Translate this page into:
Role of Intralesional Vitamin D3 in the Treatment of Cutaneous Warts
Address for correspondence: Insha Latif, Medical Officer (Dermatologist), District Hospital, Ganderbal, Kashmir, J&K. E-mail: Insha.latif@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Cutaneous warts are common benign skin lesions caused by human papillomavirus. Various treatment options are available for these but immunotherapy is becoming more and more popular over the past few years. It stimulates cell-mediated immunity causing clearance of warts.
Aims and Objectives:
The aim is to determine the role of intralesional vitamin D3 in the treatment of warts.
Materials and Methods:
Consecutive patients with verruca vulgaris attending OPD were included. Around two to three warts were injected first with 0.2 mL of lignocaine (20 mg/mL). After a few minutes, the same warts were injected with 0.2 mL (4 U) of vitamin D3 (15 mg/mL). The injections were given2 weeks apart for a maximum of six sessions, and the patient was followed up 3 months after the last injection.
Results:
A total of 41 patients of cutaneous warts completed the study. Complete clearance was seen in 27 (65.85%) patients, partial response was seen in 11 (26.83%) patients, and three patients (7.32%) showed no response at all. The mean number of injections required for complete response was four. Recurrence was seen in two patients (4.88%) and one patient had keloid formation at the sight of injection.
Limitation:
This is a small sample sized study and lacks a control group.
Conclusion:
Intralesional vitamin D3 is an effective treatment option for common warts.
Keywords
Treatment
vitamin D3
warts
INTRODUCTION
Cutaneous warts are common benign skin lesions caused by human papillomavirus (HPV) infection. They are usually asymptomatic and spontaneous resolution occurs in about 65–78% of the cases. Most patients with warts seek treatment due to their cosmetic unacceptability and sometimes for the pain or tenderness, caused by certain types of warts (e.g. periungual and plantar warts).[12] Various modalities including topical agents (e.g. salicylic acid, trichloroacetic acid, silver nitrate, phenol, etc.) and physical destructive methods (e.g. electrocoagulation, cryotherapy, or laser therapy) are available for the treatment of warts.[34] However, these treatment options are not suitable for multiple and refractory warts and might be associated with pain, scarring, and frequent recurrences.[12] Due to their proven efficacy and ease of therapy, immunotherapeutic modalities are becoming more and more popular in the treatment of warts. These act by stimulating the host cell-mediated immunity to eliminate the virus rather than just clearing the skin lesions.[12,5] Several immunotherapeutic agents have been used via topical, intralesional, and systemic routes in the treatment of warts. These include imiquimod, Mycobacterium w vaccine, bacillus Calmette–Guérin vaccine, measles, mumps, and rubella vaccine, Candida antigen, Trichophyton antigen, tuberculin, zinc, cimetidine, levamisole, HPV vaccine, and autoimplantation therapy.[67]
Recently, various studies have shown intralesional vitamin D as a potential therapeutic option for the treatment of cutaneous warts. We conducted this study to determine the role of intralesional vitamin D3 in the treatment of cutaneous warts.
MATERIALS AND METHODS
This study was conducted in the Department of Dermatology, Venerology, and Leprosy, Government Medical College, Srinagar from November 2018 till October 2019. Clearance was sought from Institutional Ethics Committee before starting the study. A total of 45 consecutive patients attending the outpatient department were included in the study after a written informed consent.
Inclusion and exclusion criteria
All male and female patients having cutaneous warts (single or multiple) and who had not received any topical or destructive treatment modalities for at least 3 months prior were included in the study.
Patients having age less than 18 years or more than 60 years, immunocompromised patients or patients on immunosuppressants, patients with any other systemic illness, pregnant and lactating females, patients with known keloidal tendency, and patients with history of hypersensitivity to vitamin D3 were excluded from this study.
The diagnosis was made by history and clinical examination. Baseline investigations were done in every patient and all demographic data were recorded in a pre-formed questionnaire. The number, size, and location of warts were recorded in every patient. Photographs were taken at each visit for assessing the response.
Administration of vitamin D3
Vitamin D3 is available as vials of 60,000 U of cholecalciferol in 1 mL (15 mg). Around two to three warts were injected first with 0.2 mL of lignocaine (20 mg/mL). After a few minutes, the same warts were injected with 0.2 mL (4 U) of vitamin D3 slowly at their base. No oral or topical treatment was given after injection.
Assessment of response
The injections were given at an interval of 2 weeks for a maximum of six injections. Clinical response was assessed by the decrease in the number and size of warts, and response was recorded by taking photographs at each visit and after 3 months of last injection for any recurrence or side effects. The response was graded as complete if all warts resolved completely, partial if there was 50% reduction in size and number of warts, and no response if there was less than 50% reduction in number and size of warts.
RESULTS
A total of 45 patients of common warts were included in the study out of which four were lost to follow up. Thus, 41 patients completed the study, out of which 23 were males and 18 were females. The age of patients ranged from 18 to 60 years with the mean age of 32.51 ± 11.20 years. The duration of warts ranged from 1 to 11 months with the mean duration of 4.46 ± 2.58 months. The number of warts ranged from 1 to 15 with a mean of 6.68 ± 3.78. The mean number of injections required for complete response was four [Table 1].
| Total patients | 45 |
|---|---|
| Patients completed the study | 41 |
| Gender ratio (male: female) | 1.27:1 |
| Age of patient (years) | 18–60 |
| Mean age ± SD (years) | 32.51 ± 11.20 |
| Duration of warts (months) | 1–11 |
| Mean duration of warts ± SD (months) | 4.46 ± 2.58 |
| No. of warts | 1–15 |
| Mean of no. of warts ± SD | 6.68 ± 3.78 |
| No. of injections for complete response (mean) | 4 |
Out of 41 patients, 23 had verruca vulgaris, 11 had periungual warts, and seven had plantar warts. All patients had warts on hands or feet. Out of 23 patients of verruca vulgaris, 16 (69.6%) showed complete response [Figure 1], five (21.7%) showed partial response, and two (8.7%) showed no response. Out of 11 patients of periungual warts, six (54.5%) showed complete response [Figure 2], four (36.4%) showed partial response and one (9.1%) showed no response. Out of seven patients of plantar warts, five (71.4%) showed complete response, whereas two (28.6%) showed partial response [Table 2].

- A. Common wart before treatment. B. Photograph taken after two sessions of intralesional vitamin D3. C. Photograph showing complete resolution of wart after four sessions of intralesional vitamin D3
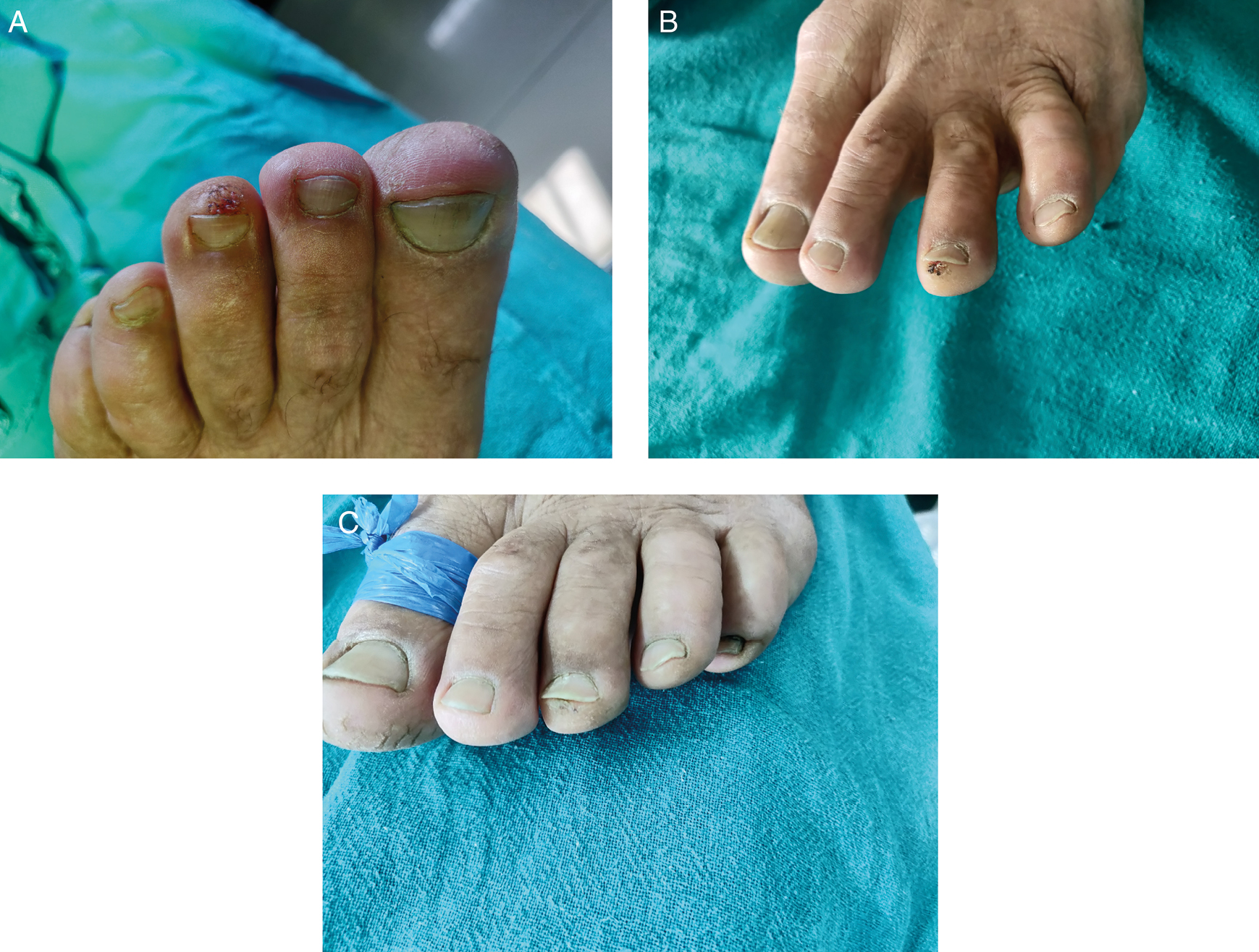
- A. Photograph before starting treatment. B. Photograph taken after two sessions of intralesional vitamin D3. C. Complete resolution after four intralesional vitamin D3 injections
| Type of warts | Complete response | Partial response | No response |
|---|---|---|---|
| Periungual warts | 6 (54.5%) | 4 (36.4%) | 1 (9.1%) |
| Plantar warts | 5 (71.4%) | 2 (28.6%) | 0 |
| Verruca vulgaris | 16 (69.6%) | 5 (21.7%) | 2 (8.7%) |
Complete clearance was seen in 27 (65.85%) patients, partial response was seen in 11 (26.83%) patients [Figure 3], and no response was seen in three (7.32%) patients.
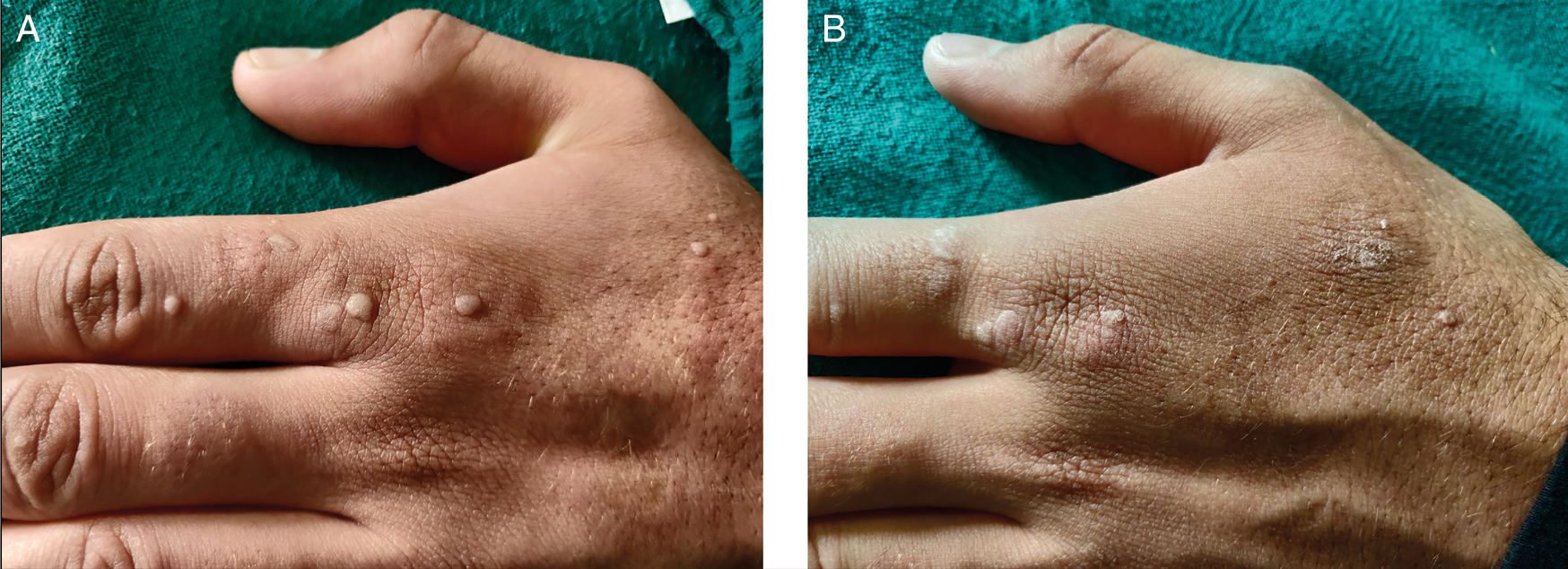
- A. Photograph showing common warts before treatment. B. Photograph showing partial resolution after four injections of vitamin D3
The only major adverse effect seen in our study was the development of injection site keloids in one patient 3 months after the last injection [Figure 4].
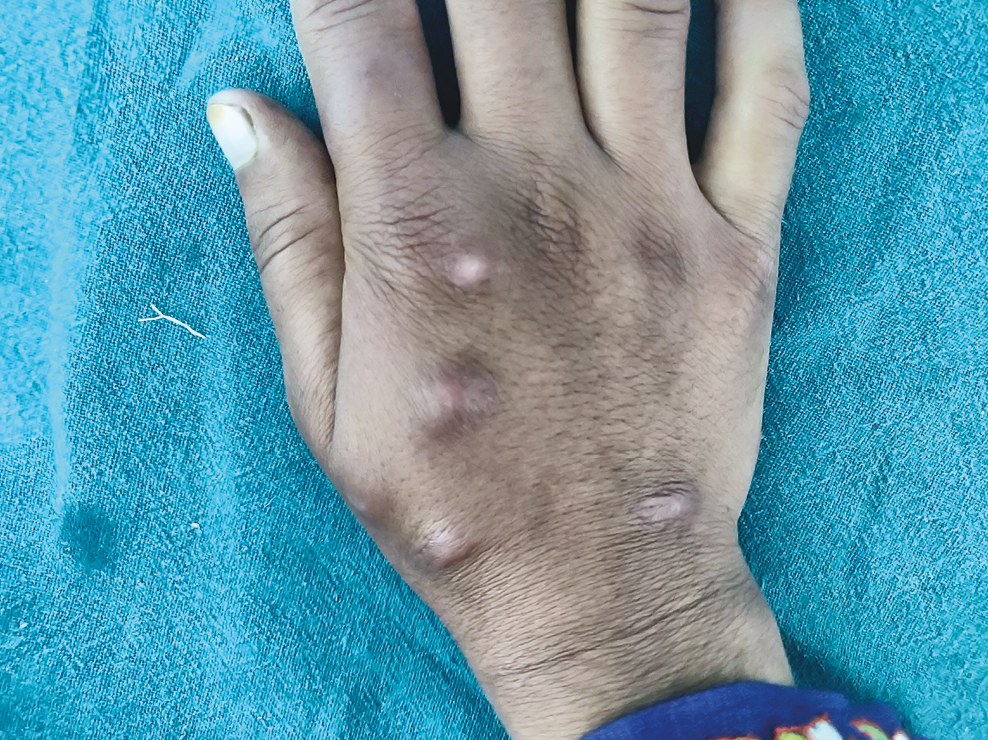
- Keloid formation after six intralesional injections of vitamin D3
Recurrence was seen in two (4.88%) patients after a follow-up of 3 months.
DISCUSSION
Although many treatment modalities are available for treating cutaneous warts, most of these treatment options are present with limitations. Destructive procedures such as electrocoagulation, cryotherapy, laser surgery, 5-fluorouracil, salicylic acid, to name a few, have been extensively used for the treatment of cutaneous warts. Apart from being associated with scarring and frequent recurrences, such treatment modalities are cumbersome, expensive, consume more time, and are painful to the patient.[18,9] Such treatment lines are limited to local application and do not act systemically, thus making them unsuitable for the treatment of multiple warts.[110] These reasons make immunotherapy a suitable treatment option for the treatment of cutaneous warts. Immunotherapy has become a popular treatment modality not only for the treatment of solitary cutaneous warts but also in the management of recalcitrant and recurrent warts. Periungual and palmoplantar warts which are difficult to treat sites have also been successfully treated with immunotherapy.[11] Immunotherapy acts via recognition by the immune system of antigens produced by virus and wart tissue causing delayed-type hypersensitivity response resulting in the eradication of HPVs.[12]
Intralesional vitamin D3 is a recent and effective immunotherapy for the treatment of warts. Vitamin D3 acts via regulation of epidermal cell proliferation and differentiation. There is also modulation of cytokine production. Activation of Toll-like receptor also takes place resulting in human macrophages upregulation and expression of VDR and VD1-hydroxylase genes, leading to production of the antimicrobial peptide.[1013]
Several studies have evaluated the efficacy of intralesional vitamin D in the treatment of cutaneous warts. The results of our study are comparable with most of these studies. In a study by Singh et al.,[14] involving 40 patients, 29 (72.5%) had complete response, eight (20%) had partial response, and three (7.5%) had no response. In another study by Kavya et al.[15] involving 42 patients with multiple warts, 33 (78.57%) patients showed complete response, six patients (14.28%) showed moderate response, and three patients (7.14%) showed mild response. Raghukumar et al.[10] reported complete clearance in 54 (90%) patients, partial response in four (6.66%) patients, and no response in two (3.33) patients from their study involving 60 patients. Aktas et al.[13] used intralesional vitamin D3 for plantar warts in 20 patients and reported complete clearance in 80% of the patients at the end of 8 weeks. The maximum response rate achieved in our study is higher than that seen with MMR vaccine, Candida albicans, Mycobacterium indicus pranii vaccine, comparable to bleomycin and PPD.[712,161718] Complications such as fever and erythema along with swelling and formation of ulcer at the site of injection as seen with the use of Mycobacterium w vaccine for the treatment of cutaneous warts were not seen in our study, making intralesional vitamin D3 a safer option for the treatment of cutaneous warts.[19]
CONCLUSION
Intralesional vitamin D3 is a promising option for the treatment of cutaneous warts. Though our study had a smaller sample size and lacked randomization, still results are encouraging for intralesional vitamin D3 to be used as immunotherapy for the treatment of cutaneous warts. Its cost-effectiveness and lack of any major side effect add to the utilization of this treatment modality. However, large-sized sample well-designed studies showing the efficacy of intralesional vitamin D3 for the treatment of cutaneous warts are needed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Intralesional immunotherapy for the treatment of warts: A network meta-analysis. J Am Acad Dermatol. 2019;80:922-30.e4.
- [Google Scholar]
- British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol. 2014;171:696-712.
- [Google Scholar]
- Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2015;28:194-200.
- [Google Scholar]
- Immunotherapy with intralesional Candida albicans antigen in resistant or recurrent warts: A study. Indian J Dermatol. 2013;58:360-5.
- [Google Scholar]
- Successful treatment of plantar warts with very diluted bleomycin using a translesional multipuncture technique: Pilot prospective study. J Cutan Med Surg. 2012;16:250-6.
- [Google Scholar]
- Cryotherapy with liquid nitrogen versus topical salicylic acid application for cutaneous warts in primary care: Randomized controlled trial. Can Med Assoc J. 2010;182:1624-30.
- [Google Scholar]
- Intralesional vitamin D3 injection in the treatment of recalcitrant warts: A novel proposition. J Cutan Med Surg. 2017;21:320-4.
- [Google Scholar]
- Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016;7:364-70.
- [Google Scholar]
- Intralesional antigen immunotherapy for the treatment of warts: Current concepts and future prospects. Am J Clin Dermatol. 2013;14:253-60.
- [Google Scholar]
- Intralesional vitamin D injection may be an effective treatment option for warts. J Cutan Med Surg. 2016;20:118-22.
- [Google Scholar]
- A comparative study between intralesional PPD and vitamin D3 in treatment of viral warts. Int J Res Dermatol. 2018;4:197-201.
- [Google Scholar]
- Safety and efficacy of intralesional vitamin D3 in cutaneous warts: An open uncontrolled trial. J Cutan Aesthet Surg. 2017;10:90-4.
- [Google Scholar]
- Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol. 2014;80:509-14.
- [Google Scholar]
- Immunotherapy using purified protein derivative in the treatment of warts: An open uncontrolled trial. Indian J Dermatol Venereol Leprol. 2016;82:42-6.
- [Google Scholar]
- Preliminary study of intralesional bleomycin injection for the treatment of genital warts. Ann Dermatol. 2015;27:239-41.
- [Google Scholar]
- Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg. 2014;7:203-8.
- [Google Scholar]






