Translate this page into:
Role of Burn Blister Fluid in Wound Healing
Address for correspondence: Dr. Ravi K. Chittoria, Department of Plastic Surgery, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry 605006, India. E-mail: drchittoria@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Blisters are characteristic finding of second-degree superficial burns. Varied opinions for the management of burn blisters are available in literature. Most accepted one is to puncture it in a sterile way, keep the overlying skin as a biological cover, and over that put a moist sterile biological dressing. Fluid in the blister is ultrafiltrate of the plasma, which is rich in proteins such as immunoglobulins, various cytokines, prostaglandins, and interleukins. This fluid is pro-inflammatory, and the evidence regarding its effect on wound healing is varied. Instead of drainage, the burn blister fluid can be aspirated and immediately sprayed over the other areas of the same wound. We found this method feasible as an adjuvant therapy for second-degree superficial burn wounds. In this case report, we share our experience of the same.
Keywords
Burn blister fluid
second-degree superficial burns
wound healing
• There is no consensus for burn blister management.
• It is feasible to aspirate the burn blister fluid and to spray it over the same wound immediately.
• Role of burn blister fluid in wound healing needs further research.

INTRODUCTION
Blisters are characteristic finding of second-degree superficial burns. Burn blister is formed in stratum spinosum layer of epidermis. Blister separates epidermis from the dermis. In acute burn injury, vasodilation and increased capillary leakage in the zone of hyperemia leads to ultrafiltration of the plasma into the wound due to hydrostatic and plasma oncotic pressure changes.[1] This fluid gets collected beneath intact superficial layers of epidermis, forming a blister. Management of burn blisters is controversial. Recommendations for the management of blisters are varied and range from leaving blisters intact to immediate complete debridement of the blisters, followed by biological dressing.[2] Another more accepted approach is to puncture the blister to drain the blister fluid and keep the overlying skin intact as a biological cover to the wound.[3]
Blister fluid is rich in proteins and arachidonic acid metabolites (prostaglandins and leukotrienes).[4] In some studies, blister fluid is shown to cause vasodilation, increased inflammatory reaction, and increased keratinocyte and fibroblast activity. Moreover, burn blister fluid is similar to autologous plasma, thus rich in cytokines and growth factors. Numerous studies have found beneficial effect of local application of autologous plasma in wound healing.[5] Hence, the local application of burn blister fluid may improve wound healing.
We suggest that instead of discarding burn blister fluid, it may be collected in a sterile way and sprayed over the wound of the same patient. Here, we share a case report where we applied the same principle.
CASE REPORT
This is a single case report of a 3-year-old girl child with 20% total body surface area with scald burns over trunk and right thigh, managed at the tertiary burn care center of our institute. The depth of scald burn wound was mixed, having areas of second-degree superficial and second-degree deep burns. There were multiple blisters formed over second-degree superficial burn areas. Some of the second-degree superficial burn and all of the second-degree deep burn wounds were exposed and were not having intact skin or blisters over them. On arrival to the burn center, the child was managed according to the WHO (World Health Organization) burn management guidelines. After stabilization, she was shifted to burns procedure room for wound management. All the wounds were cleaned with warm saline carefully keeping the blisters intact. Intact burn blisters were aspirated in sterile way using 1-mL syringes, and the overlying skin was kept intact over the wound [Figure 1]. Quantity of burn blister fluid aspirated was 3 mL. The aspirated blister fluid was sprayed over the exposed parts of second-degree superficial burn areas immediately [Figure 2]. These areas were around 5% of the total body surface area. After that, all the wounds were covered with bovine collagen dry sheets, followed by a non-adherent layer, then a layer of absorbing dressing with connections for regulated oxygenation and negative pressure therapy, and a last layer of occlusive dressing. During postoperative period, fluid resuscitation and supportive treatment was provided to the child. After 5 days of burn injury, the child was taken to the operation theater, and her wounds were reassessed. Wounds were cleared of debris from dead skin and collagen sheets, using hydrojet debridement. It was found that the second-degree superficial wounds were healing well [Figure 3]. Only patchy areas were having deep burns, which were debrided, and pixel grafting was carried out to minimize donor-site problems. All wounds were provided adjuvant wound therapy using low-level laser, autologous platelet-rich plasma (APRP), and regulated oxygenation and negative pressure therapy. On 11th post-burn day, the wound was again opened, and it was found that all areas of second-degree superficial burns were completely epithelialized [Figure 4]. The pixel graft had started proliferating over the deep burn areas. On 18th post-burn day, complete wound was found to be healed [Figure 5]. Postoperative scar therapy was provided using silicone sheets and compression garments. The child was discharged with physiotherapy and nutrition counseling.
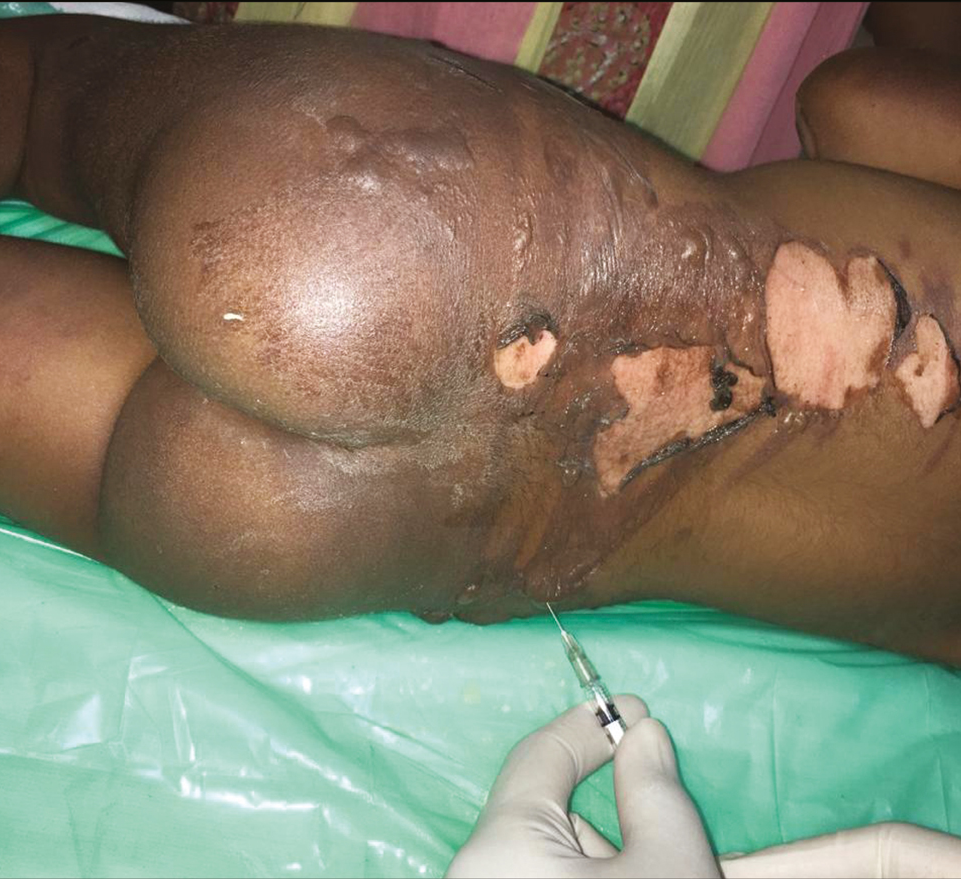
- Aspiration of burn blister fluid and keeping the overlying skin intact
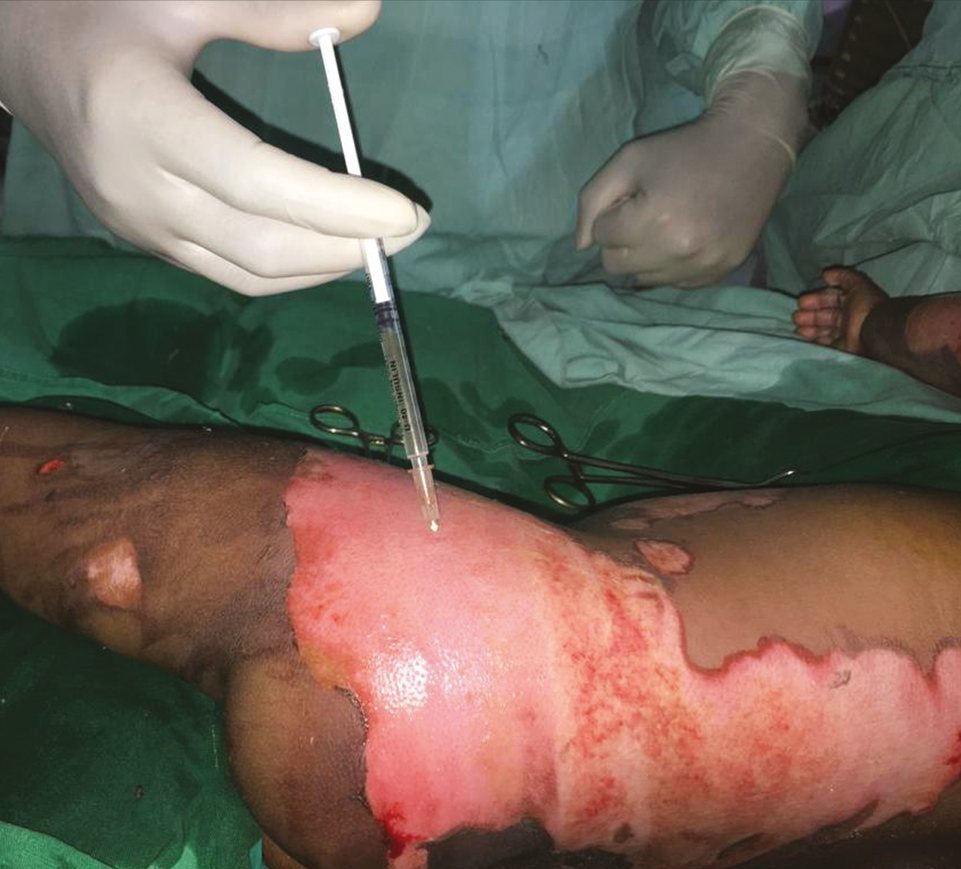
- Spraying of burn blister fluid over second-degree superficial burns without intact skin
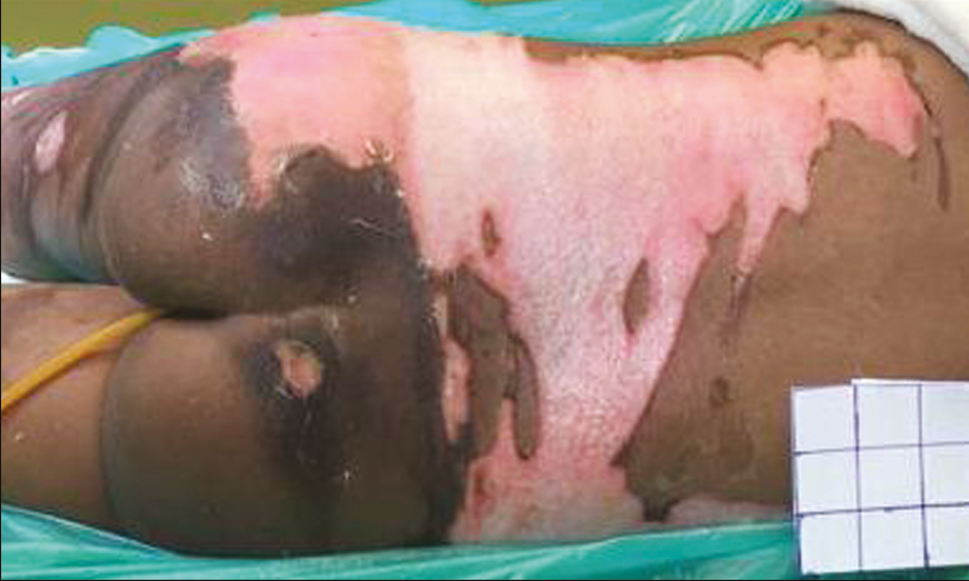
- Post-burn day 5: second-degree superficial wound healing well with patchy white areas of deep burns
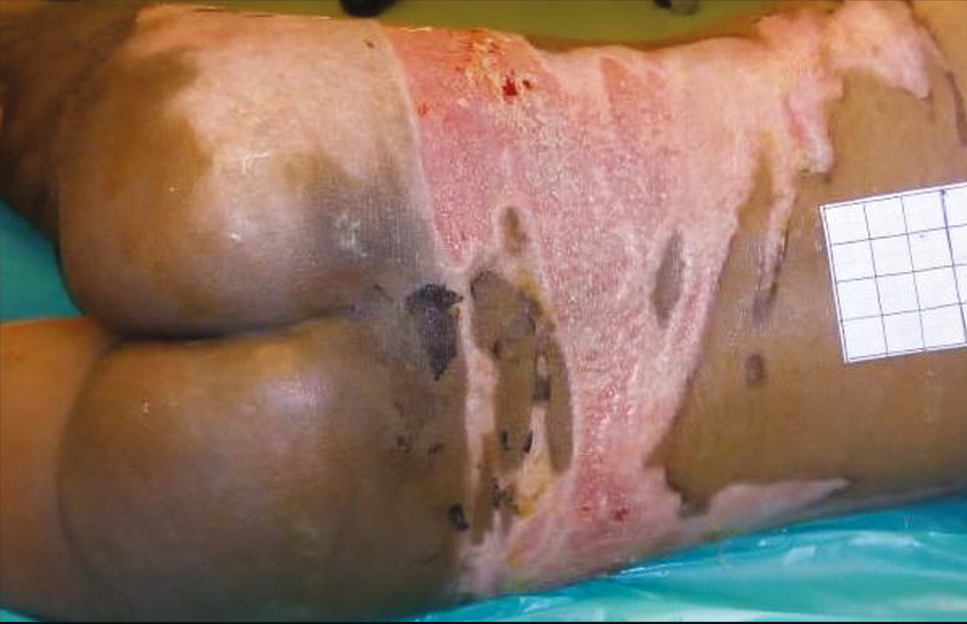
- Post-burn day 11: second-degree superficial burns are completely epithelialized and deep burns are healing with pixel graft in situ
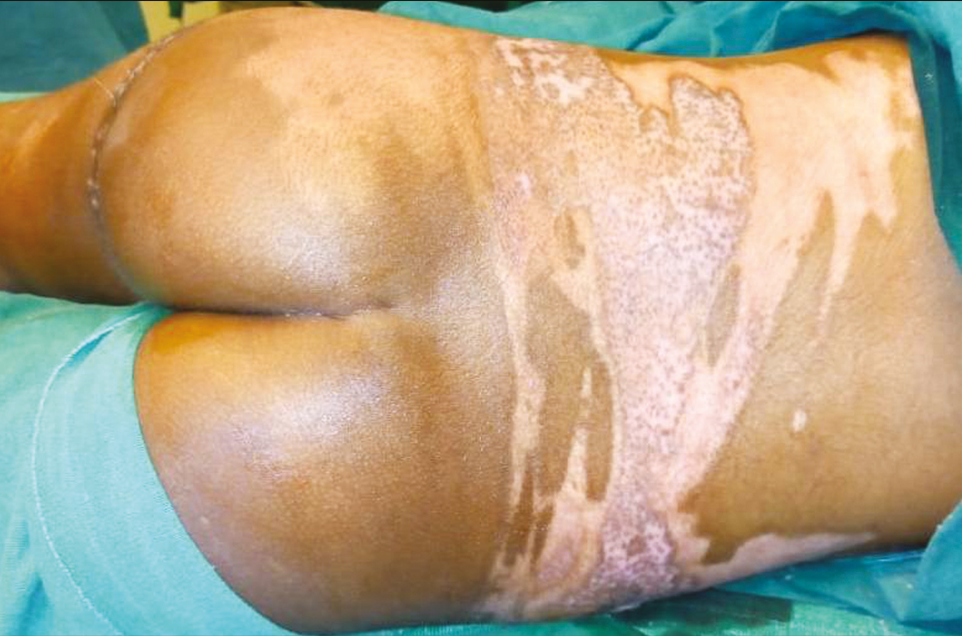
- Post-burn day 18: completely healed wound
DISCUSSION
Arturson et al.[1] attributed prostaglandins in the burn blister fluid for vasodilatation, increased microvascular permeability, and accumulation of polymorphonuclear leucocytes following burn injury. Heggers et al.[4] showed that burn blister fluid is similar to plasma and contains immunoglobulins along with prostaglandins and other proteins. They recommended keeping the blister intact.[4] Studies have found that the burn blister fluid provides good environment for fibroblasts proliferation and myofibroblast-mediated wound contraction. Rockwell and Ehrlich[6] found it to inhibit fibrinolysis. Burn blister fluid is rich in interleukin-6, transforming growth factor (TGF) α and TGF β, and other cytokines. In another study, Inoue et al.[7] showed that theses cytokines are bound to serum proteins. Later on, Pan et al.[8] found angiogenin activity in burn blister fluid, which stimulates neoangiogenesis. Thus, burn blister fluid has a potential to facilitate wound healing when applied locally. Also the vasodilation induced by burn blister fluid may improve circulation in the zone of stasis and may limit the spread of coagulation zone.
However, contradictory evidences are also present in the literature such as a study by Rockwell and Ehrlich,[6] which is in favor of debriding burn blister completely due to its negative effects on wound healing. In an in vitro study by Garner et al.,[9] they found that burn blister fluid suppresses keratinocyte replication. But they have used stored blister fluid for the study.
Most of the proponents of burn blister fluid debridement are mainly concerned with the chances of infection and pressure over the wound.[23] Deitch[10] found that the burn blister fluid has retained opsonic activity against Staphylococcus aureus, which is similar to normal human serum; however, opsonic activity against Pseudomonas was impaired. Sterile manner of handling burn wounds and culture sensitivity-based antibiotic treatment will help to avoid the infection. Aspirating the blister will decompress it, and the effect of pressure will be nullified. Keeping the intact skin as dressing will provide biological cover below which epithelialization will occur.
Beneficial effects of APRP in wound healing have been shown in various studies.[5] The burn blister fluid is similar to APRP and is supposed to have same biological effects.[47,8] It is needed to perform an analytical study for the assessment of growth factor concentration in burn blister fluid. It is supposed to provide a protein-rich environment favorable for wound healing.
We have used burn blister fluid on second-degree superficial burn wounds only. These were the areas from where blisters were lost during transportation of the patient. The application of burn blister fluid on these areas was to support body’s wound-healing process and thus to reduce the time of healing. We found in this particular case that second-degree superficial burn wounds were healed in 11 days.
There is a risk of introduction of infection to burns wounds while aspirating. Absolute aseptic precaution should be taken while collecting and spraying the burn blister fluid, and the procedure should be carried out in well-equipped procedure room or operation theater only. All wounds including those with blister skin cover should be covered with a moist biological dressing. The dressing should be capable of retaining cytokines activity. This is a single case report introducing an innovative idea of using patient’s own body fluid as adjuvant for wound therapy. Detailed systematic studies are needed to validate this finding.
To conclude, there is no consensus of management of burn blisters at present. Immediate aspiration of burn blister fluid and spraying over other areas of same wound is feasible to be used as adjuvant local therapy for second-degree superficial burns. Its beneficial effects need to be validated.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Financial support for this study was provided by the Department of Plastic Surgery, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Management of blisters in the partial-thickness burn: an integrative research review. J Burn Care Res. 2006;27:66-81.
- [Google Scholar]
- Fibrinolysis inhibition in human burn blister fluid. J Burn Care Rehabil. 1990;11:1-6.
- [Google Scholar]
- Effects of cytokines in burn blister fluids on fibroblast proliferation and their inhibition with the use of neutralizing antibodies. Wound Repair Regen. 1996;4:426-32.
- [Google Scholar]
- Angiogenin expression in burn blister fluid: implications for its role in burn wound neovascularization. Wound Repair Regen. 2012;20:731-9.
- [Google Scholar]
- The effects of burn blister fluid on keratinocyte replication and differentiation. J Burn Care Rehabil. 1993;14:127-31.
- [Google Scholar]
- Opsonic activity of blister fluid from burn patients. Infect Immun. 1983;41:1184-9.
- [Google Scholar]





