotes wound healing,[910] employing the intralesional administration of 75mg lyophilized powder thrice per week; gel 150g / g or spray rhEGF topically twice a day until complete healing.[11]
Of the 21 publications in regenerative medicine, only 14 are clinical or preclinical trials [Table 3]. Korea is the country that has most investigated rhEGF in regeneration and the year in which it was most published was 2013.
| Type of study | Number of articles | Reference |
|---|---|---|
| Clinical trials | 8 | [1617182122232425] |
| Preclinical trials | 6 | [1112,1415,1920] |
| Total | 14 |
Source: Prepared by the authors from the study results.
The main clinical uses of rhEGF in regenerative medicine include the treatment of alopecia and dermatitis after chemotherapy, burns, diabetic foot ulcers, postsurgical ulcers, oral mucositis, pharyngeal ulcers, and tympanic membrane perforation. This evidence is summarized in [Table 4] next.
| Authors and year of publication | Type of study | Country of origin | Number of participants | Intervention protocol | Follow-up period | Results | Adverse effects |
|---|---|---|---|---|---|---|---|
| Jeon YJ et al 2019[11] | In vitro preclinical trial | Korea | Dermal cultures | Skin penetration study of CTP-EGF recombinant protein using fluorescent imaging techniques and synthesis of hyaluronic acid study by immunoblotting and ELISA | Between 6 and 48 hours according to the corresponding laboratory test | CTP-EGF has superior ability, compared with EGF alone, to penetrate the skin and induce hyaluronic acid synthesis and collagen formation. | - |
| Paik SH et al.2013[12] | In vivo preclinical trial | Korea | 24 Mice | Four days before chemotherapy, topical pretreatment with EFG was performed on hair follicles. | Days 2 and 4 after chemotherapy | EGF is effective as an anagen inducer protecting against chemotherapy-induced alopecia. | - |
| Niiyama H And Kuroyanagi Y, 2014[14] | Invitro preclinical trial | Japan | Cell cultures | EGF wound dressing, wound dressing with Vit. C, and dressing with EGF + Vit. C have probed into cytokine production. Fibroblasts were assessed in vitro and cultured. | 7 Days for cell culture | The dressing with EGF and Vit. C improved the production of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) compared with the dressing without EGF. | - |
| Yamamoto A et al 2013[15] | In vitro preclinical trial | Japan | 40 Mice | The effectiveness of wound dressings with EGF, Vit. C, and EGF + Vit. C was assessed in diabetic mice. | 1 Week (clinical and histologic assessment) | The dressing with EGF promoted best granulation tissue and major angiogenesis being more effectively than the other wound dressings.. | No report |
| Esquirol-Caussa J and Herrero-Vila E. 2019[16] | Quasi-experimental clinical trial | Spain | 77 Patients | Ulcers are cured by gel using rhEGF gel once per day. | 7 Weeks | The surrounding skin improved in 93.5% of cases. The appearance of the wound improved by 92.2%. The size of the wound was reduced from 66.7% in 43.3% in 4 weeks. | They were not found. |
| Martínez -Peñalver I and Cuevas -Pérez I. 1998[17] | Quasi-experimental clinical trial | Cuba | 12 Patients | Topical application twice a day until healing or improvement that would allow successful reoperation | 7 Weeks | 8 cases of complete cicatrization and 4 partial closures. Closures are completed in 34 days. The EGF is an option for patients who cannot undergo pharyngoplasty or where it has not been effective. | They were not found. |
| Kim JW et al. 2017[18] | Clinical trial | Korea | 138 Patients | Spray rhEGF (experimental) or placebo was applied in oral mucosa twice a day from the day of chemotherapy until oral mucositis resolved. | 28 Days | The spray does not reduce grade 2 of oral mucositis in patients receiving chemotherapy. However, the oral spray reduces pain induced by mucositis. | Transient nausea |
| Shi HX, et al. 2013[19] | In vitro and in vivo preclinical trial | China | 30 Rats | 1ml of EFG every 2 days in skin wounds; the control group received saline at 0.9% | 14 Days | EGF regulated the synthesis and degradation of the extracellular matrix. EGF regeneration was promoted in animals and in human cells in vitro. | - |
| Lee JH et al.2013[20] | In vivo preclinical trial | Korea | 40 Animals | Once the burn was induced 14 days after laser irradiation, the gel was started with EFG once a day. | 22 Days | EGF stimulated granulation tissue with accelerated wound closure and minimization of scars, compared with the group without treatment. | It was not observed |
| Kong Mand Hong SE.2013[21] | Clinical trial | Korea | 40 Patients | EFG cream in the irradiated area thrice a day from the start of radiotherapy until 2 weeks after completion of radiotherapy | Weekly during radiotherapy and 6 weeks after completion | Cream with EGF has a beneficial role in preventing or minimizing radiation dermatitis in patients with breast cancer. | They were not recorded |
| Hwang IG et al 2016[22] | Clinical trial | Korea | 52 Patients | Cream twice a day in skin lesions (ERSEs or skin-related effects of erlotinib) | 8 Weeks | It is effective in improving all types of ERSEs, regardless of the dose of erlotinib | They were not reported |
| Hernández-Cañete CM et al 2017[23] | Quasi-experimental clinical trial | Cuba | 17 Patients | Intralesional application thrice a week until complete granulation or healing | - | EGF avoided amputation in Wagner grade 4 diabetic foot ulcers, and 100% evolved to healing. | Muscle spasms |
| Kahraman M et al 2019[24] | Quasi-experimental clinical trial | Turkey | 34 Patients | Intralesional injections thrice per week for 8 weeks or until healing | 8 Weeks or until healing follow-up to 5 years | Wound closure in 87.9% of injuries. It is effective in reducing the recurrence of long-term ulcers. | Burning pain |
| Lou Z.2019[25] | Quasi-experimental clinical trial | China | 24 Patients | Tympanic membrane application once a day | Twice per week for 6 months or until the closure of the tympanic perforation | The closing rate was 100% in 6 days | They were not recorded. |
Source: Prepared by the authors from the study results.
In alopecia treatment, the topical EGF-liposomal solution by transfollicular route favored primary hair recovery via the dystrophic anagen pathway.[12] In this regard, the mechanism of action consists of EGF, promotes the proliferation and migration of hair follicle outer root sheath cells, and modulates the expression of several follicle-regulatory genes via Wnt/β-catenin signaling.[13]
The rhEGF in aesthetic medicine
From fibroblast cell cultures with rhEGF, it was determined that rhEGF promotes the migration and contractility of aged fibroblasts[10] and increases the production of hyaluronic acid and the synthesis of collagen.[11] Hence, it possesses potential as a regenerator of skin aging.
Peptides with rhEGF that penetrate the skin when applied topically have been developed[11]; cosmeceuticals with rhEGF that prevent or improve rhytids and hydrate the skin without significant side effects are being formulated.[26]
Of the 28 publications in Aesthetic Medicine, 23 are clinical or preclinical trials [Table 5] with a higher level of evidence in aesthetics than in regenerative medicine. The country that has researched this matter the most is Korea, followed by the United States, and the periods of greatest publication were 2015 and 2017.
The rhEGF to treat hyperpigmentation
The EGF is a noninvasive and effective treatment for melasma. Topical serum twice a day for 8 weeks decreased melasma in 73.4% cases of the experimental group without any side effects.[27]
The EGF acts on the melanocytes by reducing the expression of melanogenesis‐associated proteins (e.g. tyrosinase/MITF microphthalmia-associated transcription factor), in consequence inhibiting or regulating melanin synthesis.[28] It is also effective in preventing postinflammatory hyperpigmentation after fractional carbon dioxide laser treatment; its daily application resulted in significant stimulation of healing with slight pruritus.[29] Other research confirmed that rhEGF prevents inflammatory hyper pigmentation by laser treatment at 3, 7, and 35 days after its use.[30]
The rhEGF for restructuring the skin
The EGF contributes to restructuring the skin tissue; it improves facial acne, both inflammatory and noninflammatory, when it is applied as an rhEGF cream for 6 weeks. It also decreases sebum production and increases hydration, whereby the topical rhEGF may be an effective and safe adjuvant treatment option for mild to moderate vulgar acne.[31]
The EGF also restructures the skin with stretch marks; it is being used as a complementary treatment to the ablative fractional carbon dioxide laser, twice a day until one month after the last session, showing significant improvements in stretch marks. Skin biopsy revealed an increased epidermal thickness and a decrease in elastic fragmentation.[32]
The rhEGF to treat skin aging
Most clinical trials reported the efficacy of rhEGF to reverse signs of skin aging,[3334] such as rhytids, grooves, hyper pigmentation and other senile pigmentations, hydration loss, and a decrease in epidermal and dermal thickness; test topical formulations such as cream, serum, and gel, either as a single therapy applied daily at home or as a complementary treatment after other treatments for facial rejuvenation such as the ablative laser.
Only a few recent publications have studied its incorporation with classic injections of mesotherapy or through the use of patches with microspicles for the transdermal release of the factor.[3536] Clinical applications of the EGF factor in facial aesthetics are shown in [Table 6].
| Authors and year of publication | Type of study | Country of origin | Number of participants | Intervention protocol | Follow-up period | Results | Adverse events |
|---|---|---|---|---|---|---|---|
| McKnight B et al 2015[37] | Clinical trial | USA | - | Topical daily application of the human epidermal growth factor in the senile purpura | 6 Weeks | Topical rhEGF decreases the appearance of senile purpura by thickening the skin, and it prevents late-stage dermatoporosis. | They did not report. |
| Kwon SB et al 2017[38] | Clinical trial | Korea | 40 Patients: 20 in the control group and 20 in the experimental group | Cream with phytosphingosine-1-phosphate and EGF twice a day for 4 weeks | 6 Weeks | Improved elasticity, density, and dermal hydration; reduction of periocular wrinkles; and phytosphingosine-1-phosphate showed synergistic effects with EGF. | They were not observed. |
| Vivó-Sesé I et al 2015[26] | Clinical trial | Spain | 18 Patients in A or experimental group and in B or control group | Before peeling, A group received EGF HA product by digital transdermal introduction, and B group received treatment by manual mesotherapy in 2 sessions (day 7 and day 21). | Days 0, 21, and 35 | The product effectively attenuates wrinkles. The digital transdermal application produced effects faster, and it was the only one that had effects on the depth of wrinkles. | They were not observed. |
| Ha JM et al 2017[35] | Clinical trial | Korea | 20 Patients on the left and right sides of the face | For 4 weeks, a patch of microspicles with EGF was applied on the experimental side and the control side. The EGF cream was applied on periocular wrinkles. | Days 0 and 1, 2, 4, and 8 weeks | The patch of EGF produced a statistical increase in dermal density compared with the control group at 4 and 8 weeks. | They were not observed. |
| Barone F et al 2019[33] | Quasi-experimental clinical trial | USA | 41 Patients | Serum with EGF twice per day for 12 weeks. Expert qualifications and evaluations with a corneometer and cutometer were made. | 12 Weeks | Improved the firmness and hydration of the skin; improved the appearance of periocular wrinkles. Ultrasound showed an increase in dermal restructuring. | They did not report. |
| TechapichethvanichT et al 2018[29] | Clinical trial | Thailand | 19 Patients on the right and left side of the face for control and experimental groups | Patients received laser carbon dioxide fractional treatment on both cheeks. They applied EGF ointment daily on one side of the face and Vaseline on the other side. | The hyper pigmentation was evaluated at 2, 3 weeks and at 1, 2 months. | There were no statistically significant differences. However, topical EGF provides significant repair stimulation. | Pruritus |
| Schouest JM et al 2012[39] | Quasi-experimental clinical trial | USA | 21 Patients | Topical application twice a day for 3 months | Every 30 days up to 3 months | Statistical improvement was found in the rhytids, in the reduction of pore size and pigmentary alterations by photodamage. | Mild inflammation controlled |
| Gawdat HI et al 2017[40] | Clinical trial | Egypt | 20 Patients on the experimental side (EGF) and the control side (PRP autologous) | Intradermotherapy every 2 weeks for 3 months, EGF side, and PRP side. The thickness of the epidermis and dermis was evaluated by tomography. | 1 Month to 6 months after the previous session. | Both procedures increased skin turgidity and vitality. The thickness of the epidermis and dermis increased in both groups without differences. | Transient burning sensation in both groups |
| Park GH et al 2015[30] | Clinical trial | Korea | 25 Patients | A 532 nm laser was applied. In addition, the experimental group applied the cream with growth factor and the control group applied the control cream. | Days 0, 3, 7, and 35 | The cream with EGF showed statistically significant differences at the end of the study, being more effective in preventing inflammatory hyper pigmentation after laser treatment. | They did not report. |
| Draelos ZD 2016[41] | Clinical trial | USA | 60 Patients | Daily application of serum hyaluronic acid with serum growth factor | Weeks 2, 4, 8, and 12 | Both products improved the skin, with statistically greater differences in serum AH + EGF | They did not report. |
| Lee DH et al 2014[42] | Quasi-experimental clinical trial | Korea | 23 Patients | EGF serum and hyaluronic acid were applied to the entire face. Photo damage was assessed with photographs and skin and rhytids with a visiometer. | Days 0, 4, and 8 weeks | Periorbital wrinkles improved with statistically significant differences, captured by both the physician and the visiometer. | They were not observed. |
| An JH et al 2019[36] | Clinical trial | Korea | 50 Patients divided into experimental and control groups | Patches with acetyl hexapeptide, control patch, and patch with EGF were applied in the nasolabial fold and periorbital area, once a week. | Days 0, 1, 3, 5, 8, and 29 | On day 29, there were statistically significant improvements in wrinkles and skin hydration with the microneedle patch / AHP-8. | They were not observed. |
| Ruri P.2018[43] | Number of cases | Indonesia | 8 Patients | rhEGF gel using microneedling, 3 sessions every 10 days | Before and after 4 weeks of treatment | Seven patients showed improvement in texture, fine lines, and wrinkles, especially in the periorbital region, reducing the signs of photoaging | Erythema associated with the microneedling |
| Seidel R and Moy RL 2015[34] | Quasi-experimental clinical trial | USA | 8 Patients | Application twice a day of serum with EGF in the areas of atrophic acne scars for 12 weeks | Before and after, 3 times at 4-week intervals | Overall, 25% of patients improved their hypertrophic scars mid-level Goodman scale. Researchers surveyed a perceived improvement of 49% in 6 patients. | They were not recorded. |
| Kim J et al 2012[44] | Preclinical trial in vitro and in vivo | Korea | 32 Mice | Application of the EGF once a day in the photodamaged and hyperpigmented area for 4 weeks. Previously applied microporation twice a week | 28 Days | Hyperpigmentation improved with EGF. EGF decreased wrinkles, and in-depth and histological analysis indicated re-densification. | - |
| Kim D et al 2015[10] | Preclinical trial in vitro | Korea | Cell cultures of young fibroblasts and aged fibroblasts in collagen 3d matrices | Later cultivation was made: Proliferation assay (72h); migration assay (4h); contractility assay (2h); immunohistochemicals (3h) | EGF increased migration and contraction of aged fibroblasts. It improved the collagen matrix more efficiently than young fibroblasts. | - | |
| Park B et al 2011[45] | Preclinical trial in vivo | Korea | 12 Rabbits | The inguinal fat was grafted with EGF or with saline (control) solution in the auricular pavilion to observe the volume and changes of adipose tissue. | The grafted fat was collected 3 months later. | In EGF, the survival rate was higher than in the control, increased neovascularization, and maintained fat cell morphology. | - |
| Draelos ZD et al 2017[46] | Quasi-experimental clinical trial | USA | 40 Patients | Serum EGF obtained from a culture in dextran to low-tension oxygen tension for 8 weeks was applied daily for 90 days. | At 90 days | Statistically significant improvements were found in skin hydration through corneometry, as well as in the global evaluations of researchers and patients. | Not reported |
| Yamamoto A et al 2016[47] | Preclinical trial in vitro in hyaluronic acid (HA), collagen (Col), EGF, vitamin C (VC), glucosylceramide (GC), poly (γ-glutamic acid) (PGA), and Argentina (Arg) | Japan | The proliferation of fibroblasts in culture cells with the product HA, Col, EGF, Vit. C, PGA, and Arg | The amount of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) produced by fibroblasts was evaluated in an in vitro wound model (cultured dermal substitute: CDS) with cultured EGF for 7 days | The media were prepared and stored at 4 ° C or 37 ° C for a different period of 1 day, 2 weeks, and 4 weeks, respectively. | The effectiveness of EGF remained even after 4 weeks. CDS fibroblasts released more VEGF and HGF in culture with EGF. Fibroblast density increased more in the medium with EGF than in the control medium without EGF. | - |
Source: Prepared by the authors from the study results.
DISCUSSION
A skin wound is similar to skin aged by inflammation mediated by reactive oxygen species.[48] Besides, in wound healing there is angiogenesis and replacement of extracellular matrix, leading to re-epithelialization, but it is in the final phase that the collagen fibers are remodeled and elastin is restructured.[49] This last phase is characterized by antiaging treatments, and the use of growth factors evokes such dermal restructuring.[50]
When topical or injectable growth factors are administered, the depleted levels are replenished and the activity of the cells responsible for dermal remodeling is regulated, reversing skin aging.[4851,52] The rhEGF replenishes this balance and facilitates wound healing and dermatological conditions, as evidenced in some studies.[53]
In 1972 Savage et al.[54] completed the sequentiation of EGF for the first time. In 1995 Parries et al.[55] they worked into their isolation and purification. Since this, the mass production of rhEGF has become a formidable task.
From 2002, a heterologous protein such as EGF with an adequate molecular size (6kDa) started being produced in large quantities based on recombinant DNA technology. This placed an increased impetus on the development of more effective and economical methods for industrial purposes.[5657] Taking advantage of this technology, in the past two decades, the EGF has been produced and exploited in the cosmetic industry for the purpose of skin treatment.[58]
For some authors, it is controversial that a growth factor that is applied topically can rejuvenate the skin; however, due to its large molecular size (> 15,000Da), its ability to penetrate the stratum corneum and to reach viable keratinocytes in the basal stratum is limited.[5960] This limitation was overcome from 2002 onward, because since then the majority of dermatologically and cosmetically commercialized formulations contain an rhEGF of 6kDa (or 6,000Da) with excellent potential for topical penetration into the stratum corneum.[6162]
The rhEGF entry into the skin is through hair follicles and sweat glands. Also, it may be possible to improve their penetration by chemical modification with lipophilic molecules or by facilitating their diffusion by compromised skin, for example, after using microneedling or laser resurfacing,[63] as observed in all studies cited earlier, in which the EGF exhibits a low molecular size.
The evidence is clear as to the success or effectiveness of rhEGF for facial rejuvenation; however, quasi-experimental, uncontrolled, or randomized clinical trials still abound. The studies assessed its effects while it was applied topically, but the greatest efficiency of the EFG was obtained intradermally, with a greater reduction of rhytids, folds, and a longer response over time. Besides, to guarantee its effectiveness, a possible route of entry for this molecular size is through intradermotherapy.
In regenerative medicine, the EGF has been studied under preclinical trials and clinical trials, mostly quasi-experimental ones that are not uniform in terms of the route of administration, dose, and therapeutic regimen, which indicates the need to shield studies in this area by promoting the control and randomization to give more precise results on the resounding efficacy of the factor in wound regeneration.
CONCLUSIONS
Products with EGF are an important topical therapeutic modality to treat aging skin efficiently and to treat hyper-pigmentation, rhytids, dryness, and laxity.
The EGF is effective in the advanced healing of skin wounds, according to the results of multiple investigations in severe cases, although studies are required to establish concentrations and indications of use for each case.
Injected rhEGF exerts a higher antiaging effect, inducing collagen, elastin, and hyaluronic acid, which are responsible for skin elasticity and turgor. However, more controlled, randomized, and long-term follow-up clinical trials are needed to specify the dose and therapeutic protocol to ensure its efficacy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Factor de Crecimiento Epidérmico (EGF) y geles de silicona en el abordaje de heridas, quemaduras y cicatrices: Revisión de la literatura. 2017. Cir Plást Iberolatinoam [Internet]. 43:387-94. doi: http://dx.doi.org/10.4321/s0376-78922017000500009
- [Google Scholar]
- Topically applied physiologically balanced growth factors: A new paradigm of skin rejuvenation. J Drugs Dermatol. 2009;8:4-13.
- [Google Scholar]
- Fibroblast growth factors: A controlling mechanism of skin aging. Skin Pharmacol Physiol. 2019;32:275-82. 10.1159/000501145
- [Google Scholar]
- Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Pannonica Adriat. 2007;16:156-65.
- [Google Scholar]
- Reversal of photodamage with topical growth factors: A pilot study. J Cosmet Laser Ther. 2003;5:25-34.
- [Google Scholar]
- New hydrogels enriched with antioxidants from saffron crocus can find applications in wound treatment and/or beautification. Skin Pharmacol Physiol. 2018;31:95-8.
- [Google Scholar]
- Advanced growth factor delivery systems in wound management and skin regeneration. Molecules. 2017;22:E1259. 10.3390/molecules22081259
- [Google Scholar]
- Recombinant human growth hormone for treating burns and donor sites. Cochrane Database Syst Rev. 2014;9:CD008990. 10.1002/14651858.CD008990.pub3
- [Google Scholar]
- Epidermal growth factor improves the migration and contractility of aged fibroblasts cultured on 3D collagen matrices. Int J Mol Med. 2015;35:1017-25.
- [Google Scholar]
- Increased synthesis of hyaluronic acid by enhanced penetration of CTP‐EGF recombinant in human keratinocytes. J Cosmet Dermatol. 2019;18:1539-45.
- [Google Scholar]
- Pretreatment of epidermal growth factor promotes primary hair recovery via the dystrophic anagen pathway after chemotherapy-induced alopecia. Exp Dermatol. 2013;22:496-9.
- [Google Scholar]
- Epidermal growth factor promotes proliferation and migration of follicular outer root sheath cells via wnt/β-catenin signaling. Cell Physiol Biochem. 2016;39:360-70.
- [Google Scholar]
- Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J Artif Organs. 2014;17:81-7.
- [Google Scholar]
- Potential of wound dressing composed of hyaluronic acid containing epidermal growth factor to enhance cytokine production by fibroblasts. J Artif Organs. 2013;16:489-94.
- [Google Scholar]
- Human recombinant epidermal growth factor in skin lesions: 77 cases in epitelizando project. J Dermatolog Treat. 2019;30:96-101.
- [Google Scholar]
- Efecto del factor de crecimiento epidérmico humano (EGF) recombinante sobre fístulas faríngeas y faringostomas. 1998. Rev Cubana Oncol. 14:77-8. Available from: http://imbiomed.com/1/1/articulos.php?method=showDetail&id_articulo=15298&id_seccion=473&id_ejemplar=1574&id_revista=74
- [Google Scholar]
- Topical recombinant human epidermal growth factor for oral mucositis induced by intensive chemotherapy with hematopoietic stem cell transplantation: Final analysis of a randomized, double-blind, placebo-controlled, phase 2 trial. PLoS One. 2017;12:e0168854. 10.1371/journal.pone.0168854
- [Google Scholar]
- The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. Plos One. 2013;8:e59966.
- [Google Scholar]
- Evaluation of a highly skin permeable low-molecular-weight protamine conjugated epidermal growth factor for novel burn wound healing therapy. J Pharm Sci. 2013;102:4109-20.
- [Google Scholar]
- Topical use of recombinant human epidermal growth factor (EGF)-based cream to prevent radiation dermatitis in breast cancer patients: A single-blind randomized preliminary study. Asian Pac J Cancer Prev. 2013;14:4859-64.
- [Google Scholar]
- Phase II trial of epidermal growth factor ointment for patients with erlotinib-related skin effects. Support Care Cancer. 2016;24:301-9.
- [Google Scholar]
- Resultados y reacciones adversas en pacientes tratados con Heberprot-P® en la comunidad. 2017. Revista Cubana de Angiología. 18:35-42. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1682-00372017000100004
- [Google Scholar]
- The long-term outcomes following the application of intralesional epidermal growth factor in patients with diabetic foot ulcers. J Foot Ankle Surg. 2019;58:282-7.
- [Google Scholar]
- The effect of epidermal growth factor on the pseudo-healing of traumatic tympanic membrane perforations. Braz J Otorhinolaryngol. 2021;87:53-8.
- [Google Scholar]
- Estudio de eficacia del producto factor de crecimiento epidérmico + ácido hialurónico fórmula. Actual Med. 2015;100:76-80. 10.15568/am.2015.795.or03
- [Google Scholar]
- A randomized, double-blind, placebo-controlled, split-face study of the efficacy of topical epidermal growth factor for the treatment of melasma. J Drugs Dermatol. 2018;17:970-3.
- [Google Scholar]
- Topical treatments for melasma: A systematic review of randomized controlled trials. J Drugs Dermatol. 2019;18:S1545961619P1156X. PMID: 31741361
- [Google Scholar]
- The effects of recombinant human epidermal growth factor containing ointment on wound healing and post inflammatory hyperpigmentation prevention after fractional ablative skin resurfacing: A split-face randomized controlled study. J Cosmet Dermatol. 2018;17:756-61.
- [Google Scholar]
- Effect of an epidermal growth factor-containing cream on postinflammatory hyperpigmentation after Q-switched 532-nm neodymium-doped yttrium aluminum garnet laser treatment. Dermatol Surg. 2015;41:131-5.
- [Google Scholar]
- Topical epidermal growth factor for the improvement of acne lesions: A randomized, double-blinded, placebo-controlled, split-face trial. Int J Dermatol. 2014;53:1031-6.
- [Google Scholar]
- Comparison between topical recombinant human epidermal growth factor and aloe vera gel in combination with ablative fractional carbon dioxide laser as treatment for striae alba: A randomized double-blind trial. Lasers Surg Med. 2020;52:166-75.
- [Google Scholar]
- Clinical evidence of dermal and epidermal restructuring from a biologically active growth factor serum for skin rejuvenation. J Drugs Dermatol. 2019;18:290-5.
- [Google Scholar]
- Improvement in atrophic acne scars using topical synthetic epidermal growth factor (EGF) serum: A pilot study. J Drugs Dermatol. 2015;14:1005-10.
- [Google Scholar]
- The effect of micro spicule containing epidermal growth factor on periocular wrinkles. 2017. Ann Dermatol. 29:187-93. doi: https://doi.org/10.5021/ad.2017.29.2.187
- [Google Scholar]
- Anti-wrinkle efficacy of cross-linked hyaluronic acid-based microneedle patch with acetyl hexapeptide-8 and epidermal growth factor on Korean skin. 2019. Ann Dermatol. 31:263-71. Available from: https://synapse.koreamed.org/search.php?where=aview&id=10.5021/ad.2019.31.3.263&code=0140AD&vmode= PUBREADER#!po=73.5294
- [Google Scholar]
- Topical human epidermal growth factor in the treatment of senile purpura and the prevention of dermatoporosis. J Drugs Dermatol. 2015;14:1147-50. PMID: 26461827
- [Google Scholar]
- Phytosphingosine-1-phosphate and epidermal growth factor synergistically restore extracellular matrix in human dermal fibroblasts in vitro and in vivo. Int J Mol Med. 2017;39:741-8.
- [Google Scholar]
- Improved texture and appearance of human facial skin after daily topical application of barley produced, synthetic, human-like epidermal growth factor (EGF) serum. J Drugs Dermatol. 2012;11:613-20.
- [Google Scholar]
- Autologous platelet-rich plasma versus readymade growth factors in skin rejuvenation: A split face study. J Cosmet Dermatol. 2017;16:258-64.
- [Google Scholar]
- The effect of a combination of recombinant egf cosmetic serum and a crosslinked hyaluronic acid serum as compared to a fibroblast-conditioned media serum on the appearance of aging skin. J Drugs Dermatol. 2016;15:738-41. PMID: 27272082
- [Google Scholar]
- Improvement in skin wrinkles using a preparation containing human growth factors and hyaluronic acid serum. J Cosmet Laser Ther. 2015;17:20-3.
- [Google Scholar]
- Topical growth factors for the treatment of facial photoaging: A clinical experience of eight cases. J Clin Aesthet Dermatol. 2018;11:28-9. PMCID: PMC6334836
- [Google Scholar]
- Enhanced topical delivery of small hydrophilic or lipophilic active agents and epidermal growth factor by fractional radiofrequency microporation. Pharm Res. 2012;29:2017-29.
- [Google Scholar]
- The effect of epidermal growth factor on autogenous fat graft. Aesthetic Plast Surg. 2011;35:738-44.
- [Google Scholar]
- The anti-aging effects of low oxygen tension generated multipotent growth factor containing serum. J Drugs Dermatol. 2017;16:30-4. PMID: 28095530
- [Google Scholar]
- Evaluation of epidermal growth factor-incorporating skin care product in culture experiment using human fibroblasts. Open J Regenerative Med. 2016;5:44-54. 10.4236/ojrm.2016.52004
- [Google Scholar]
- The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30:157-71.
- [Google Scholar]
- Treatment of venous ulcers with growth factors: Systematic review and meta-analysis. Rev Bras Enferm. 2019;72:200-10.
- [Google Scholar]
- Advances in surgical applications of growth factors for wound healing. Burns Trauma. 2019;7:10.
- [Google Scholar]
- Topical growth factors for skin rejuvenation. In: Farage M, Miller K, Maibach H, eds. Textbook of aging skin. Berlin, Heidelberg: Springer; 2016. 10.1007/978-3-642-27814-3_100-3
- [Google Scholar]
- Plasma rico en factores de crecimiento plaquetario. Una nueva puerta a la Medicina regenerativa. 2015. Rev Hematol Mex. 16:128-42. Available from: https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=59346
- [Google Scholar]
- Epidermal growth factor therapy impact on scar tissue resilience of diabetic lower limbs ulcers-an enlightening hypothesis. J Diabetes Metab. 2018;9:798. 10.4172/2155–6156.1000798
- [Google Scholar]
- The human urinary epidermal growth factor (EGF) precursor. Isolation of a biologically active 160-kilodalton heparin-binding pro-EGF with a truncated carboxyl terminus. J Biol Chem. 1995;270:27954-60.
- [Google Scholar]
- Purification of recombinant human epidermal growth factor secreted from the methylotrophic yeast hansenula polymorpha. Protein Expr Purif. 2002;24:117-22.
- [Google Scholar]
- Purification of soluble human epidermal growth factor (hegf) from recombinant escherichia coli culture broth by using expanded-bed adsorption chromatography. Biotechnol Appl Biochem. 2003;38:9-13.
- [Google Scholar]
- Review article: Reasons for underrating the potential of human epidermal growth factor in medical applications. J Anal Pharm Res. 2017;4:00101. 10.15406/japlr.2017.04.00101
- [Google Scholar]
- Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: A review of the literature. Clin Cosmet Investig Dermatol. 2016;9:411-9.
- [Google Scholar]
- Topical recombinant human epidermal growth factor for diabetic foot ulcers: A meta-analysis of randomized controlled clinical trials. Ann Vasc Surg. 2020;62:442-51.
- [Google Scholar]
- Kindlin-1 contributes to EGF-induced re-epithelialization in skin wound healing. Int J Mol Med. 2017;39:949-59.
- [Google Scholar]
- The role of follicular penetration. A differential view. Skin Pharmacol Appl Skin Physiol. 2001;14:23-7. 10.1159/000056386
- [Google Scholar]





