Translate this page into:
Low-Level Laser and Bovine Amniotic Fluid-derived Cream Accelerating Skin Neck Wound Healing and Reducing Inflammation and Wound Scar in a Rat Animal Model
Address for correspondence: Dr. Hossein Abdali, Department of Surgery, School of Medicine, Esfahan University of Medical Sciences, Esfahan, Iran. E-mail: abdali@med.mui.ac.ir
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Nowadays, wound healing is one of the main problems of patients. Therefore, extensive research is underway to discover mechanisms associated with non-scarring of wounds. Using amniotic fluid and laser may potentially play a key role in wound healing and scar reduction due to its presence in tissue growth and repair agents.
Aim:
The present study evaluated the effect of bovine amniotic fluid (BAF)-derived cream and low-power laser (LPL) on accelerating skin wound healing and reducing scarring in an animal model.
Materials and Methods:
Therefore, 72 male Wistar rats were randomly divided into three groups (each group: 24). A wound 6 mm in diameter was then inflicted on the rats’ backs. In the first group that was the control group, the wound was only used. Moreover, BAF was implemented for the second group, and in the third group, LPL radiation was utilized. On the 1st, 3rd, 5th, 14th, and 21st days, the healing condition of the wound and scar created were examined.
Results:
Hence, evaluation of wound healing status on days 5 and 14 showed that the wound healing scale in the BAF group and LPL group was significantly better than that of the control group. On the 21st day, the average Scar Scoring Scale in the BAF and LPL groups was significantly lower than that of the control group. Histological images showed a significant repair in the LPL and BAF groups.
Conclusion:
To conclude, considering the positive effect of LPL and BAF on wound healing and less scarring, it seems that LPL and BAF can heal wounds faster. Moreover, they can be used to prevent scarring after wound healing.
Keywords
Amniotic fluid
low-power laser
scar
wound healing
INTRODUCTION
The skin is an effective barrier against adverse external conditions and has properties that can prevent microbial, mechanical, chemical, osmotic, and thermal damage.[12] Wound healing is a complex and dynamic process that affects the quality of life during recovery. It leads to high costs for the health system worldwide.[3] Adverse wound healing, especially in exposed areas of the body, is not attractive in appearance; however, it predisposes a person to tissue infection, necrosis, and other severe consequences. Scar formation and delay in wound healing are the main challenges in skin tissue trauma treatment.[4] Scar formation is caused by increased fibroblast activity during the wound healing process. When a wound forms, the epidermis thickens, collagen and glycoprotein deposition increase, and collagen fibers become thicker than normal and parallel to the epithelium. Higher levels of β1-TGF, I-Col, III-Col, fibronectin, and SMA-α have been observed in wounds, compared with normal skin.[5] Despite advances in wound healing, the complexity of this process remains a significant clinical barrier. Some common treatments used to reduce or eliminate scars include surgery, corticosteroid injections, radiation therapy, topical silicone gel, and interferon injections. Due to the unknown mechanism of scar formation, treatment has been unsuccessful in most cases, so they have encountered various side effects.[6] Some substances with specific conditions such as tissue oxygenation, small blood vessels, age, underlying diseases, decrease or increase in nutritional status, or drug interactions can reduce or increase the time required for tissue repair by affecting each step in the wound healing process.[7] One of the substances that possibly contain a variety of molecules that may be involved in wound healing and scarring is amniotic fluid.[8] This substance has been used in the healing of bone lesions. It has also been effective in healing damage to peripheral nerves and tendons. The effect of amniotic fluid on wound healing in the fetus has also been studied, and the presence of hyaluronic acid has been suggested as a possible reason.[9] In addition, the presence of stem cells in this fluid indicates the richness of the substances in it for cell growth. The advantages of this material are easy to access and low cost of its preparation, which can be used to repair skin wounds if it proves its effectiveness in accelerating the wound healing process and reducing the resulting scar.[10] Low-power laser (LPL) has recently been used to treat skin wounds, and positive results have been obtained. Due to the importance of this physiological process and to weave new factors affecting this process, by conducting this experimental study, the effect of LPL and bovine amniotic fluid (BAF) in each stage of tissue repair in the animal model will be studied.
MATERIALS AND METHODS
Amniotic fluid preparation
Amniotic fluid was prepared from a pregnant cow in the 20th week after pregnancy using a veterinarian. In addition, the cow underwent veterinary examinations for no specific disease (tuberculosis or brucellosis).
Experimental groups
In this study, 72 male Wistar rats weighing 200–250 g were randomly divided into three groups (each group: 24) and kept in temperature-controlled units and 12-h light–dark cycle and standard feeding in the nest, after anesthesia with intravenous intraperitoneal injection of ketamine/xylocaine, and hair removal in the lumbar region was made with a skin puncture of the wound with a diameter of 6 mm. After examining and observing the wound area for infection and bleeding, the closed containers were used on different days to maintain sterile conditions. On the 1st, 3rd, 5th, 14th, and 25th days, a group of LPL (diode laser, 810 nm, 60 s, 4 J/cm2)[11] and a group of BAF rats were sacrificed according to the standard method, and skin sampling was performed on the wound area.
Sampling and analysis
Samples from the 1st, 3rd, and 5th days after wounding were counted in five microscopic fields: in terms of inflammatory cells and fibroblasts, neutrophils on the first day; macrophages and lymphocytes on the third day; and lymphocytes and fibroblasts on the 5th and 15th days, respectively. The standard deviation of each percentage was calculated. Also, to examine the scar tissue in the samples related to the 5th day, a microscopic field count was performed. Besides, its mean and standard deviation were calculated. To evaluate the collagen accumulation in the samples related to the 14th day, pathology analysis was carried out using Image Analysis Software (the pathology analysis software registered in the Supreme Informatics Council of Iran affiliated to President No. 102543). Wound healing scores were evaluated in samples related to days 5, 14, and 21 by 3 physicians without knowing the control, LPL, and BAF groups, according to Krasner scoring. Finally, in the samples for the 21st day, the Oscar scoring criteria (20 and 16) were evaluated according to the Vancouver Burn Scar Scoring [Table 1].
| Condition of sore bed | Epithelialization | Ready for autograft | Infection | Depth | Area | Score |
|---|---|---|---|---|---|---|
| Healed | Complete | — | — | — | — | 2 |
| Excellent | Well in minimum time | Yes | No | Decreased | Decreased | 2 |
| Good | Good | No | No | Unchanged | Unchanged | 1 |
| Bad | Has not done well | No | Yes | Increased | Increased | 0 |
Histological examination
Wound sampling was performed after sacrificing mice. First, the samples were fixed in 10% formalin for fixation. The tissues were then fixed, and 5-µm sections were prepared from the skin. Hematoxylin and eosin staining was finally performed.
Real-time PCR
In this assay, gene RT-qPCR was performed on samples PE (Applied Biosystems, CA, USA). For a whole volume of 20 μL, the sample reactions contain SYBR Green PCR Master Mix (TaKaRa), cDNA template, primer forward and reverse, and distilled water. The level of relative gene (Bax, Bcl2, Caspase 3, GAPDH) expression was measured using the ΔΔCq method.[1213] Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize gene expression. The primers sequence used are listed as follows in Table 2.
| Gene name | Product size | Sequence | Accession no. |
|---|---|---|---|
| GAPDH | 175 | GTCTCCTCTGACTTCAACAGCG ACCACCCTGTTGCTGTAGCCAA |
NM_001256799.3 |
| BAX | 210 | TCAGGATGCGTCCACCAAGAAG TGTGTCCACGGCGGCAATCATC |
NM_001291428.2 |
| BCL2 | 194 | ATCGCCCTGTGGATGACTGAGT GCCAGGAGAAATCAAACAGAGGC |
NM_000633.3 |
| CASPASE3 | 220 | GGAAGCGAATCAATGGACTCTGG GCATCGACATCTGTACCAGACC |
NM_004346.4 |
Statistical analysis
After collecting and editing, the data were entered into a computer and analyzed by SPSS software version 24. The following tests were implemented to analyze the data. Student’s t-test to compare between quantitative data of two samples, the χ2 test to compare between qualitative data, and the Mann–Whitney test for comparison between ranking data were utilized.
RESULTS
In the present study, 72 male rats were selected and randomly assigned to three groups. Then, a 6 mm wound was made on the back of the mice, no treatment was given for the control group, and 1 mL of BAF was applied topically for the experimental group. Then, on the 1st and 3rd days, the type and severity of inflammation were evaluated. On the 5th day, the type and severity of inflammation were re-evaluated. In addition, fibroblast, angiogenesis, and wound healing scale were evaluated. On the 14th day, collagen accumulation was examined, and the wound healing scale was re-evaluated. Finally, on the 21st day, the scar storing scale was evaluated.
Inflammation
On the 1st day, the severity of inflammation was evaluated in the LPL, BAF, and control groups, in which for 4 samples the severity of inflammation was +4 and for 2 samples it was +3. If in the control group all six samples had inflammation of +3 and according to the Whitney test, the severity of inflammation on the 1st day was significantly different in the experimental and control groups (P = 0.019). On the 1st day, the type of inflammation was acute in all the samples and there was no case of subacute or chronic inflammation. The results are shown in Figure 1.
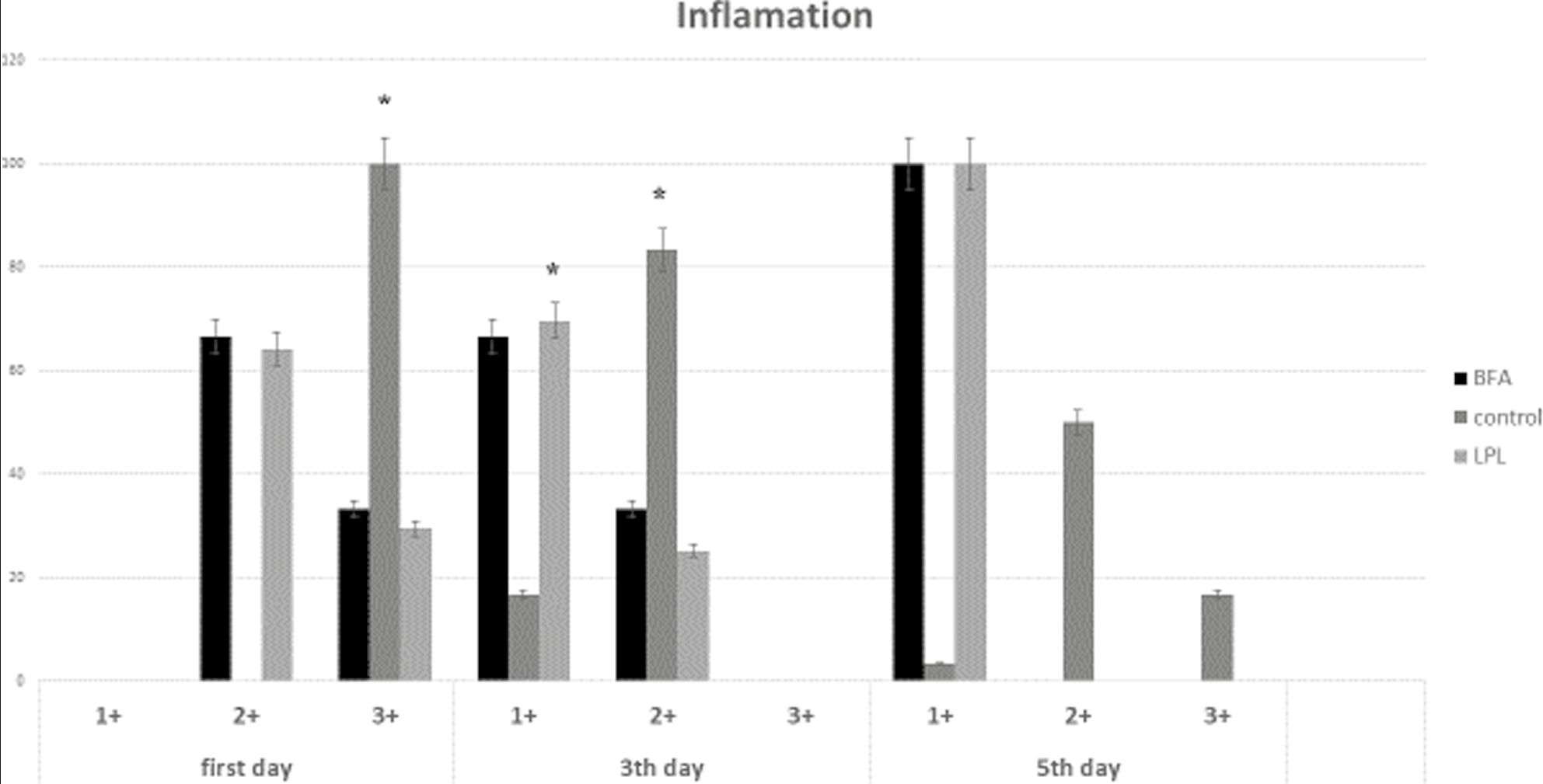
- Frequency distribution of inflammation intensity on the 1st day in control and experimental groups (*P < 0.05)
On the 3rd day, in LPL, BAF, and control groups, inflammation observed was +1 and also +2. In addition, there was no case of severity of inflammation +3 in any of the samples. The Mann–Whitney statistics on these data showed that the severity of inflammation on the 3rd day was not significantly different between the two groups (P = 0.093). On the 3rd day, some samples of the BAF and LPA groups had subacute inflammation (66.7%) and some had chronic inflammation (33.3%). There was also no case of acute inflammation in any of the samples [Figure 2]. On the 5th day, all samples in the LPL and BAF groups had +1 inflammations, whereas in the control group, two samples had +1 inflammation, three samples had +2 inflammation, and one case had inflammation of +3. The Mann–Whitney test also showed that the severity of inflammation on the 5th day in the LPL group was significantly lower (P = 0.021). On the 5th day, there were no samples with acute inflammation in the experimental groups, but in the control group, one sample had acute inflammation. Also, on the fifth day, there was no case of subacute inflammation, whereas in the control group, three samples had subacute inflammation. All samples in the LPL and BAF groups had chronic inflammation, but in the control group, only two samples had chronic inflammation [Figure 3].
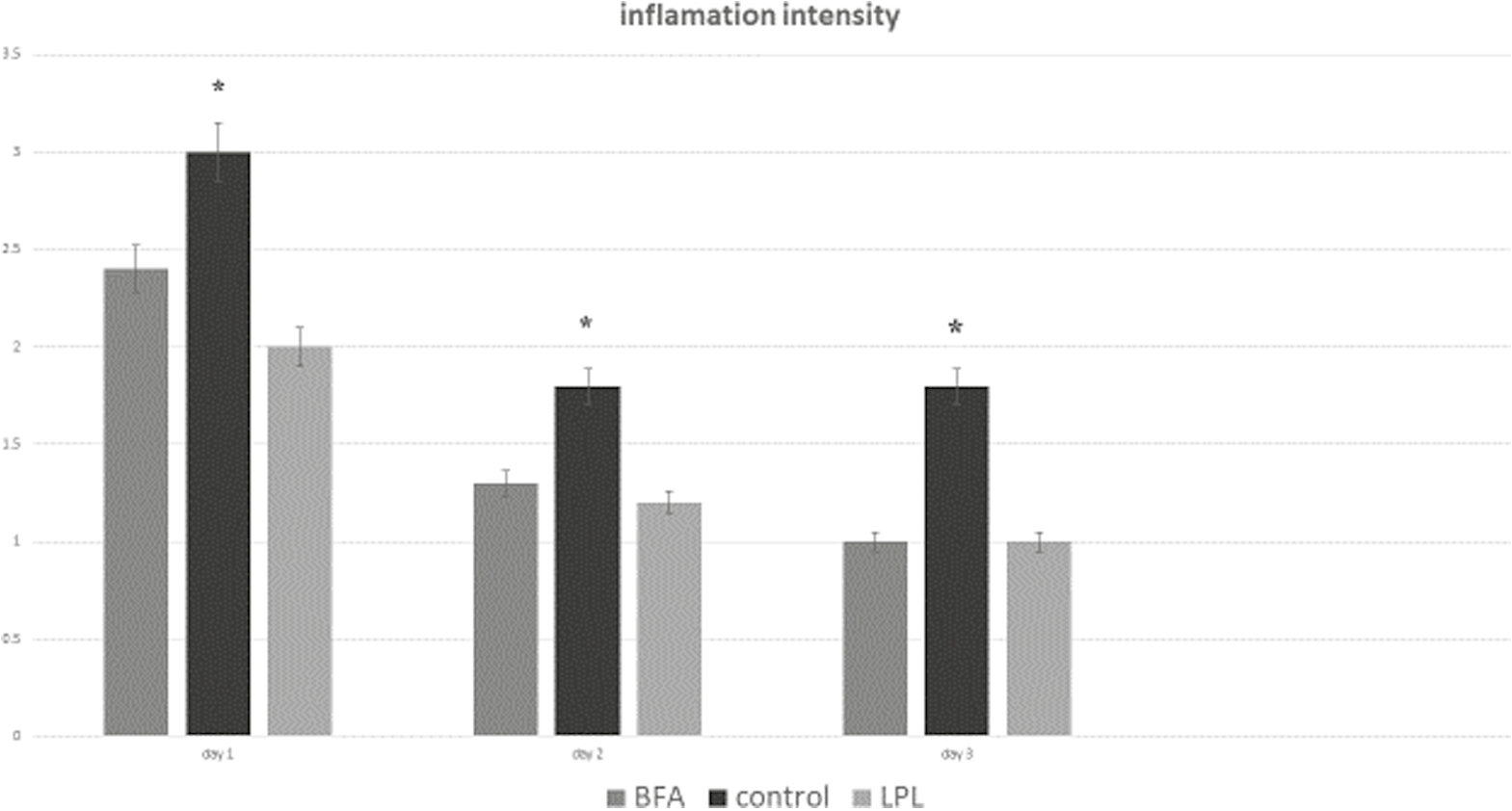
- Frequency distribution of inflammation intensity on the 1st, 2nd, and 3rd days in control and experimental groups (*P < 0.05)
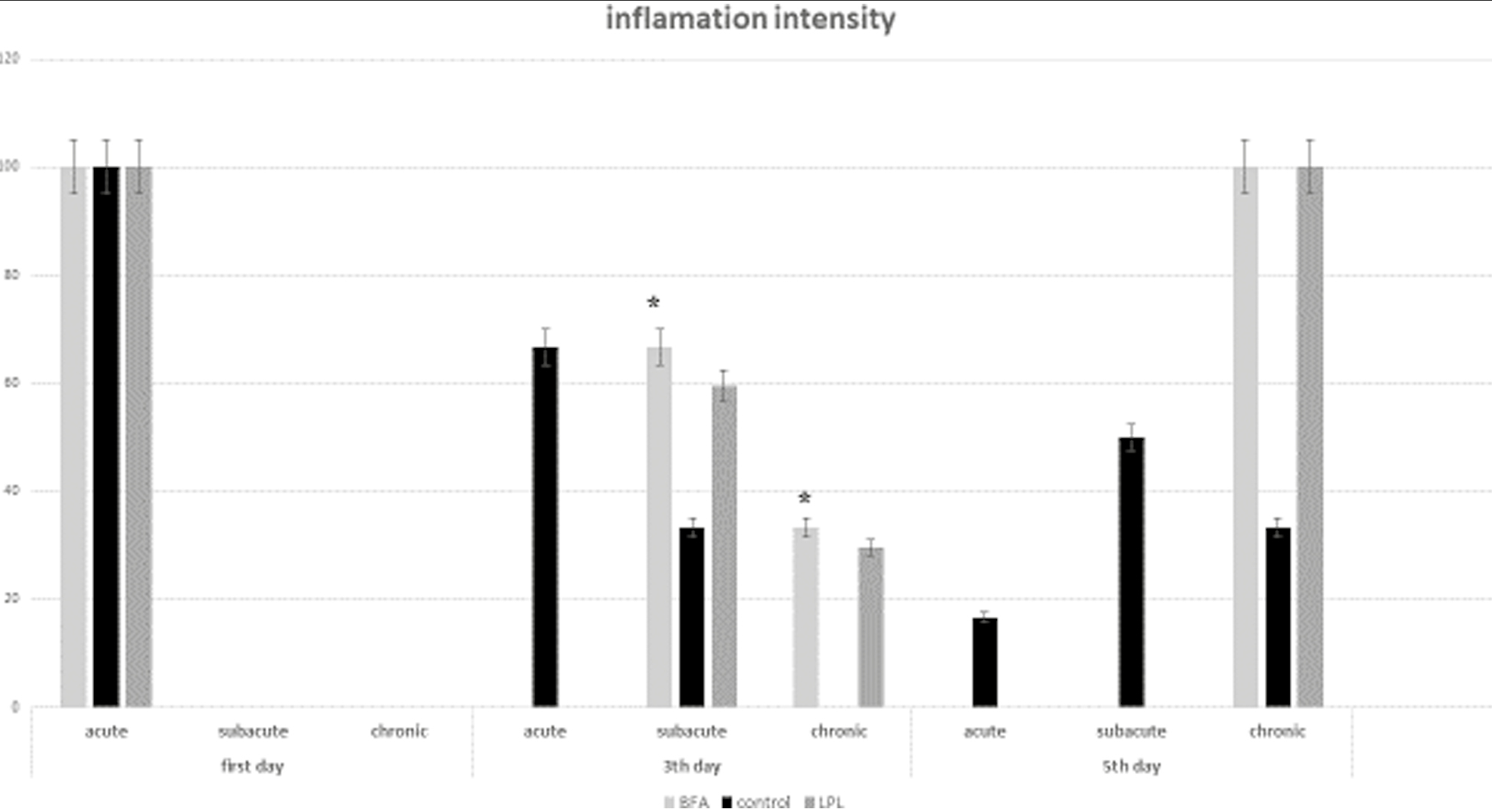
- Frequency distribution of inflammation intensity on the 5th day in control and experimental groups (*P < 0.05)
Fibroblast formation
On the 5th day of the intervention [Figure 4], the samples were examined for fibroblast formation, angiogenesis, and wound healing score. The status of fibroplasia was examined in all the groups, of which two samples from the experimental groups had +3 fibroblasts and four samples had +3 fibroblasts. In the control group, five samples had +1 fibroblasts and one sample had no fibroblasts. Fisher’s exact test on these data also showed that fibroblast formation was significantly higher in the LPL and BAF groups in comparison to the control group (P = 0.002).
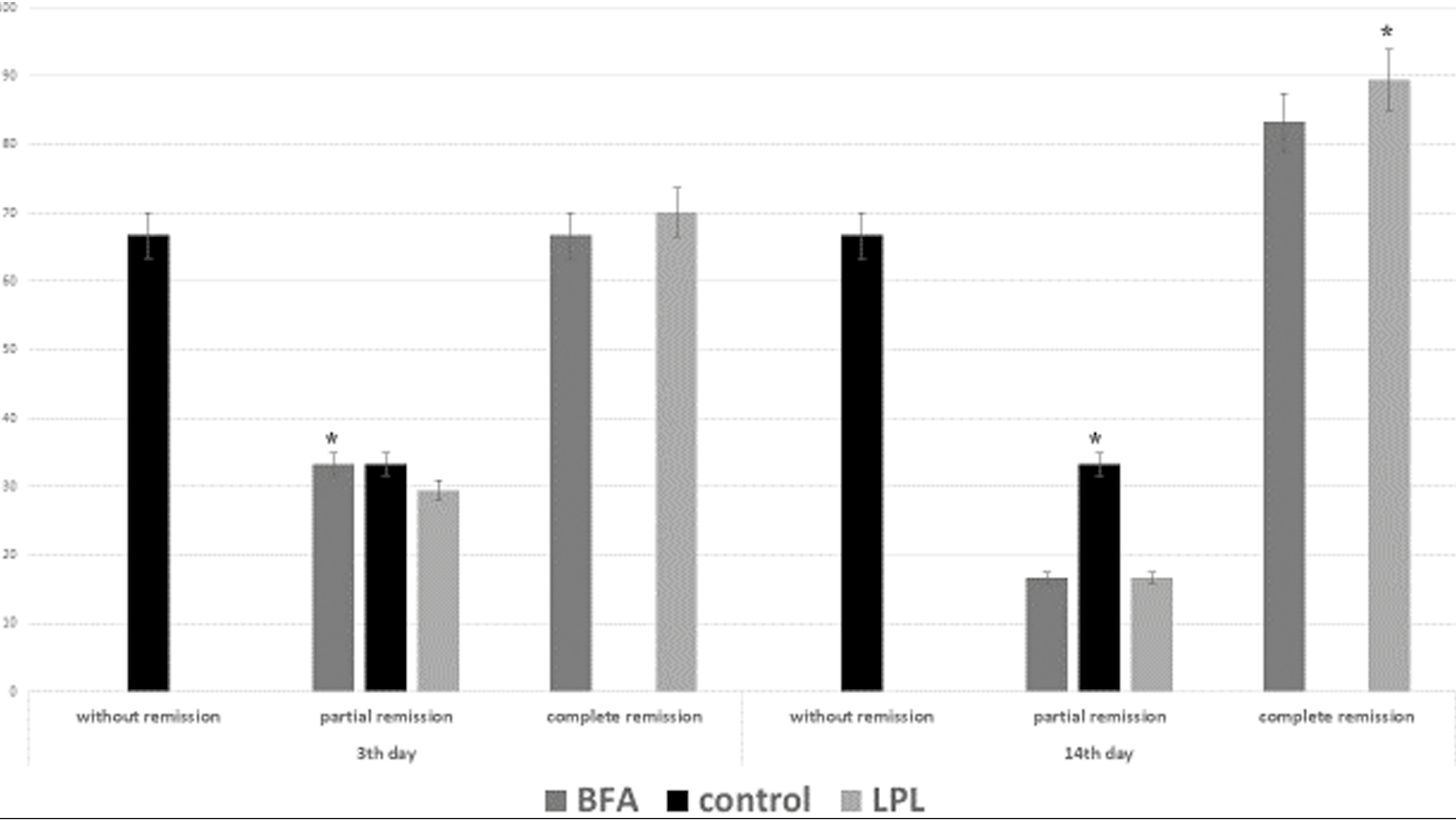
- Frequency distribution of fibroblast formation on the 5th day in experimental and control groups (*P < 0.05)
Angiogenesis
The mean angiogenesis in the experimental and control groups was 11.7, 3.7, 6.5, 1.9, 9.1, and 3.9, respectively, and according to Student’s t-test, the mean angiogenesis in the case group was significantly higher (P = 0.013). The results are illustrated in Table 3.
| Standard deviation | Angiogenesis percent | Group |
|---|---|---|
| 7/3 | 7/11 | LPL |
| 9/1 | 5/6 | Control |
| 9/3 | 1/9 | BAF |
Wound healing status
On the 5th day, out of the 6 samples from the experimental group, two samples had partial wound healing and four samples had complete wound healing, but in the control group, four samples had no wound healing and two samples had partial wound healing. Fisher’s exact test also showed that the wound healing status in the case group was significantly better (P = 0.039). On the 14th day, six samples from the experimental groups and six samples from the control group were examined for wound healing status and collagen tissue formation at the wound site. Five samples from the experimental group had complete wound healing. The sample had a relative wound healing. In the control group, four samples had no wound healing and three samples had relative wound healing. Fisher’s exact test also showed that the wound healing status on the 14th day in the case group was significantly more favorable (P = 0.006).
Collagen tissue formation
Table 4 shows the frequency distribution of collagen accumulation intensity in the two groups; according to the Mann–Whitney test, the collagen accumulation intensity in experimental groups was significantly higher than that in the control group (P = 0.037).
| LPL | Control | BAF | Groups Collagen |
|||
|---|---|---|---|---|---|---|
| Percent | Number | Percent | Number | Percent | Number | |
| 7/16 | 2 | 3/33 | 2 | 0 | 0 | 1+ |
| 25 | 3 | 50 | 3 | 0 | 0 | 2+ |
| 7/16 | 2 | 0 | 0 | 3/33 | 2 | 3+ |
| 7/41 | 5 | 7/16 | 1 | 7/66 | 4 | 4+ |
| 100 | 12 | 100 | 6 | 100 | 6 | Total |
Wound scar score
On the 21st day, the rest of the samples, which included 12 samples from the experimental groups and 13 samples from the control group, were examined and two Scar Scoring Scales were examined in them; the mean Scar Scoring Scale in the control and experimental groups was 4.1 and 1.2, 5.6, 6, 1.6, respectively, according to the scar scoring test. Student’s t-test in the case group was significantly lower (P = 0.025). The results are presented in Table 4.
Histological result
Figures 5 and 6 showed macroscopical and histological changes in LPL, BAF, and control groups. The results showed that after skin lesion and healing in different groups, the rate of improvement in the laser and fluid group was faster than that in the control group. Histological images showed that fibroblasts and collagen increased in the wound healing area, indicating a positive effect of LPL and BAF.
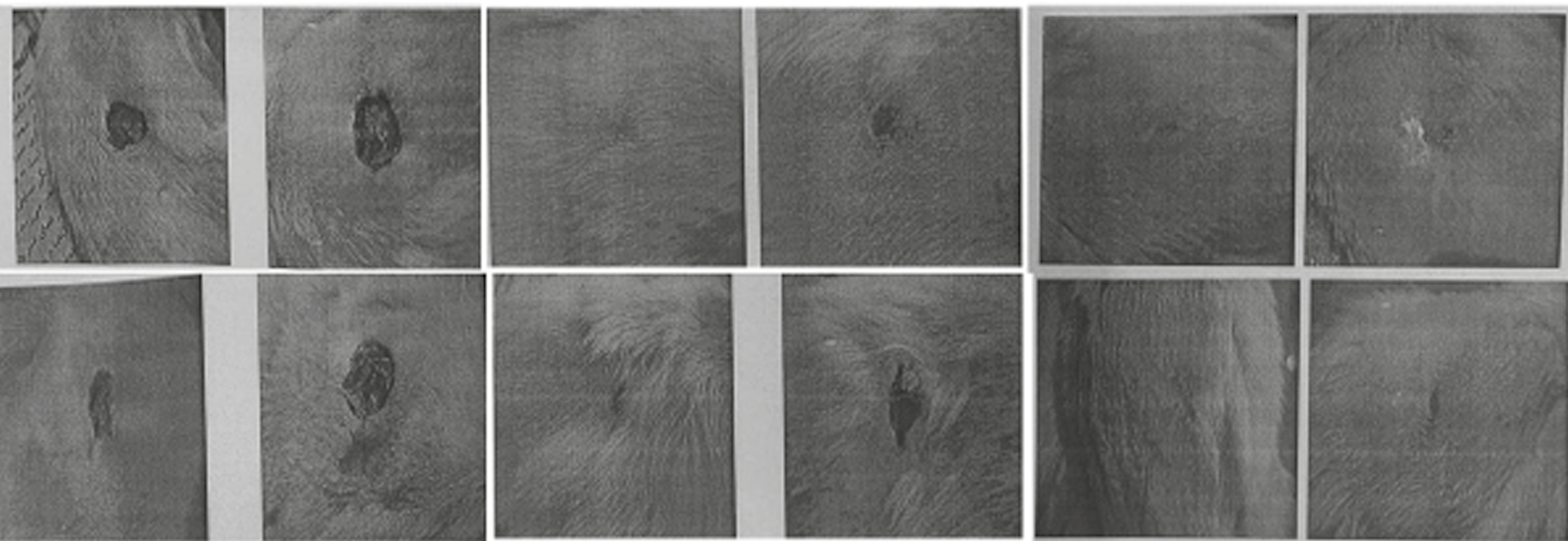
- Results showed that recovery and wound healing in rat groups: control (A and B), BAF (C and D), and LPL (E and F)
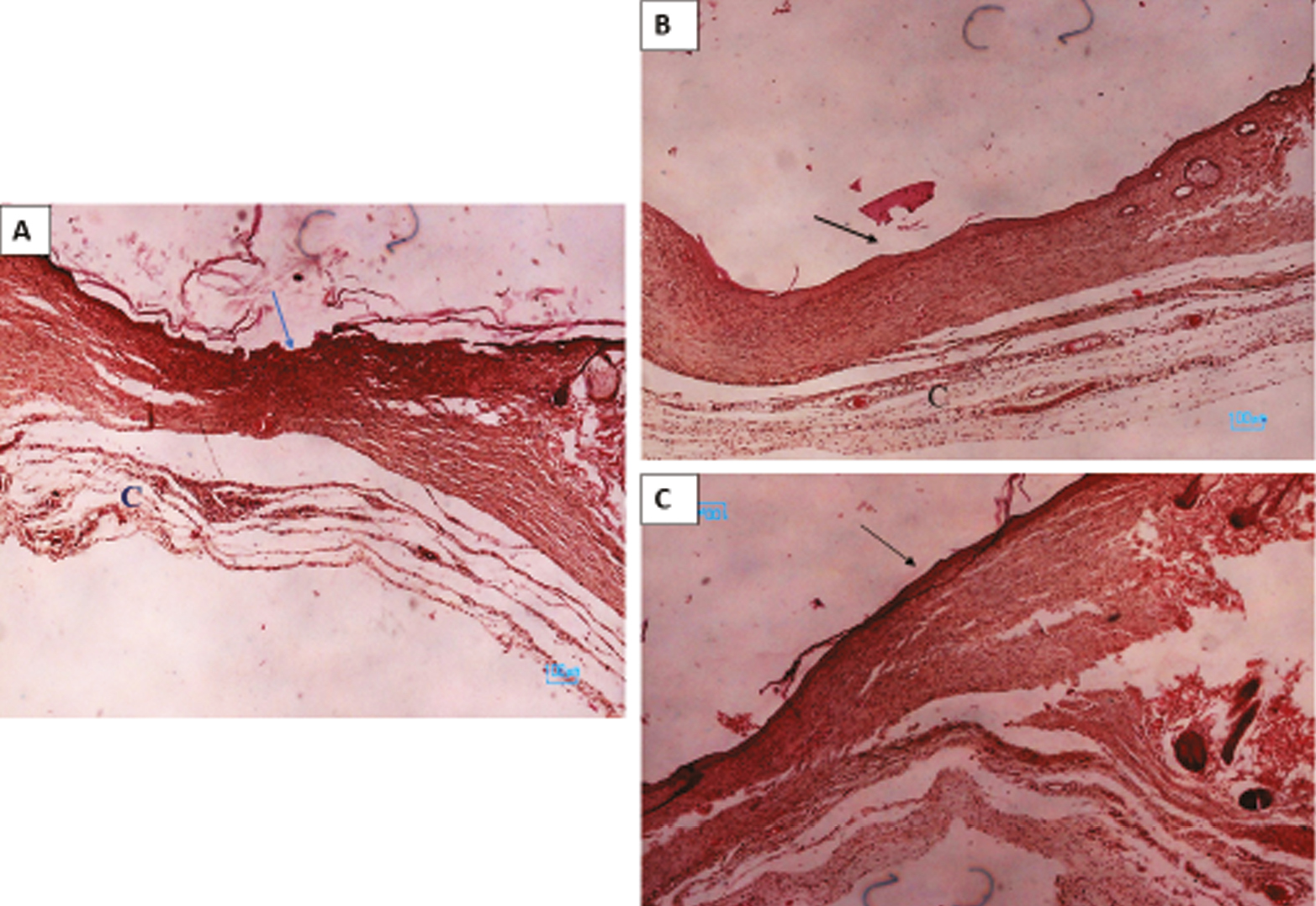
- Histological images show skin repair in the control (A) and BAF (B), and LPL groups. Arrows showed wound healing in different groups and (C) collagen fiber
Real-time PCR
To estimate the mRNA expression of target genes at the molecular level, the number of transcriptions of three genes which were involved in apoptosis Bcl2, Bax, and Caspase3 was examined in the samples. Bcl2 expression levels in the control group were remarkably lower than those in the experimental group (P < 0.5). The experimental group’s Bax and Caspase3 expression levels were greater than those of the control group (P < 0.01) [Figure 7].
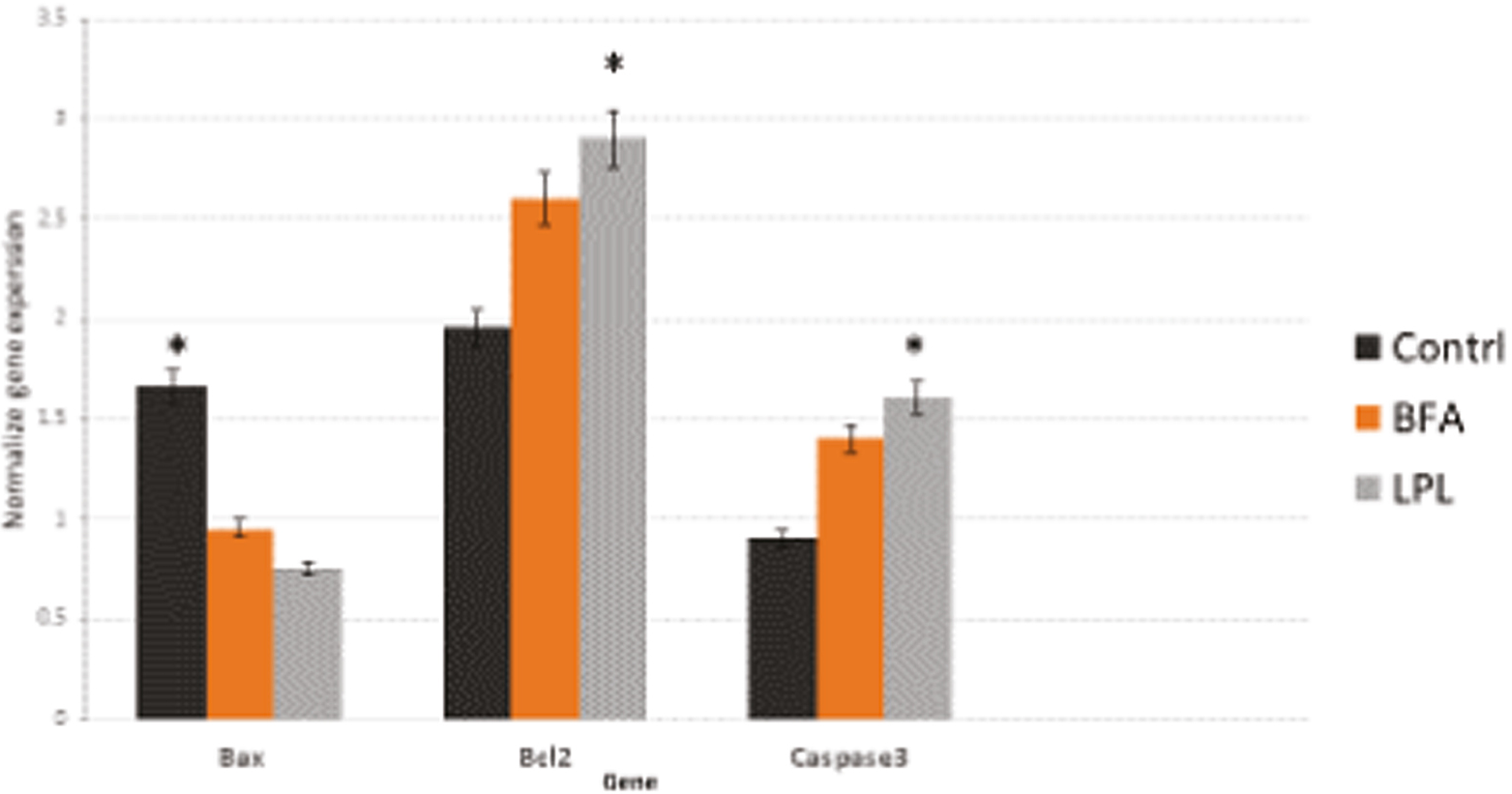
- Real-time PCR result shows that apoptosis marker in control and LPL and BAF groups
DISCUSSION
The overall aim of this study was to determine the effect of LPL and BAF in accelerating skin wound healing and reducing wound scarring in an animal model. Recent clinical and empirical evidence suggests a fundamental difference between fetuses and adults in terms of injury; fetal skin lesions heal quickly without scarring. This phenomenon was first observed clinically during fetal surgery experience, and a new trade in several animal embryo models has supported the clinical observation of scar-free fetal wound healing. Progressive research has begun to elucidate the unique mechanism of wound healing in the fetus, and the process of fetal wound healing may provide a model for ideal wound healing in adults.[1415] Unlike chronic non-healing bone healing wounds, there is usually little concern about the time it takes for tissue to re-integrate and its tensile strength after injury, but the important point is to restore tissue structure and normal function without scarring. In one study, the effect of amniotic fluid on preventing intra-abdominal adhesions after laparotomy in rats was investigated and its effectiveness was emphasized. In another study, amniotic fluid improved peripheral nerve regeneration and had a preventive effect on epineuria scars. Another study demonstrated the effectiveness of the topical application of amniotic fluid in the re-acceleration of corneal epithelialization. In another study, topical application of amniotic fluid after tenorrhaphy had a significant effect on preventing the formation of adhesions around the tendon without impairing tendon healing in the animal model.[16] Numerous other studies have been performed on each of the growth factors that are abundant in amniotic fluid and are effective in wound healing.[1718] One possible mechanism for accelerating wound healing and scarring in the fetus is its placement in amniotic fluid rich in hyaluronic acid and other growth factors are TGF-B, VEGF, PDGF, and so on. Therefore, it seems that one of the substances with different types of molecules that may be effective in the wound healing process and reducing its scar is amniotic fluid.[1920] Also, the presence of stem cells in this fluid indicates that it is rich in cell growth. Given the importance of the physiological process of wound healing and intending to find new factors affecting this process, we undertake an experimental study of the effect of using BAF on the wound healing process and its scarring in the animal model. In this study, in a group of rats that used BAF and LPL in their wounds, the type and severity of inflammation were significantly lower and the inflammation disappeared in a shorter time in the control group. Therefore, the effect of BAF and LPL can be considered effective in reducing inflammation. The effects of LPL in the treatment of various diseases have been reported to be consistent with the results of our study.[2122] The status of fibroblast formation was examined in different groups, and the results showed that LPL and BAF have positive effects on this process. The mean angiogenesis in LPL, BAF, and control groups was 11.7, 3.7, 6.5, 1.9, 9.1, and 3.9 respectively, and the mean angiogenesis was higher in the case group. However, angiogenesis should be lower in the group receiving BAF, which may be related to other interfering factors during wound healing. Other variables such as collagen tissue formation and wound healing status and wound scar score in the LPL and BAF groups were significantly more favorable than the control group. Finally, this study indicates the effectiveness of BAF and LPL in reducing inflammation, increasing fibroplasia, increasing angiogenesis, increasing collagen formation, as well as improving wound healing and reducing scarring, but for a definitive conclusion, this study needs to be performed on a larger scale. Considering the positive effect of BAF and LPL in improving wound healing and less scarring, it seems that with the continuation of research, it will be possible in the future for a drug containing amniotic fluid with similar synthetic compounds to heal wounds faster and prevent scarring.
CONCLUSION
Considering the positive effect of BAF cream on wound healing and less scarring compared with the laser group, it seems that with the continuation of research, in the future, a drug containing amniotic fluid or similar synthetic compounds can heal wounds faster and be used to prevent scarring after wound healing.
Statement of ethics
The protocols of this study are confirmed by the Ethical Committee of Esfahan University of Medical Sciences.
Financial support and sponsorship
No funding sources.
Conflicts of interest
There are no conflicts of interest.
Authors’ contribution
Each of the authors read and confirmed the resulting paper.
Data availability statement
All data generated or analyzed during this study are included in this article.
Acknowledgement
We are thankful for the funding provided by the School of Medicine, Esfahan University of Medical Sciences, Esfahan, Iran.
REFERENCES
- Repairing injured skin: Biologics, skin substitutes, and scaffolds. J Skin Stem Cell. 2018;5:e86162.
- [Google Scholar]
- Comparison of the effects of tretinoin, adapalene and collagenase in an experimental model of wound healing. Eur J Dermatol. 2002;12:145-8.
- [Google Scholar]
- Effects of an aqueous extract from the leaves of Chromolaena odorata (Eupolin) on the proliferation of human keratinocytes and on their migration in an in vitro model of reepithelialization. Wound Repair Regen. 2001;9:305-13.
- [Google Scholar]
- Wound healing property review of Siam weed, Chromolaena odorata. Pharmacogn Rev. 2017;11:35-8.
- [Google Scholar]
- The invasive weed with healing properties: A review on Chromolaena odorata. Int J Pharm Sci Res. 2012;3:80.
- [Google Scholar]
- Efficacy of Chromolaena odorata leaf extracts for the healing of rat excision wounds. Veterinární medicína. 2017;62:565-78.
- [Google Scholar]
- The combined effects of mesenchymal stem cell conditioned media and low-level laser on stereological and biomechanical parameter in hypothyroidism rat model. J Lasers Med Sci. 2018;9:243-8.
- [Google Scholar]
- Human amniotic fluid stem cells attract osteoprogenitor cells in bone healing. J Cell Physiol. 2020;235:4643-54.
- [Google Scholar]
- Comparative evaluation of the efficacy of laser therapy and fibroblastic growth factor injection on mucosal wound healing in rat experimental model. J Lasers Med Sci. 2018;9:194-9.
- [Google Scholar]
- Low level of autophagy-related gene 10 (ATG10) expression in the 6-hydroxydopamine rat model of Parkinson’s disease. Iran Biomed J. 2018;22:15-21.
- [Google Scholar]
- Creatine and retinoic acid effects on the induction of autophagy and differentiation of adipose tissue-derived stem cells into GABAergic-like neurons. J Babol Univ Med Sci. 2017;19:41-9.
- [Google Scholar]
- Hyaluronic acid, an important factor in the wound healing properties of amniotic fluid: In vitro studies of re-epithelialisation in human skin wounds. J Plast Surg Hand Surg. 2013;47:89-92.
- [Google Scholar]
- Achilles tendon disorders including tendinopathies and ruptures. Baxter’s The Foot and Ankle in Sport. 2009;37:1223-34.
- [Google Scholar]
- Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol. 2007;177:369-76.
- [Google Scholar]
- Therapeutic effect of total ginseng saponin on skin wound healing. J Ginseng Res. 2011;35:360-7.
- [Google Scholar]
- Direct comparison of reproducibility and reliability in quantitative assessments of burn scar properties. Burns. 2021;47:466-78.
- [Google Scholar]
- Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater Sci Eng C Mater Biol Appl. 2017;79:533-40.
- [Google Scholar]
- Skin wound healing and scarring: Fetal wounds and regenerative restitution. Birth Defects Res C Embryo Today. 2012;96:325-33.
- [Google Scholar]
- The effect of low-level laser therapy and curcumin on the expression of Lc3, Atg10 and Bax/Bcl2 ratio in Pc12 cells induced by 6-hydroxide dopamine. J Lasers Med Sci. 2020;11:299-304.
- [Google Scholar]






