Translate this page into:
Monotherapy of Biofiller for Atrophic Acne Scars: A Prospective Nonrandomized Study
Address for correspondence: Dr. Balkrishna Pralhadrao Nikam, Department of Dermatology, Krishna Institute of Medical Sciences, Karad, Maharashtra 415110, India. E-mail: mangeshnikam@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Atrophic acne scarring is an unpleasant and often permanent complication and a therapeutic challenge for dermatologists. Platelet-poor plasma (PPP) gel injections are derived from the patient’s own blood and used as a “biofiller” for skin rejuvenation.
Objectives:
The objective was to study the efficacy and safety profile of PPP gel in atrophic acne scars.
Materials and Methods:
Thirty patients with atrophic acne scars were included in the study. Topical anesthesia was applied on the area of interest 45 min prior to the procedure. 20 mL of blood was collected in eight sodium citrate bulbs and centrifuged to get PPP that is coagulated with heat to form gel. This gel (biofiller) was injected in the scarred areas monthly for 6 months. Patients were evaluated using Goodman and Baron Scar (GBS) scale (quantitative and qualitative), Physician Global Assessment, and Visual Analogue Scale (VAS) at each visit. The final visit was after 3 months of the last procedure.
Results:
The mean value of GBS at the first visit was 28, which reduced to 8.2 at the final visit. The analysis of variance test was applied to the quantitative scale from the baseline visit to the final visit. The F value was 462.55 with a P value < 0.0001. The paired t-test was applied for the GBS quantitative scale, which showed a value of 22.86 with a P value of <0.001. Transient local side effects were noted.
Conclusion:
Biofiller is efficacious in improving atrophic acne scars. It is a simple, minimally invasive, cost-effective procedure with no risk of immunogenic reaction.
Keywords
Atrophic acne scars
autologous filler
biofiller
dermal filler
platelet-poor plasma
INTRODUCTION
Atrophic acne scarring is always a therapeutic challenge. Various modalities available for acne scars: device-based treatments such as dermaroller, microneedling radiofrequency, fractional lasers; surgical modalities such as punch excision and suturing, subcision, dermabrasion; and rejuvenation modalities such as autologous fat grafting, autologous dermal grafts, autologous platelet-rich plasma (PRP), injectable platelet-rich fibrin (iPRF), etc. To achieve the volumetric filling of atrophy, methods used are autologous fat, dermal grafts, and synthetic fillers. However, synthetic fillers are costly, and fat grafting is an invasive procedure and needs expertise to harvest the fat. Platelet-poor plasma (PPP) gel (biofiller) is a new development, which has probable collagen remodulation and tissue regeneration activity. The platelets in PPP are less than 104/μL[1] and then processed into a viscous gel before injecting. This is a source of many soluble proteins (growth factors) and platelet factor 3, the activity of which can induce tissue regeneration and collagen remodulation[23] in addition to the immediate volumetric filling up of the atrophic scars. Being autologous in nature, there is no risk of rejection or immunogenic reactions.
MATERIALS AND METHODS
Patients
The aim was to study the efficacy and safety profile of PPP gel in the treatment of atrophic acne scars. The approval from the ethics committee was taken (approval code: 260/2019–2020).
Patients of age 18 years and above with acne scars and no active acne or other skin lesions and who did not receive any other treatment for the same in the past were included in the study. Pregnant and lactating women, patients with keloidal tendency, a history of recurrent herpes simplex infection, any other dermatological, systemic, autoimmune, or bleeding disorders, and patients on anticoagulant drugs and with unrealistic expectations were excluded. This study was conducted at the tertiary hospital as uncontrolled, prospective interventional study. Thirty patients were enrolled in the study after written informed consent. All patients were informed about the complications, risks, and benefits of the treatment before taking the consent. All patients were subjected to complete history-taking, full general and dermatological examination, and routine blood investigation. At each visit, digital photographs using 18.1 megapixel Canon EOS 1200D camera (Taiwan) were taken.
Procedure
For the preparation of PPP gel, 20 mL of venous blood was drawn from each patient under aseptic precautions in eight sterile sodium citrate bulbs. These eight bulbs were centrifuged by REMIR-8C centrifuge (India) at 1500 RPM for 10 min, giving rise to three layers: upper straw-colored plasma, middle fibrin, and lower red cells [Figure 1A]. The plasma from all the eight bulbs were collected in a single sterile tube and centrifuged again at 3000 RPM for another 10 min to get the upper 4–5 mL of PPP [Figure 1B]. The PPP was collected in two lever-lock syringes of 3 mL each without disturbing the platelet clot. These syringes were placed in a hot bath of 80°–100° for 5 min [Figure 1C] till the PPP became opaque signifying gelation and then placed immediately in a cold bath of 0°–6° for another 5 min [Figure 1D]. The PPP was then converted into a viscous gel (biofiller) [Figure 1E].
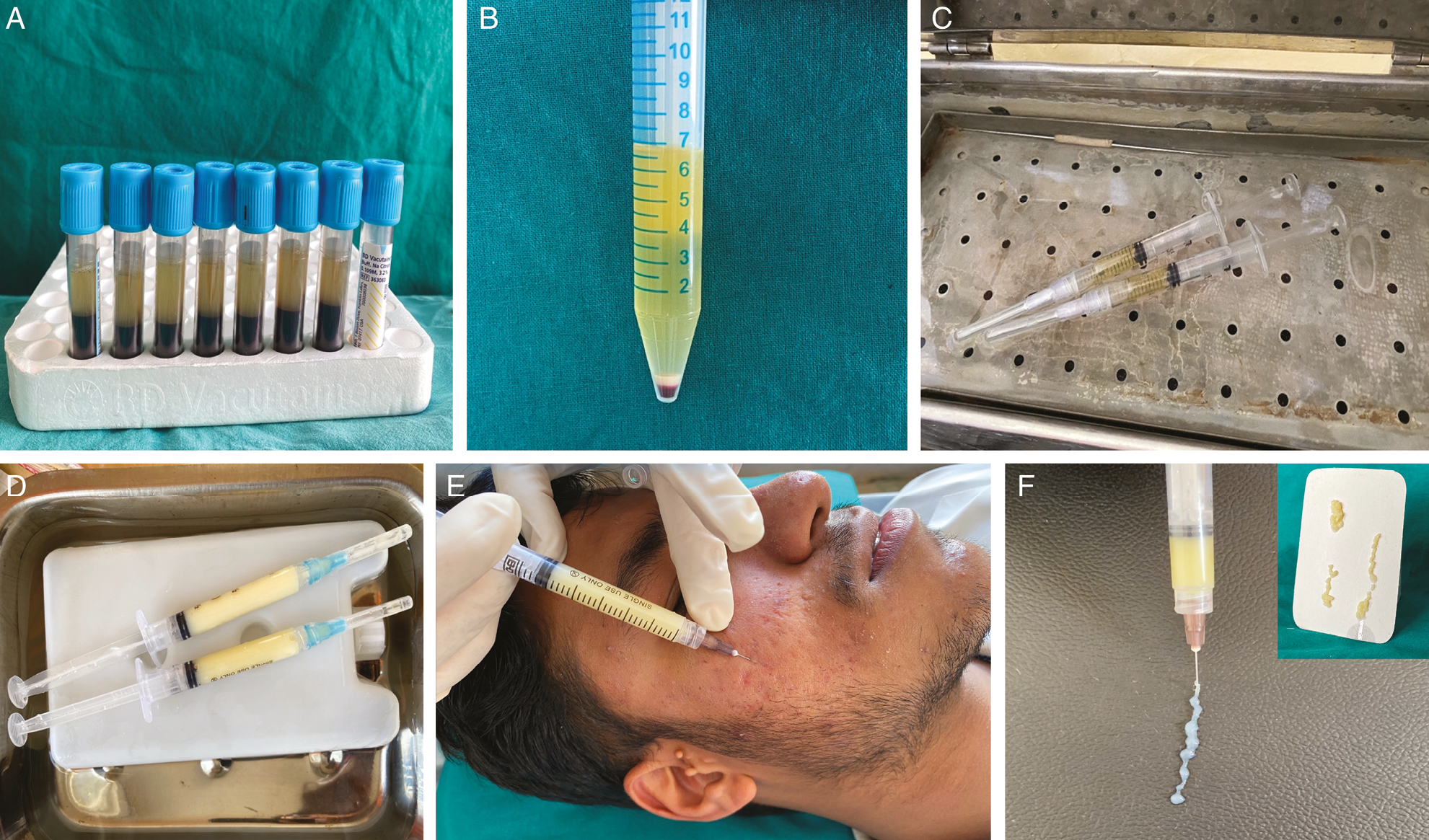
- (A) After the first centrifugation, the plasma separates from the whole blood. (B) After the second centrifugation, we get upper 4–5 mL of PPP and a platelet-rich clot settled. (C) The PPP is heated at 80°–100° for 5 min. (D) Biofiller formed after the heated plasma is placed in a cold bath of 0°–6° for 5 min. (E) Typical semisolid consistency of the biofiller. (F) PPP gel is injected below each scar with to-and-fro motion to deposit the depot and massaged gently
Injection technique
Topical anesthetic, 2.5% prilocaine plus 2.5% lidocaine (EMLA), was applied under occlusion 1 h prior to the procedure over the area of interest. The needle of the lever-lock syringe was changed to 26½ number needle. The treatment area was cleaned with spirit and povidone-iodine, and then the scars were marked with a skin marker. The needle was inserted 1–2 mm proximal to the scar at 30°–45° angle with the pointed end facing downward. The filler was injected under each marked scar at the level of the dermo–hypodermal junction and the needle was withdrawn slowly while injecting the filler till the exit point to achieve uniform filling of the scar [Figure 1F]. The area was gently massaged to maintain the contour of the surrounding tissue. The patient was advised not to use ice packs because it may interfere with the platelet function and avoid applying pressure on the treated area for a week. The procedure was repeated monthly for 6 months.
Clinical assessment and analysis
Patients were evaluated at each visit and 3 months after the last procedure.
Grading of the scars was done using Goodman and Baron scar (GBS) scale of both qualitative and quantitative [Tables 1 and 2].[45]
| Level of disease | Characteristics | Examples |
|---|---|---|
| Macular disease | Erythematous, hyper- or hypopigmented flat marks visible to the patient or observer irrespective of distance | Erythematous, hyper- or hypopigmented flat marks |
| Mild disease | Mild atrophy or hypertrophy that may not be obvious at social distances of 50 cm or greater and may be covered adequately by make-up or the normal shadow of shaved beard hair in males or normal body hair if extrafacial | Mild rolling, small, soft papular scars |
| Moderate disease | Moderate atrophic or hypertrophic scarring that is obvious at social distances of 50 cm or greater and is not covered easily by make-up or the normal shadow of shaved beard hair but is still able to be flattened by manual stretching of the skin | More significant rolling, shallow “boxscar,” mild-to-moderate hypertrophic or papular scars |
| Severe disease | Severe atrophic or hypertrophic scarring that is obvious at social distances of 50 cm or greater and is not covered easily by make-up or the normal shadow of shaved beard hair in males or body hair (if extrafacial) and is not able to be flattened by manual stretching of the skin | Punched out atrophic deep “boxscar,” “ice pick,” bridges and tunnels, gross atrophy, dystrophic scars significant hypertrophy or keloid |
Source: Goodman GJ, Baron JA. Postacne scarring: A qualitative global scarring grading system. Dermatol Surg[4]
| Grade or type | Number of lesions 1 (1–10) | Number of lesions 2 (11–20) | Number of lesions 3 (>20) |
|---|---|---|---|
| (A) Mild scarring (1 point each) | 1 point | 2 points | 3 points |
| Macular erythematous or pigmented | - | - | - |
| Mild atrophic dish-like | - | 2 | - |
| (B) Moderate scarring (2 points each) | 2 points | 4 points | 6 points |
| Moderately atrophic dish-like | - | - | 6 |
| Punched out with shallow bases, small scars (<5 mm) | - | - | 6 |
| Shallow but broad atrophic areas | - | 4 | - |
| (C) Severe scarring (3 points each) | 3 points | 6 points | 9 points |
| Punched out with deep but normal bases, small scars (<5 mm) | 3 | - | - |
| Punched out with deep abnormal bases, small scars (<5 mm) | - | - | - |
| Linear or troughed dermal scarring | - | 6 | - |
| Deep broad atrophic areas | 3 | - | - |
| (D) Hyperplastic | 2 points | 4 points | 6 points |
| Papular scars | - | - | - |
| (D) Hyperplastic | Area: <5 cm2 | Area: 5–20 cm2 | Area: >20 cm2 |
| 6 points | 12 points | 18 points | |
| (E) Keloidal/hypertrophic scars | - | - | - |
| 6 points | 12 points | 18 points |
Source: Goodman GJ, Baron JA. Postacne scarring: A quantitative global scarring grading system. J Cosmet Dermatol[5]
Physician Global Assessment (PGA) was done using four grades.[6] Grade 1 is almost clear, grade 2 is given to mild scars, grade 3 for moderate scars, and grade 4 for severe scars.
Visual Analogue Scale (VAS)[7] was graded as grade 0 (not satisfied), slightly satisfied (grade 1), very satisfied (grade 2), and extremely satisfied (grade 3). At the first visit, all the patients were given grade 3.
Analysis methods used were the analysis of variance (ANOVA) test and paired t test applied for GBS quantitative scales.
RESULTS
Among 30 patients of acne scars, 17 patients (56.6%) were males and 13 patients (43.3%) were females. Mean age was 26 years.
Scars of all patients were assessed at every visit using GBS scale (qualitative and quantitative), PGA, and VAS.
GBS qualitative grading system showed a gradual reduction in grades of scars at each visit [Table 3]. Based on the GBS qualitative grading, we observed that at the first visit, eight patients (26.6%) had severe scars, 13 patients (43.3%) had moderate scars, and nine patients (30%) had mild scars. After 3 months of the last treatment, i.e., at the final visit, three patients (10%) belonged to severe scars (grade 4), eight patients (26.6%) belonged to moderate scars (grade 3), and 11 (36.6%) had mild scars (grade 2). At the baseline, no patients were in grade 1 scar severity, whereas the final visit showed eight patients reduced to macular scar (grade 1) severity.
| First visit | Seventh visit | ||||
|---|---|---|---|---|---|
| Grade of acne scars | Number of patients | Scars reduced by three grades (%) | Scars reduced by two grades (%) | Scars reduced by one grade (%) | No reduction in scars (%) |
| Grade 4 | 8 | 1 (12.5) | 4 (50.0) | 3 (37.5) | 0 (0) |
| Grade 3 | 13 | 0 (0) | 8 (61.53) | 5 (38.46) | 0 (0) |
| Grade 2 | 9 | 0 (0) | 0 (0) | 8 (88.88) | 1 (11.11) |
The GBS quantitative scale was used to assess the acne scars at each visit. The mean value of the first visit was found to be 28, which reduced to 8.2 by the end of the last visit. The ANOVA test was applied for the mean scale from the baseline visit to the final visit. The high F value and very low P value showed an intergroup significance in all visits. There is a significant reduction in the mean GBS scales at each visit. The paired t-test was applied for the mean GBS quantitative scale for the first and last visit, which showed statistically significant difference suggesting high efficacy of the biofiller [Table 4].
| Visits | Mean value | Standard deviation |
|---|---|---|
| First | 28 | 3.3 |
| Second | 23.7 | 2.8 |
| Third | 19.7 | 3.2 |
| Fourth | 16.7 | 3.3 |
| Fifth | 13.7 | 2.9 |
| Sixth | 10.9 | 2.9 |
| Seventh | 8.2 | 3.3 |
| ANOVA | F value = 462.55 | P value < 0.0001 |
| Paired t-test | F value = 22.86 | P value < 0.001 |
The assessment of improvement in the GBS quantitative scales was further considered as minimal, moderate, good, and very good depending on the reduction of acne scars scores [Table 5]. A total of 13 patients showed a good to very good improvement [Figures 2,3–4].
| Grades | Number of patients (%) | Improvement |
|---|---|---|
| 0–5 | 3 (10) | Minimal reduction in GBS scores |
| 5–10 | 14 (46.66) | Moderate reduction in GBS scores |
| 10–15 | 8 (26.66) | Good reduction in GBS scores |
| >15 | 5 (16.66) | Very good reduction in GBS scores |
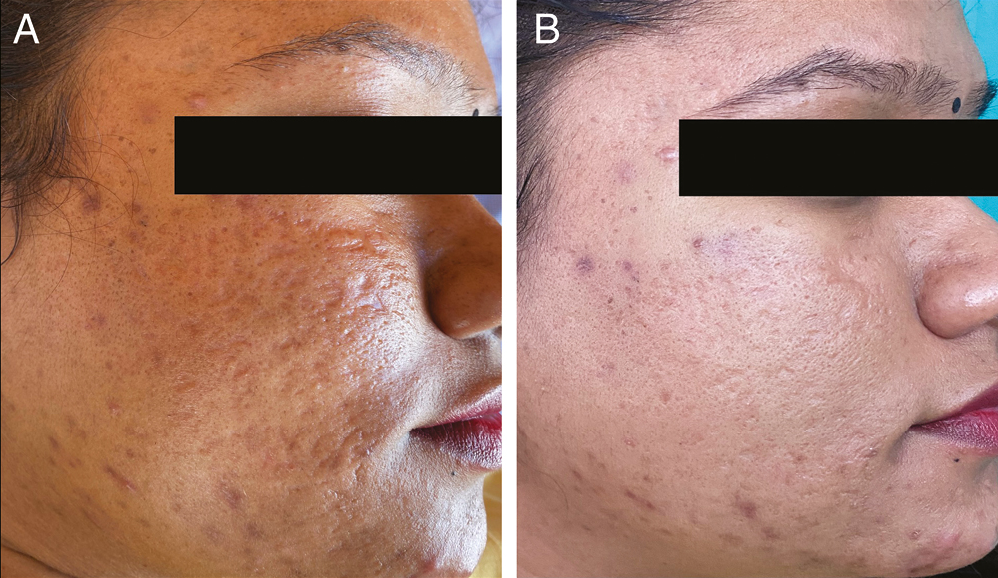
- (A) Pretreatment photograph of the patient showing acne scars of GBS grade 4, GBS scale 28, PGA-grade 4, and VAS-0. (B) At the seventh visit, GBS grade 2, scale reduced to 8, PGA-grade 2, and VAS-3 (extremely satisfied)
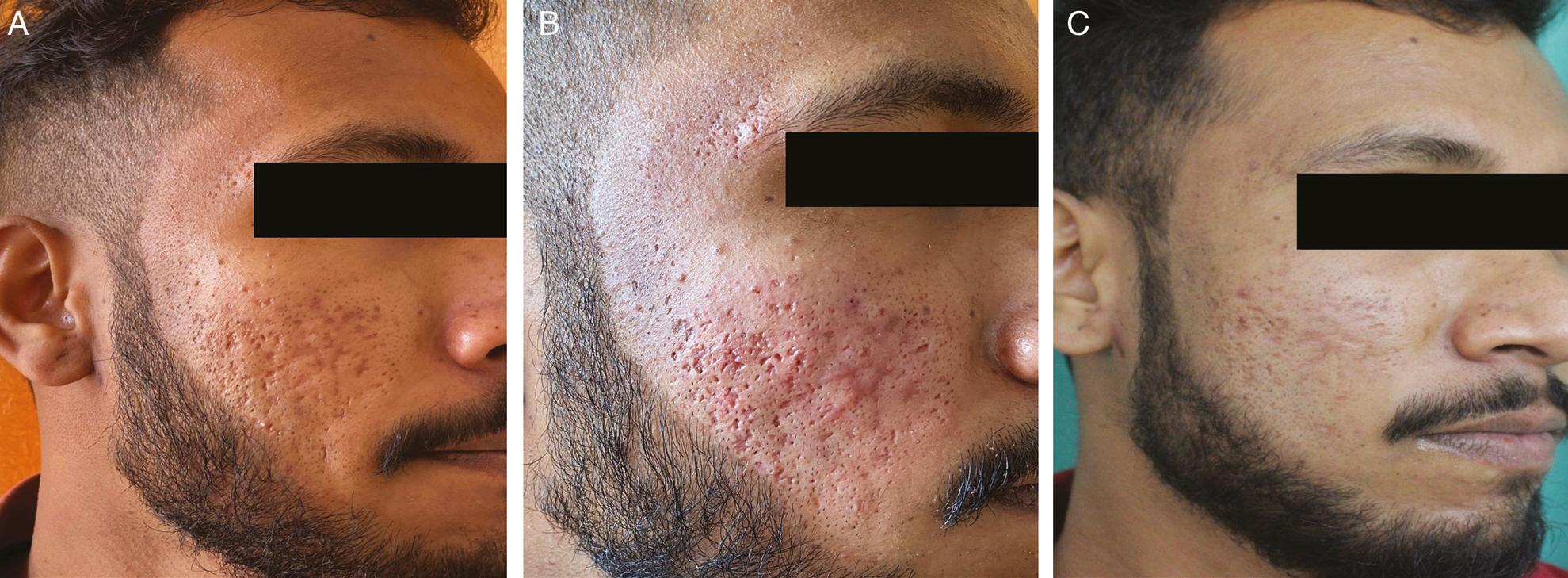
- (A) Pretreatment photograph of the patient showing acne scars of GBS grade 3, GBS scale 24, PGA-grade 3, and VAS-0. (B) Immediate volumetric effect and transient erythema. (C) At the seventh visit, GBS grade 2, scale reduced to 10, PGA-grade 2, and VAS-3 (extremely satisfied)
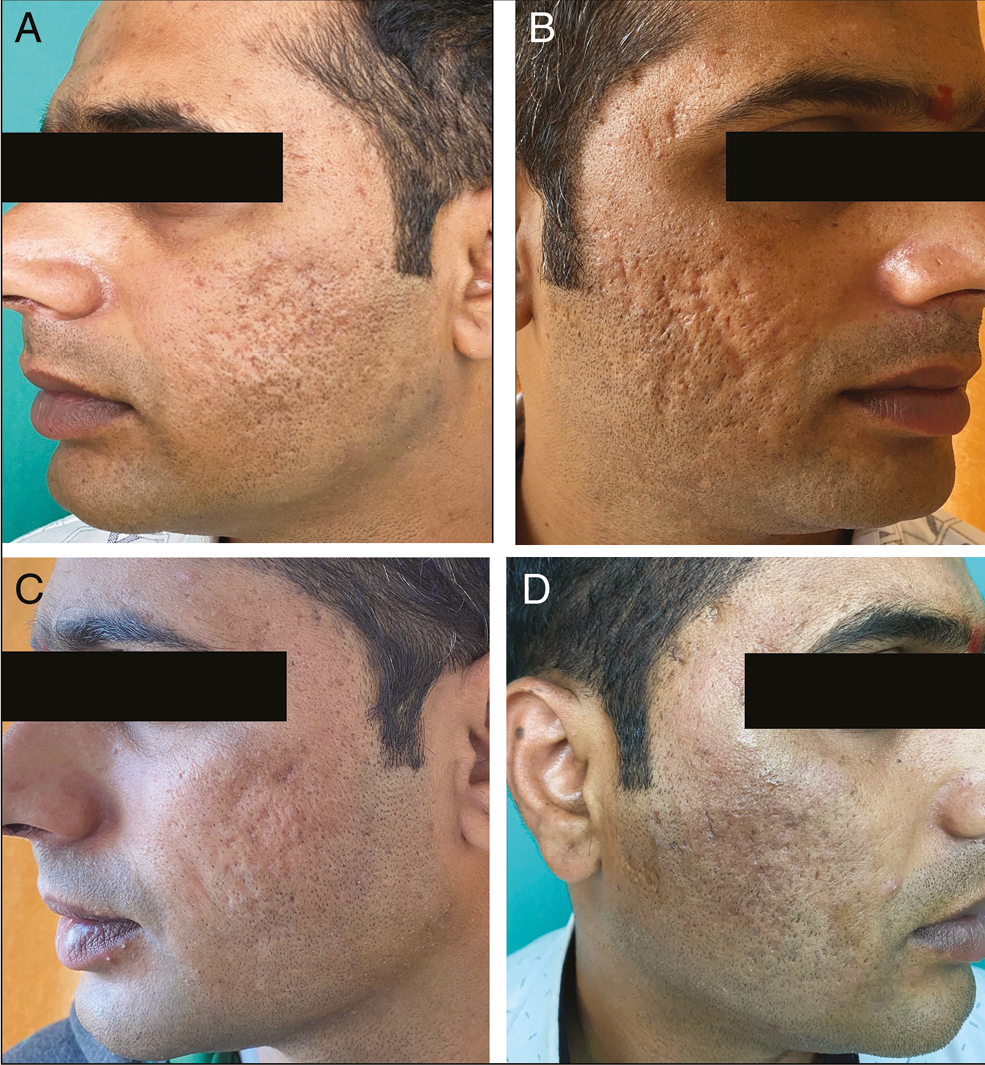
- (A) and (B) Both cheeks pretreatment photograph of the patient showing acne scars of GBS grade 4, GBS scale 26, PGA-grade 4, and VAS-0. (C) Mild postinflammatory hyperpigmentation on the right cheek. (D) At the sixth visit, GBS grade 2, scale reduced to 7, PGA-grade 2, and VAS-3 (extremely satisfied)
At the baseline visit, PGA showed zero patients in grade 1, five patients were labeled as grade 2, 12 had grade 3, and 13 patients had grade 4. By the seventh visit, 10 patients (33.33%) had scars that were almost clear (grade 1), 16 patients (53.33%) had mild scars (grade 2), and only four patients (13.33%) had moderate scars (grade 3).
The patients were asked to self-assess their scars based on the VAS at each visit. All the patients were given grade 0 (not satisfied) at the start of the study. At the seventh visit, we observed three patients (10%) were not satisfied (grade 0), 11 patients (36.6%) were slightly satisfied (grade 1), 13 patients (43.3%) were very satisfied (grade 2), and three patients (10%) were extremely satisfied (grade 3).
Side effects noted were transient erythema [Figure 3B] seen in 22 patients (73.33%), transient edema in 14 patients (46.6%), and transient pain in 16 patients (53.3%). It disappeared within 12–24 h of the procedure. Postinflammatory hyperpigmentation was seen in two patients [Figure 4C] and resolved within month. No permanent side effects were noted in any of the patients. No patient reported any fibrosis, irregularity, hardness, or lumpiness at the injection site at any time.
DISCUSSION
The current study evaluated the efficacy and safety profile of injectable PPP gel (biofiller) in atrophic acne scars. Autologous plasma was used in the past in dermal filler called fibrel[8] (a denatured porcine collagen that is reconstructed with the patient’s plasma) and was approved by the US Food and Drug Administration for the treatment of depressed wrinkles in 1990. However, its high cost and the risk of developing immune reaction due to the xenogeneic origin of the product were restrictions of its use.
Man[9] in the year 2000 used autologous PRP gel and PPP “glue” for hemostasis of capillary bleeding in various flap and cosmetic surgeries. The author mixed PPP in thrombin chloride solution to prepare fibrin glue. In recent past years, PPP gel has been used by otorhinolaryngologist, orthopedicians, and oral surgeons as a biofiller, hemostats, as well as for postoperative rapid healing and pain relief. Woo et al.,[10] in 2013, used PPP gel in the treatment of vocal cord palsy, and his study showed the autologous plasma gel remained in situ for 6 months in animals with minimal inflammation. The clinical study showed that vocal cord palsy was well compensated for with the plasma gel in all patients at 2 months after injection with no significant complications.
Doghaim et al.[11] in the year 2018 studied PPP gel as an autologous dermal filler for facial rejuvenation. The authors treated 52 female patients with facial wrinkles (group A) and tear-trough deformities (group B) with two sessions of plasma gel injections 2 weeks apart and followed up for 3 months. They showed an immediate significant clinical improvement after the plasma gel injections and was maintained till the end of follow-up period of 3 months. This finding was confirmed by a significant reduction in the mean values of Wrinkle Severity Rating Scale in group A and Tear Trough Rating Scale in group B, and a significant improvement of skin homogeneity and texture in both groups. The authors reported few side effects of plasma gel injection such as transient minor pain, burning sensation, erythema, and edema at the site of injection, which disappeared spontaneously within few hours. Gupta in 2020 used PPP gel as a biofiller instead of synthetic filler in a 52-year-old woman with a mid-facial volume loss and nasolabial fold correction.[12] PPP gel is also used in chicken pox scars and atrophic and rolling acne scars anecdotally.[13]
Although biofiller’s volume retention reported is 3 months, its rejuvenation effects persist longer. Recently PPP gel is gaining popularity in rejuvenation over PRP.
Neinaa et al. in the year 2020[14] studied PPP gel versus PRP for infraorbital rejuvenation and concluded that a significant reduction of the degree of hyperpigmentation and mean values of tear trough rating scale in response to PPP gel injection than to PRP injection. Moreover, the degree of clinical and dermoscopic improvements, skin texture, and patients satisfaction were more statistically significant in response to PPP gel injection than to PRP injection. Thus, PPP gel has advantages of both volumization and rejuvenation, which can be used in atrophic acne scars.
Nashwa et al.[15] studied the effects of plasma gel in 60 patients of atrophic acne scars. It was a randomized controlled study in which three groups of 20 patients each were treated with intradermal PPP gel injections, dermaroller, and a combination of both dermaroller with PPP gel monthly for 4 months. The authors observed the efficacy of PPP gel alone reduced at 3 months after the last procedure. In the current study, PPP gel was given monthly for 6 months and found persistent results in acne scar rejuvenation even after 3 months. This suggests the repeated sessions might be necessary for the longevity of results.
Further, posttreatment histopathological analysis done by Nashwa et al. in acne scars and Gad et al.[16] in striae distensae showed a significant improvement in collagen bundles thickness, condensation, and orientation. PPP gel at the site of the injection induces a slight inflammatory response without producing tissue necrosis and granuloma hypersensitivity reaction, suggesting tolerability and biocompatibility of the plasma gel. PPP gel injection increased the content and distribution of the newly formed collagen and elastic fibers giving the strength to the extracellular matrix. At the end of the treatment, newly formed collagen bundles became more organized, dense, and parallel to epidermis.
Shahidi et al.[17] conducted a comparative study between PRP and PPP effects on angiogenesis, which demonstrated that PPP could promote endothelial cell alteration to angiogenic cells in human vascular endothelial cell culture and in vivo experiments. In contrast, an inhibitory effect of PRP on vascular endothelial growth factor receptor 2 expression by endothelial cells was evidenced. Therefore, the data might propose a greater angiogenic effect of PPP, when compared with PRP, suggesting PPP as a more potential activator in this regard.
The advantage of PPP gel over PRP or iPRF is immediate volumetric filling effect. Hatakeyama et al.[2] evaluated the ultrastructural morphology and components of both PRP and PPP and found that although the concentration of platelets and their growth factors in the PRP is much higher than in PPP, fibrinogen concentration in PPP is much higher compared with PRP. The fibrin fibers are usually formed in bundles in PPP unlike in PRP. The direct volumetric filling effect of the gel is due to the denaturized gelled proteins and fibrin bundles, providing constant stability and volume.[3] This fibrous network of insoluble fibrin provides a scaffold for platelets that serve as a source for the sustained release of growth factors.[18] This scaffolding helps localize the growth factors and increase their concentration at the desired location to facilitate tissue regeneration.[11] These growth factors interact with the undifferentiated adipose-derived stem cells and dermal fibroblasts by binding to their specific cellular receptors, promoting neovascularization and neocollagenesis, resulting in a soft-tissue augmentation and reduction of wrinkles.[19] It is suggested that the continual release of growth factors from the trapped platelets at the injection site may be responsible for the sustained therapeutic effects of plasma gel for several months after the treatment session.[2] These growth factors also enhance the synthesis of extracellular matrix components such as hyaluronic acid. The contraction of myofibroblasts around wrinkles causes skin tightening and strengthening.[20]
Pigmentary improvement was noted too, which may be due to the increase in skin volume after plasma gel injection, in addition to the inhibitory effect of transforming growth factor-β1 on melanogenesis via delayed extracellular signal-regulated kinase activation.[17] PPP gel has a reduced postinflammatory hyperpigmentation of CO2 laser in the treatment of striae distensae.[16]
The limitations of the current study are that it is not a comparative study, and the follow-up period is short (9 months).
CONCLUSION
PPP gel injection as a dermal filler is a cost-effective, well-tolerated, and very simple nonsurgical clinic procedure. It is an autologous material easily obtained from the patient’s own blood, and therefore risks of immunogenic reactions or disease transmission are eliminated. The viscous plasma gel gives an immediate significant volumetric filling effect, which can be maintained for more than 3 months after repeated injections and show with sustained rejuvenation effects. Although this method needs further validations, initial results are encouraging and promising. Further studies on bigger study population and longer duration of follow-up, in addition to comparative studies with other types of popular dermal fillers, are required.
Ethical approval
The ethical approval code was 260/2019–2020 dated January 24, 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Five-minute preparation of platelet-poor plasma for routine coagulation testing. East Mediterr Health J. 2010;16:233-6.
- [Google Scholar]
- Effects of platelet-poor plasma, platelet-rich plasma, and platelet-rich fibrin on healing of extraction sockets with buccal dehiscence in dogs. Tissue Eng Part A. 2014;20:874-82.
- [Google Scholar]
- Safety, efficacy, and utility of platelet-rich fibrin matrix in facial plastic surgery. Arch Facial Plast Surg. 2011;13:247-51.
- [Google Scholar]
- Postacne scarring: A qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-66.
- [Google Scholar]
- Postacne scarring: A quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- [Google Scholar]
- Using the physician global assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151:375-81.
- [Google Scholar]
- Visual analogue scale scoring and ranking: A suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118:909-18.
- [Google Scholar]
- The fibrel mechanism of action study. A preliminary report. J Dermatol Surg Oncol. 1994;20:586-90.
- [Google Scholar]
- The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229-37; discussion 238-9.
- [Google Scholar]
- Autologous platelet-poor plasma gel for injection laryngoplasty. Yonsei Med J. 2013;54:1516-23.
- [Google Scholar]
- Combination of fractional carbon dioxide laser with narrow band ultraviolet B to induce repigmentation in stable vitiligo: A comparative study. J Cosmet Dermatol. 2019;18:142-9.
- [Google Scholar]
- Bio-filler: An effective facial rejuvenation tool-easy on pocket. J Cutan Aesthet Surg. 2020;13:243-6.
- [Google Scholar]
- Platelet-poor plasma-based biofiller: An innovative alternative to expensive hyaluronic acid-based fillers for treatment of chicken pox scars. J Am Acad Dermatol. 2021;84:e11-3.
- [Google Scholar]
- Platelet-poor plasma gel vs platelet-rich plasma for infraorbital rejuvenation: A clinical and dermoscopic comparative study. Dermatol Ther. 2020;33:e14255.
- [Google Scholar]
- Efficacy and safety of plasma gel as a new modality in treatment of atrophic acne scars. Int J Dermatol. 2020;59:620-6.
- [Google Scholar]
- Efficacy of platelet-poor plasma gel in combination with fractional CO2 laser in striae distensae: A clinical, histological, and immunohistochemical study. J Cosmet Dermatol. 2021;20:3236-44.
- [Google Scholar]
- A comparative study between platelet-rich plasma and platelet-poor plasma effects on angiogenesis. Med Mol Morphol. 2018;51:21-31.
- [Google Scholar]
- Flow cytometric and morphological characterization of platelet-rich plasma gel. Clin Oral Implants Res. 2006;17:687-93.
- [Google Scholar]
- Efficacy of platelet rich plasma (PRP) on skin rejuvenation: A systematic review. Iran J Dermatol. 2015;18:119-27.
- [Google Scholar]






