Translate this page into:
Evaluation of Healing Effects of Poultice Containing 0.5% Fulvic Acid on Male White-Male Rats with Skin Ulcer
Address for correspondence: Nematollah Gheibi, Department of Medical Biotechnology, School of Allied Medical Sciences, Cellular and Molecular Research Center, Research Institute for Non-communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran. E-mail: gheibi284@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Chronic and acute skin wounds are an important health concern because they are very frequent during human life and affect millions of people worldwide. Shortening the wound healing process reduces treatment costs and hospitalization. Therefore, researchers have been looking for new treatment approaches to shorten the wound healing process.
Aims and Objectives:
The aim of this study was to evaluate the wound healing properties of poultice containing 0.5% fulvic acid.
Materials and Methods:
In this experimental study, a full-thickness skin wound was created on the dorsal side of 24 male rats. The animals were then randomly assigned to control, sham, and experiment groups. The skin defects were daily bandaged by using sterile gauze dipped in normal saline, carboxymethylcellulose, and 0.5% fulvic acid for 21 days, respectively. The wound healing rate was evaluated grossly and histologically at various time intervals post injury. Both descriptive and statistical analysis methods were applied (P < 0.05).
Results:
The wound healing percentage was significantly higher in the poultice treatment group at all time intervals (P < 0.001). The wound was completely closed in this group compared with other groups at the end of week 4 post treatment. The mean numbers of inflammatory cells were statistically lower, and fibroblasts and vessels were higher in the poultice group than in the other groups at various time intervals post injury (P < 0.001).
Conclusion:
Fulvic acid (0.5%) could be used as an effective therapeutic approach to improve the wound healing process because of its unique anti-inflammatory and neovascularization properties at the skin wound site.
Keywords
Anti-inflammatory treatment
fulvic acid
humic substances
wound healing
INTRODUCTION
Disruption of skin epithelial layer is named wound that leads to dysfunction and structural disorder of normal tissue.[1] Chronic and acute skin wounds are an important health concern because they are very frequent during human life and affect millions of people worldwide.[2] The wound healing (WH) process is a complicated mechanism that is evolved in humans and animals. This process consists of three phases: inflammation, proliferation, recovery or tissue remodeling.[3]
Wounds significantly impact morbidity and mortality in various patients necessitating the development of new approaches and treatments to accelerate the WH process.[4]
Nowadays, tissue engineering, growth factors therapy, nanobiotechnologic and biologic-based treatments have been widely introduced to accelerate the WH process.[5678910] However, these new therapeutic approaches are very expensive and limited. For a long time, humans have found that the plants, soil, and their derivatives are effective in WH.[11]
Humic substances, containing humic acid (HA) and fulvic acid in their structures, are mostly present in soil, lignite, peat, and water that regulate cell growth. HA has been previously demonstrated to enhance the WH process because of its antibacterial, antioxidant, immunomodulatory, and anti-inflammatory properties.[12]
Fulvic acid is derived from humic substances produced by microorganisms. The antioxidant activity of HA is due to its ability to bind to oxygen reactive.[12] The promotion function of Nrf2 transcriptions as well as its coding proteins in WH is due to their activities against oxidative stress.[13]
The carbohydrate-derived fulvic that is a main constituent of HA enhances the expression of interleukin (IL)-10. The overexpression of IL-10, which is one of the main anti-inflammatory cytokines, accelerates the WH process, and the safety, anti-inflammatory, as well as WH properties of carbohydrate-derived fulvic acid were evaluated in rats. It was demonstrated that carbohydrate-derived fulvic acid is a safe compound with anti-inflammatory and WH properties.[1415] Although several studies have investigated the substances containing fulvic acid could be used to improve the skin ulcer with bacterial infection, the anti-inflammatory properties of fulvic acid poultice have yet to be investigated, histologically.[1617181920] Therefore, the aim of this study was to evaluate the healing effects of 0.5% fulvic acid poultice on male white-male rats with skin ulcer.
MATERIALS AND METHODS
Animal design
This experimental study was approved by the ethical committee of Qazvin University of Medical Sciences (IR.QUMS.REC.1396.433) in accordance with NIH guidelines for care of the laboratory animals. Twenty-four male Sprague Dawley rats, initial bodyweight 230–250 g, were purchased from a breeding colony of an animal house at Razi Institute. The animals were allowed to adapt for two weeks before the beginning of the experiment. The animals were kept in clean solitary cages with free access to food and tap water under standard conditions (25 ± 1°C, 55% humidity) with a 12-h light:12-h dark cycle. The dorsal side of each was shaved and disinfected using povidone-iodine. The animals were anesthetized using intraperitoneal administration of ketamine and xylazine (50 mg/kg ketamine, 5 mg/kg xylazine [Merck, Germany]). Full-thickness skin wound 2 cm in diameter was created on the shaved site using a disposable punch.[9]
The animals were then randomly assigned to one of the three equal groups: control, sham, and experiment, each of eight rats. The skin defects in the control and sham groups were daily bandaged by using sterile gauze dipped in normal saline and carboxymethylcellulose (CMC) for 21 days, respectively. In the experiment group, the skin defect was daily bandaged by fulvic acid for 21 days. We did not estimate any tissue level of matrix metalloproteinase-9 (MMP-9), IL-10, or reactive oxygen species (ROS).
Preparation of 0.5% fulvic acid poultice
A 0.5% fulvic acid poultice was prepared by dissolving 0.004 g of fulvic acid (Sigma-Aldrich, USA) and 8 g of CMC (Merck, Germany) in 16 mL of normal saline within a glass beaker. Fulvic acid is not available in the market for dressing. The prepared poultice was stored at 37°C or room temperature until onset treatment. According to the previous study,[15] there are not any contraindications for fulvic acid topical usage in wound management.
WH rate
Grossly, for measuring WH rate, the animals were located in a standard crouching position and the skin defect area of each rat was blindly measured by an expert person by drawing it on a transparent paper at the end of weeks 1, 2, and 3 post treatment. Wound areas were calculated using AutoCAD software.[10] Multiple images were also taken from the wound area of each sample. The WH rate was calculated using the following formula and reported as percentage:
WH percentage = (Wound size at day 0−Wound size at desired day)/Wound size at day 0 × 100
Histopathological evaluation
At the end of weeks 1, 2, and 3 postinjury, the animals were anesthetized intraperitoneally with ketamine and xylazine (50 mg/kg ketamine, 5 mg/kg xylazine), and the samples were collected from the fresh edge of the wound for the investigation of inflammation, proliferation, remodeling, and wound closure phases.
For histological examination, the collected samples were fixed in 10% buffered formalin. The samples were embedded in paraffin. The embedded paraffin samples were sectioned at 5 µm and stained routinely using hematoxylin and eosin. The sections were blindly interpreted by an expert pathologist for the presence of angiogenesis and inflammatory cells (neutrophils, eosinophils, and mast cells), and fibroblasts using Iwf-Iox-Holland ocular lens.[8] Toluidine blue staining was also conducted for detecting mast cells.
Wound scoring
The wounds were scored histologically. First, the stained slides were descriptively reported. The number of inflammatory cells such as neutrophils and eosinophils as well as mast cells and fibroblasts was counted using an objective lens (×400). For evaluating the angiogenesis, the sections were initially examined at low magnification to find the high-density areas of new vessels. Then, three separate fields were chosen for further assay at high-magnification field with objective lens (×400). Histologically, the angiogenesis coding was according to Table 1.[2122] WH process was scored based on the number of inflammatory cells, fibroblasts, and the number of new vessels.
| Index | Coding | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Angiogenesis | No evidence of vascular channel | 4–8 blood vessels | 12–15 blood vessels | 15–20 blood vessels | More than 20 blood vessels |
Statistical analysis
Data were analyzed using SPSS software version 20. All data were expressed as mean ± standard deviation (SD). Normality of data was evaluated using a one-sample Kolmogorov–Smirnov test. The mean differences of variables were analyzed using a one-way analysis of variance (ANOVA) followed by post hoc Tukey. A repeated measures ANOVA was used for intragroup analysis. The value of P < 0.05 was considered statistically significant.
RESULTS
Macroscopic findings
Wound contraction and healing percentage
Wound contraction and healing percentage in any of the three groups at the end of weeks 1, 2, and 3 after creating skin defects are presented in Figures 1 and 2A–Q. The WH level was equal to zero in all the groups on day 1 after creating skin defects. However, the WH rate was significantly higher in the fulvic acid group than in the control and sham groups at the end of 1, 2, and 3 weeks after creating skin injury (P < 0.001).
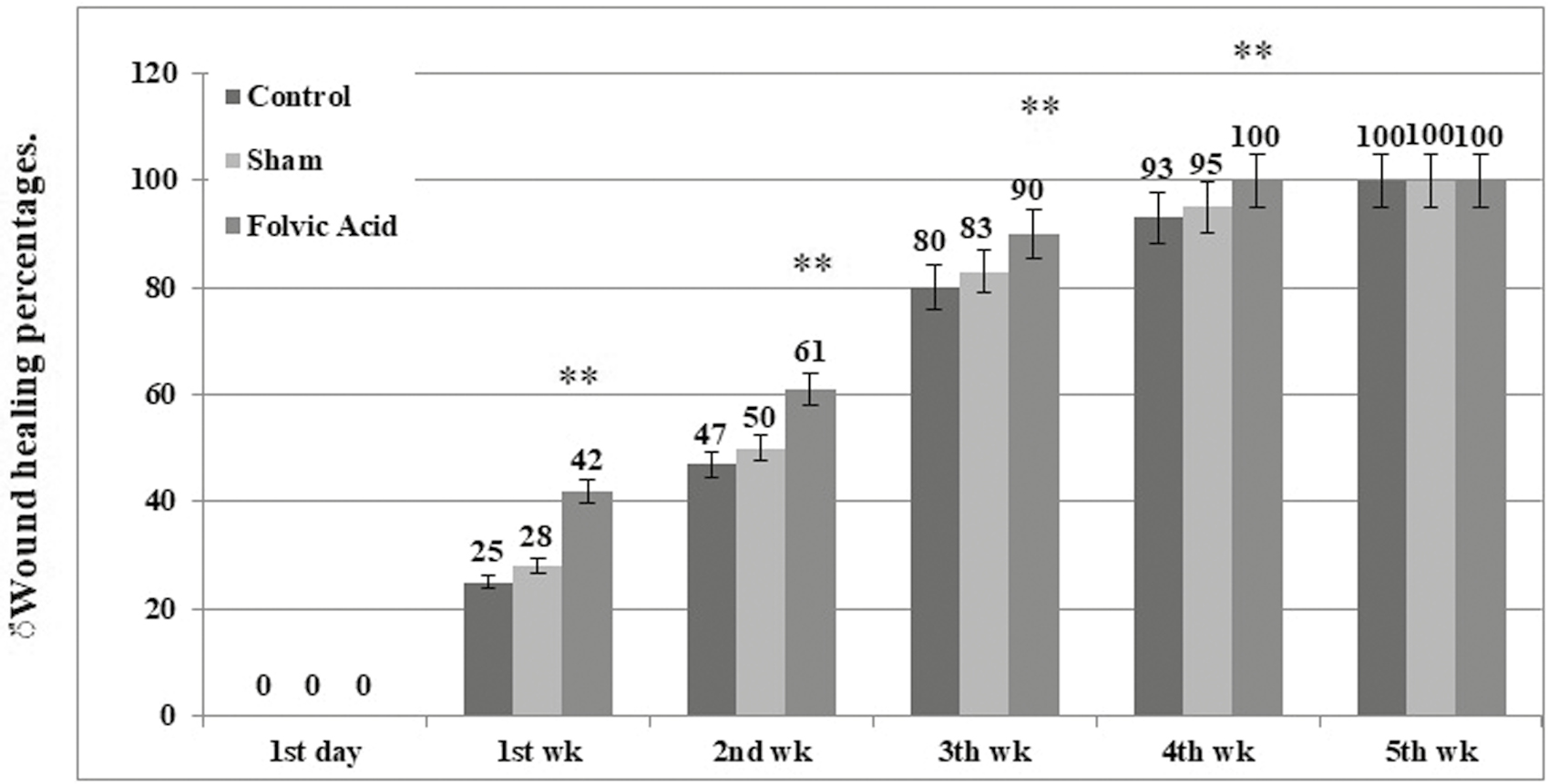
- The mean percentage of WH in three experimental groups at different time intervals after injury
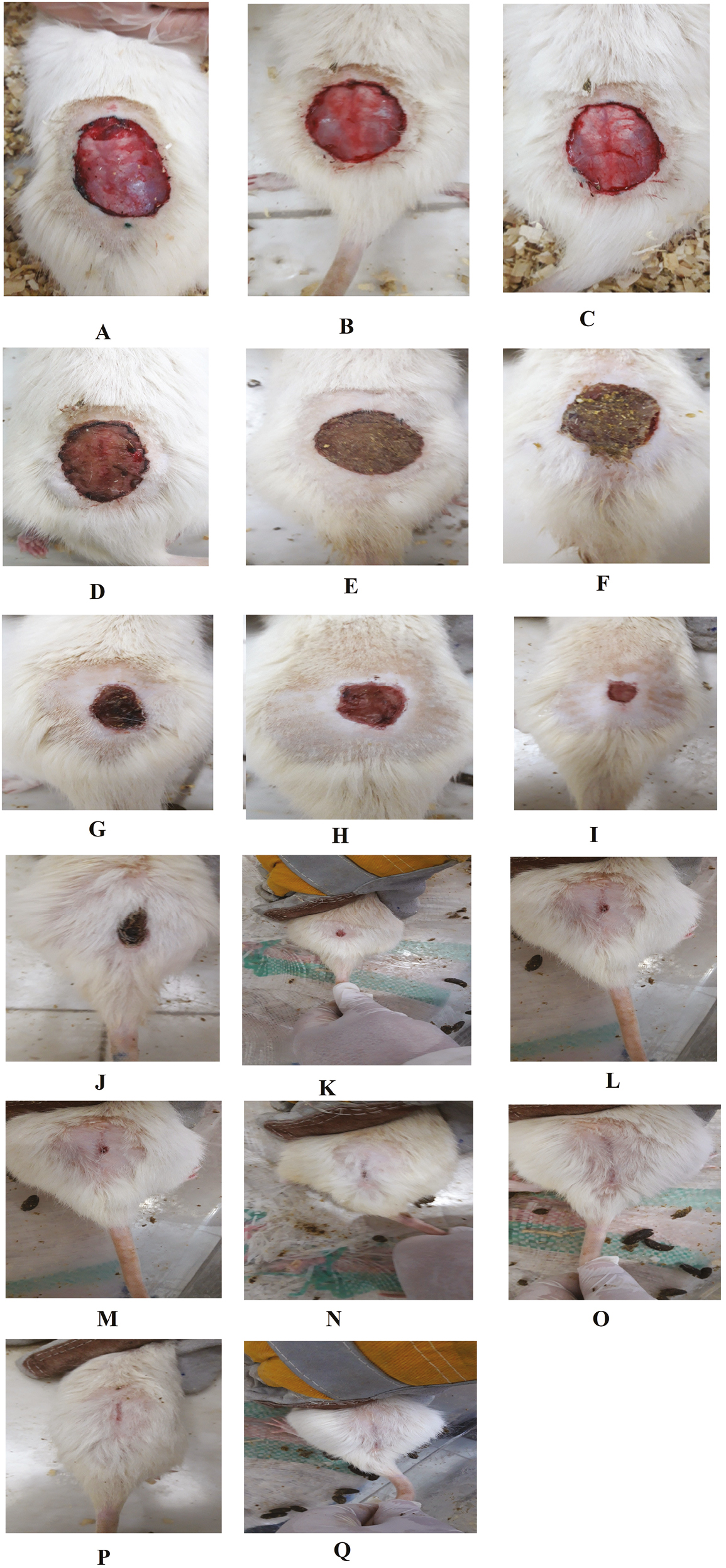
- (A–Q) Macroscopic evaluation of WH rate in all the three experimental groups at different time intervals after injury
In the 0.5% fulvic acid poultice group, the skin defect rate was more than 90% at the end of week 3 after creating a skin wound. In this group, the wound was completely closed at the end of week 4 posttreatment [Figure 2O]. The difference between the treatment groups and the control and sham groups was obvious, grossly [Figure 2A–Q]. None of the mice develop hypertrophic scar or keloid.
Microscopic findings
The mean number of inflammatory cells, including neutrophils, eosinophils, and mast cells, and the mean number of fibroblasts and vessels were obtained and compared at mentioned time intervals after skin injury.
Neutrophils
No significant difference was seen between all the three experimental groups on day 1 of the study in terms of the mean number of neutrophils (P = 0.738). At the end of weeks 1, 2, and 3 of the study, the mean numbers of neutrophils were 90.8 ± 6.2, 69.3 ± 4.8, and 50.6 ± 3.3 for the control; 87.0 ± 3.0, 60.0 ± 3.5, and 41.1 ± 6.3 for the sham; and 64.3 ± 6.2, 44.5 ± 6.6, and 23.6 ± 2.9 for the fulvic acid groups, respectively. Intragroup analysis showed that the mean number of neutrophils was significantly decreased during the study period (P < 0.001). Intergroup analysis showed a significant difference among the three experimental groups with respect to the mean number of neutrophils at various time intervals of the study (P < 0.001) [Table 2, Figure 3A–I].
| Inflammatory cells | Group time | Control (N), mean ± SD | Sham (N), mean ± SD | Fulvic acid (N), mean ± SD* |
|---|---|---|---|---|
| Neutrophils | Day 1 PS | 145.00 ± 9.47 | 146.62 ± 9.62 | 143.12 ± 7.52 |
| First week PS | 90.87 ± 6.26 | 87.00 ± 3.00 | 64.37 ± 6.23 | |
| Second week PS | 69.37 ± 4.83 | 60.00 ± 3.54 | 44.50 ± 5.68 | |
| Third week PS | 50.62 ± 3.37 | 42.12 ± 6.31 | 23.62 ± 2.97 | |
| Eosinophils | Day 1 PS | 59.50 ± 4.37 | 60.12 ± 4.35 | 59.87 ± 4.22 |
| First week PS | 50.00 ± 3.42 | 44.00 ± 2.32 | 29.37 ± 4.20 | |
| Second week PS | 38.25 ± 2.86 | 32.87 ± 3.72 | 20.00 ± 2.67 | |
| Third week PS | 29.00 ± 3.85 | 23.62 ± 3.42 | 10.00 ± 3.25 | |
| Mast cells | Day 1 PS | 45.00 ± 3.77 | 44.75 ± 3.37 | 45.25 ± 2.65 |
| First week PS | 35.00 ± 2.67 | 31.25 ± 3.24 | 24.87± 2.90 | |
| Second week PS | 27.37 ± 4.10 | 22.12 ± 3.60 | 14.87 ± 2.85 | |
| Third week PS | 20.00 ± 3.46 | 16.00 ± 3.11 | 10.00 ± 2.67 |
N = number, PS = postsurgery
*P < 0.05 = significant changes in comparison with control and sham groups
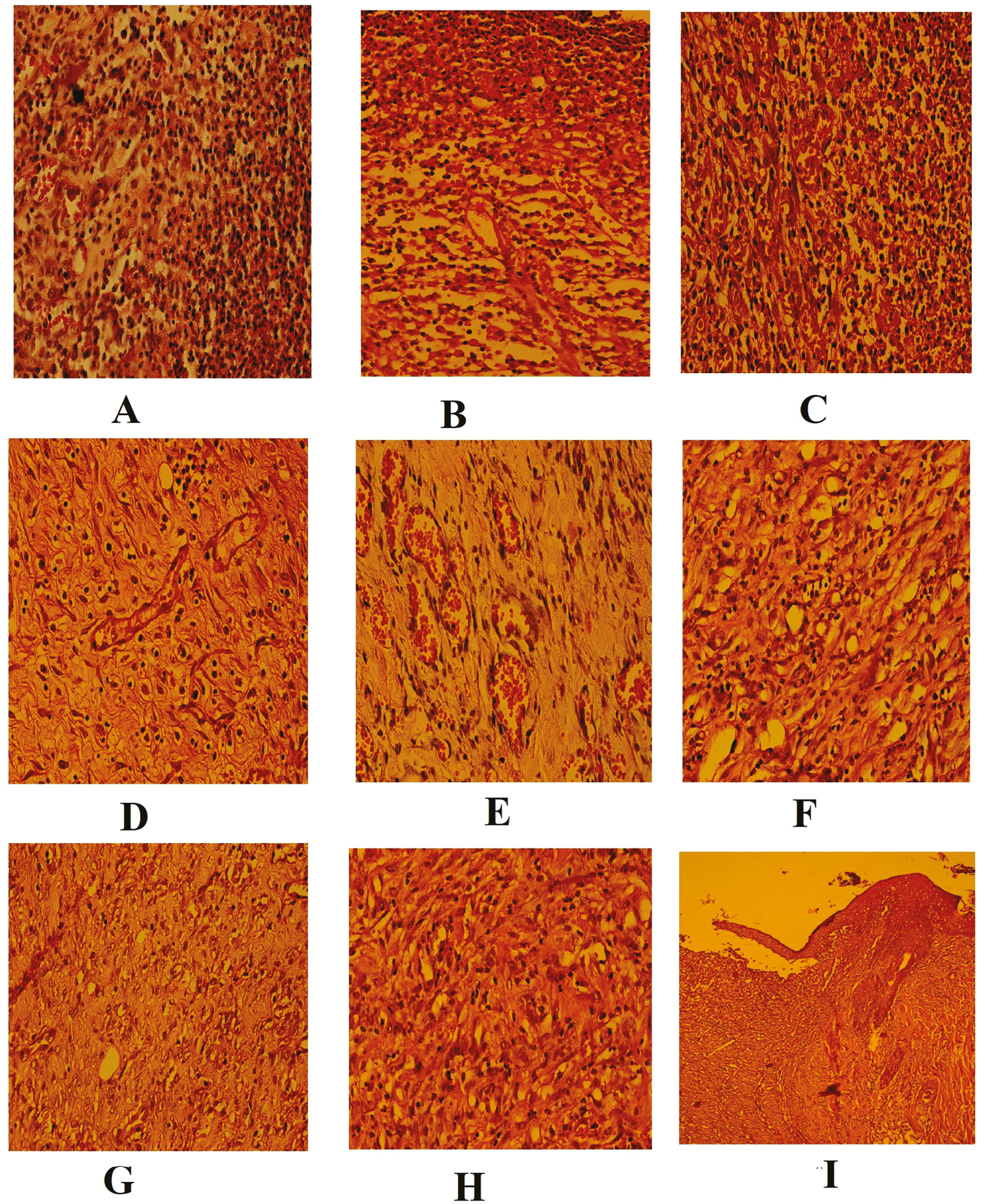
- (A–I) Histopathological skin sections of WH in all the three experimental groups
Eosinophils
No significant difference was seen between all the three experimental groups on day 1 of the study in terms of mean number of eosinophils (P = 0.958). At the end of weeks 1, 2, and 3 of the study, the mean numbers of eosinophils were 50.0 ± 3.4, 38.2 ± 2.8, and 29.0 ± 3.8 for the control; 44.0 ± 2.3, 32.8 ± 3.7, and 23.6 ± 3.4 for the sham; and 29.3 ± 4.3, 20.0 ± 2.6, and 10.0 ± 3.2 for the fulvic acid groups, respectively. Intragroup analysis showed that the mean number of eosinophils was significantly decreased during the study period (P < 0.001). Intergroup analysis showed a significant difference between the fulvic acid group and the control and sham groups with respect to the mean number of eosinophils at various time intervals of the study (P < 0.001) [Table 2, Figure 3A–I]. There was no significant difference between the control group and the sham group in terms of the mean number of eosinophils at various time intervals of the study (P > 0.05).
Mast cells
No significant difference was seen between all the three experimental groups on day 1 of the study with respect to the mean number of mast cells (P = 0.958).
The mean numbers of mast cells were 35.0 ± 2.67, 27.37 ± 4.10, and 20.0 ± 3.46 for the control; 31.25 ± 3.24, 22.12 ± 3.6, and 16.00 ± 3.11 for the sham; and 24.87 ± 2.90, 14.87 ± 2.85; and 10.0 ± 2.67 for the fulvic acid groups at the end of weeks 1, 2, and 3 of the study, respectively. Intragroup analysis showed that the mean number of mast cells was significantly decreased during the study period (P < 0.001). Intergroup analysis showed a significant difference between groups and sham groups with respect to the mean number of mast cells at various time intervals of the study (P < 0.001) [Table 2, Figure 3A–I].
Fibroblasts
There was no fibroblast in the skin tissue section of any of the three groups on day 1 of the study. The mean numbers of fibroblasts were 14.80 ± 3.1, 24.7 ± 3.2, and 45.0 ± 3.2 at the end of week 1 after skin injury in the control, sham, and fulvic acid groups, respectively. At the end of week 2 after creating skin defect, the highest and lowest mean fibroblast numbers belonged to the fulvic acid (75.0 ± 3.7) and the control groups (28.7 ± 3.6). The mean numbers of fibroblasts were 114.7 ± 3.9, 82.0 ± 3.2, and 75.1 ± 3.0 at the end of week 3 after skin injury in the fulvic acid, control, and sham groups, respectively. A significant decrease was found in the fulvic acid group compared with the sham and the control groups in terms of the mean number of fibroblasts at the end of weeks 2 and 3 after creating skin defect (P < 0.001) [Table 3, Figure 3A–I]. The sham group showed no significant difference from the control group in terms of the mean number of fibroblast at the end of weeks 2 and 3 after creating skin defect (P > 0.05).
| Index | Group time | Control (N), mean ± SD | Sham (N), mean ± SD | Fulvic acid (N), mean ± SD |
|---|---|---|---|---|
| Fibroblasts | Day 1 PS | (0.0) 0.0 | (0.0) 0.0 | (0.0) 0.0 |
| First week PS | 14.87 ± 3.13 | 24.75 ± 3.24 | 45.00 ± 3.24* | |
| Second week PS | 28.75 ± 3.61 | 37.12 ± 3.64 | 75.00 ± 3.77* | |
| Third week PS | 75.12 ± 3.09 | 82.00 ± 3.29 | 114.75 ± 3.95* | |
| Formed new blood vessels (angiogenesis) | Day 1 PS | (0.0) 0.0 | (0.0) 0.0 | (0.0) 0.0 |
| First week PS | 7.12 ± 2.16 | 15.00 ± 2.87 | 25.00 ± 3.77* | |
| Second week PS | 14.00 ± 3.29 | 18.25 ± 2.91 | 45.00 ± 3.02* | |
| Third week PS | 5.00 ± 1.19 | 9.87 ± 2.41 | 17.62 ± 3.99* |
N = number, PS = postsurgery
*P < 0.05 = significant changes in comparison with control and sham groups
The mean number of new vessels (angiogenesis)
The mean number of vessels was significantly increased in the fulvic acid (25.0 ± 3.7) compared with the sham (15.0 ± 3.8) and the control (7.1 ± 2.1) groups at the end of week 1 postinjury (P < 0.001). At the end of week 2 postinjury, the mean numbers of vessels were increased to 14.00 ± 3.2, 18.2 ± 2.9, and 45.0 ± 3.0 in the control, sham, and fulvic acid groups, respectively. The mean numbers of vessels were 5.0 ± 1.1, 7.8 ± 2.4, and 17.6 ± 3.9 in the control, sham, and fulvic acid groups, respectively. At the end of both second and third weeks postinjury, the difference between groups in terms of the mean number of vessels was statistically significant at the end of second week postinjury (P < 0.001). Intragroup analysis showed that the mean numbers of vessels are statistically significant at various time intervals [Table 3, Figure 3A–I].
DISCUSSION
The aim of this study was to evaluate the regenerative effect of a poultice containing 0.5% fulvic acid on full-thickness defect in the male rat animal model on day 1 and at the end of weeks 1, 2, and 3 after creating skin injury. The obtained data from wound contraction and healing percentage in skin defects in the fulvic acid poultice were significantly higher than those in the other groups at the end of 1, 2, and 3 weeks after creating skin injury. Hence, in the poultice group, more than 90% of the wound was closed at the end of week 3 after creating skin ulcer, and the wounds were completely closed at the end of week 4 posttreatment. The decrease in the mean number of inflammatory cells, including the neutrophils, eosinophils, and mast cells, and the increase in the mean number of fibroblast and vessels in the poultice containing 0.5% fulvic acid during the study period were the main microscopic findings in this study. In addition, the accelerative effect of poultice containing 0.5% fulvic acid on WH was confirmed grossly by measuring the wound area. The WH effect of the used poultice in our study attributes to its anti-inflammatory properties. The proliferation of inflammatory cells including neutrophils, eosinophils, and mast cells was significantly decreased in the treatment group by poultice in the wound area during the course of the study. Fulvic acid can act as an anti-inflammatory by decreasing the proinflammatory mediator release from cells.[1923]
The anti-inflammatory properties of fulvic acid resulted in a decrease in neutrophils and eosinophils in the wound area. Inflammation has been shown to cause endothelial dysfunction and tissue injury. Inflammation and the presence of polymorphonuclear cells such as neutrophils lead to induce endothelial dysfunction and tissue injury by ROS generation.[2324]
However, ROS act as secondary messengers for nonlymphoid cells and immunocytes involving in the repair process. Therefore, they appear to be important in modulating the lymphoid cell recruitment into the wound site and effective tissue repair.[25] The immunomodulatory effect of fulvic acid and its influence on the redox state have been wildly accepted.[19]
The obtained data from the mean number of vessels at the wound site showed that the poultice group had significantly higher vessels than the sham and control groups. It has been shown that ROS also possess the ability to regulate the angiogenesis or formation of blood vessels at the wound site and the optimal perfusion of blood into the WH area.[25]
The obtained data from the proliferation of fibroblasts showed that the poultice group significantly increased the fibroblast proliferation that is one of the main factors in the WH process than the control and sham groups. The possibility of an antiaging effect of fulvic acid because of enhancement of fibroblast viability as well as prevention of collagen degradation has been previously reported by Kinoshita, et al.[26]
One of the mechanisms of the used poultice containing fulvic acid to improve WH can be attributed to its immunomodulatory, anti-inflammatory, and antioxidant properties of fulvic acid. The anti-inflammatory properties of the administrated poultice can also be attributed to the function of the cytokines such as IL-10 and transforming growth factor-beta (TGF-β). IL-10 is one of the important anti-inflammatory cytokines that has been demonstrated to be upregulated by the administration of the drugs containing fulvic acid. Fulvic acid upregulates the expression of IL that accelerates the WH process. Ji, et al.[27] showed that the humic substances accelerate skin WH by activating TGF-β/Smads signaling pathway in a rat animal model. Anisyah, et al. demonstrated that the administration of fulvic acid topically expresses the gene-related MMPs and CC (proteins have two adjacent cysteines [amino acids], near their amino terminus) chemokines that are involved in the deposition of collagen needed and angiogenesis during the WH process, respectively.[28]
Kinoshita, et al. have shown that 1% fulvic acid is a good approach to accelerate WH. They also have reported that the WH rate by fulvic aid is affected by various concentrations. They suggested that the increase in fulvic acid concentration from 1% to 5% results in preventing collagen degradation from 47% to 61%.[26]
The anti-inflammatory properties and safety of carbohydrate-derived fulvic acid have been previously reported in a rat animal model. However, the accelerating effect of carbohydrate-derived fulvic acid was shown in the healing of excised wounds infected with Staphylococcus aureus.[15] Our findings are in agreement with the results reported by Sabi, et al.[15] However, this study was different with respect to some parameters such as different wound sites, lack of bacterial contamination, and duration of treatment.
The accelerating effect of fulvic acid on the WH process has been widely studied and attributed to its property to prevent collagen degradation.[27]
However, in the study by Zhao et al., the 5% carbohydrate-derived fulvic acid has been administrated topically for the WH that was in accordance with our study.[29] However, their study was different in nature of wound in which the wound had been infected by pathogens. Fulvic acid has been shown to accelerate WH by downregulation of IL-6 and MMP-9. The overexpression of IL-6 and MMP-9 leads to a delay in the WH process.[3031] The observed pattern about the accretion of WH at the initial weeks postinjury in this study was in accordance with Zho, et al. The fulvic acid compounds in the conducted study by Zaho were different when compared with our study.[29] The release of some cytokines such as IL-1 and tumor necrosis factor-alpha during inflammation by neutrophils, macrophages, and keratinocytes plays an important role in the onset of WH.[3132]
Several studies have shown that humic substances negatively regulate these cytokines leading to the acceleration of WH.[161718]
In conclusion, the evaluation of WH process, the changes of wound area, and the mean percentage of wound improvement indicated that wound was completely closed in the poultice containing 0.5% fulvic at the end of week 4 posttreatment. The results of the mean inflammatory parameters including neutrophils, eosinophils, and mast cells showed a significant difference between the fulvic acid groups and both control and sham groups at any of the three time intervals (1, 2, and 3 weeks) postinjury. The mean number of fibroblasts and the newly formed vessels at the wound site in the poultice group were significantly increased when compared with the control and sham groups at the same time. Thus, fulvic acid poultice plays a role in the WH process by modulation of inflammatory reactions, accelerating proliferation, and remodeling at the wound site. Therefore, the findings of our study demonstrated that the fulvic acid poultice can be used as a treatment strategy for the acceleration of WH and remodeling of the damaged tissue as well as an effective treatment for reducing the inflammation in the skin wound.
Financial support and sponsorship
We would like to thank the Research Deputy of Qazvin University of Medical Sciences for funding this study.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We extend our gratitude to the Clinical Research Development Center of Kosar Hospital and Dr. Samaneh Rouhi for assisting us in the online submission of this research project.
REFERENCES
- Effect of acacia honey-impregnated placenta membrane on pain and burn wound repair. Comp Clin Path. 2018;27:1457-63.
- [Google Scholar]
- Prevalence of chronic skin wounds and their risk factors in an inpatient hospital setting in Northern China. Adv Skin Wound Care. 2020;33:1-10.
- [Google Scholar]
- Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J Tissue Viability. 2016;25:98-118.
- [Google Scholar]
- Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetol. 2020;57:883-90.
- [Google Scholar]
- Comparison of topical sucralfate and silver sulfadiazine cream in second degree burns in rats. Adv Clin Exp Med. 2013;22:481-7.
- [Google Scholar]
- Accelerating dermal wound healing and mitigating excessive scar formation using LBL modified nanofibrous mats. Mater Des. 2020;185:108265.
- [Google Scholar]
- Royal jelly accelerates healing of acetate induced gastric ulcers in male rats. Gastroenterol Hepatol Bed Bench. 2020;13:14-22.
- [Google Scholar]
- Effects of the oral administration of silver nanoparticles on wound healing in male rats. Wound Repair Regen. 2020;28:8-16.
- [Google Scholar]
- The effect of oral consumption of propolis alone and in combination with silver nanoparticles on wound healing in male Wistar rats. Wound Manag Prev. 2020;66:38-46.
- [Google Scholar]
- The effect of honey-impregnated human placenta membrane on burn wound healing in rat. Comp Clin Path. 2015;24:263-8.
- [Google Scholar]
- Humic acid enhances wound healing in the rat palate. Evid Based Complement Alternat Med. 2018;2018:1783513.
- [Google Scholar]
- Regulation of wound healing by the NRF2 transcription factor—More than cytoprotection. Int J Mol Sci. 2019;20:3856.
- [Google Scholar]
- The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv Wound Care (New Rochelle). 2020;9:184-98.
- [Google Scholar]
- Carbohydrate-derived Fulvic acid (CHD-FA) inhibits carrageenan-induced inflammation and enhances wound healing: Efficacy and toxicity study in rats. Drug Dev Res. 2012;73:18-23.
- [Google Scholar]
- The antiinflammatory properties of humic substances: A mini review. Phytother Res. 2015;29:791-5.
- [Google Scholar]
- Humic acid in drinking well water induces inflammation through reactive oxygen species generation and activation of nuclear factor-κb/activator protein-1 signaling pathways: A possible role in atherosclerosis. Toxicol Appl Pharmacol. 2014;274:249-62.
- [Google Scholar]
- An in vitro investigation of the anti-inflammatory properties of potassium humate. Inflammation. 2004;28:169-74.
- [Google Scholar]
- Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J Diabetes Res. 2018;2018:1-7.
- [Google Scholar]
- Characterization of fulvic acid beverages by mineral profile and antioxidant capacity. Foods. 2019;8:605.
- [Google Scholar]
- Restorative effect of Iranian probiotic bacteria Lactobacillus casei on healing gastric stomach ulcers caused by acetic acid in male Wistar rats. J AnimBiol. 2011;4:35-45.
- [Google Scholar]
- Topical estrogen accelerates wound healing in diabetic rats. Iran J Endocrinol Metab. 2011;12:544-51.
- [Google Scholar]
- Fulvic acid–A natural and multifaceted approach to the management of inflammatory dermatosis. Indian Pract. 2019;72:28-31.
- [Google Scholar]
- Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126-67.
- [Google Scholar]
- Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14:89-96.
- [Google Scholar]
- Effect of fulvic acid on ultraviolet induced skin aging: The effect of fulvic acid on fibroblasts and matrix metalloproteinase. Nishinihon J Dermatol. 2012;74:427-31.
- [Google Scholar]
- Sodium humate accelerates cutaneous wound healing by activating TGF-β/smads signaling pathway in rats. Acta Pharm Sin B. 2016;6:132-40.
- [Google Scholar]
- Carbohydrate-derived fulvic acid is a highly promising topical agent to enhance healing of wounds infected with drug-resistant pathogens. J Trauma Acute Care Surg. 2015;79:S121-9.
- [Google Scholar]
- Comparison of the cytokine and chemokine dynamics of the early inflammatory response in models of burn injury and infection. Cytokine. 2011;55:362-71.
- [Google Scholar]
- Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10:26-37.
- [Google Scholar]
- Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585-601.
- [Google Scholar]






