Translate this page into:
Efficacy of Fractional CO2 Laser for Improvement of Limited Mouth Opening in Systemic Sclerosis
Address for correspondence: Dr. Yasmeen Jabeen Bhat, Department of Dermatology, Venereology and Leprosy, Government Medical College (GMC), Srinagar 190010, Jammu and Kashmir, India. E-mail: yasmeenasif76@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Systemic sclerosis is a multisystem auto-inflammatory disease-causing fibrosis in the skin and internal organs. A frequent complication of systemic sclerosis is the limited mouth opening (LMO), a difficult-to-treat condition with only a few treatment options available.
Aims and Objectives:
The aim of this study was to evaluate the response of fractional carbon dioxide (CO2) laser resurfacing in LMO associated with systemic sclerosis.
Materials and Methods:
This was a hospital-based prospective study in which diagnosed cases of systemic sclerosis were taken. Patients who had significant LMO and who gave informed consent were included in the study. Fractional CO2 laser treatment was performed in the perioral area. Patients were assessed at baseline, after three and six sessions, and 3 months after the last session. Assessment was done by measurement of the interincisor distance (IID) using a ruler and calculation of the mouth handicap in systemic sclerosis (MHISS) scale.
Results:
Improvement in IID occurred 3 months after the first session with a mean gain of +5 mm (range 2–7). At 6 months, a mean gain of +8.5 mm (range 7–10) in IID was observed (P < 0.001). The MHISS score decreased by a mean of 14 (range 11–17) (P < 0.001). All patients showed improvement of mouth opening, which allowed the patients to have better phonation and the patients were able to have proper dental care posttreatment in the form of brushing of teeth and other dental procedures. The adverse effects noted in these patients included erythema that resolved spontaneously or after icing posttreatment. Other adverse effects noted were stinging and burning sensations that were mild and transient.
Conclusion:
Fractional CO2 laser forms a safe, effective, and well-tolerated treatment modality for improvement of LMO in systemic sclerosis.
Limitations:
The limitations of this study were less number of patients and no long-term follow-up.
Keywords
Carbon dioxide (CO2) laser
diffuse cutaneous systemic sclerosis (dcSSc)
limited mouth opening (LMO)
mouth handicap in systemic sclerosis (MHISS)
systemic sclerosis
INTRODUCTION
Scleroderma, also called systemic sclerosis (SSc), is derived from the Greek words skleros (hard or indurated) and derma (skin). It was initially defined by Hippocrates, but a detailed description was given by Carlo Curzio.[1] It is divided into two groups: limited cutaneous and diffuse cutaneous disease.[2] Limited cutaneous SSc (lcSSc) is characterized by thickening of the skin distal to the elbows. The internal organ involvement in this type is less severe. Diffuse cutaneous SSc (dcSSc) is characterized by involvement of skin proximal to the elbows and knees in addition to distal area involvement. It is associated with more severe organ damage.[3]
Autoantibodies in SSc are produced as a result of immune system activation. This helps in classifying the type of disease and in determining the prognosis. Autoantibodies against topoisomerase I (Topo I, ATA, and Scl-70) are more often observed in patients with dcSSc. They are associated with pulmonary complications and digital ulcers.[4] In lcSSc, anti-centromere antibodies (ACAs) are commonly found and they are associated with increased risk of pulmonary fibrosis and pulmonary hypertension. Other antibodies detected in patients with dcSSc are the ones against RNA polymerase III, which carries a higher risk of a renal crisis.[45] Less-frequent antibodies include antibodies against fibrillarin (anti-U3RNP) and against Th/To (anti-Th). These antibodies are characteristic of lcSSc.[456]
SSc presents with a number of complications. The complications are divided into cutaneous and visceral. Internal organ complications are common in patients with SSc, and involve pulmonary, renal, gastrointestinal, cardiac, and musculoskeletal systems. SSc can affect the lung parenchyma leading to interstitial lung disease and the pulmonary blood vessels causing pulmonary arterial hypertension.[78] It also causes complications such as scleroderma renal crisis and cardiac complications. Cutaneous complications include Raynaud’s phenomenon, digital ulcers, pitted scars, telangiectasias, and calcinosis cutis.
Facial skin thickening can occur with the limited cutaneous and diffuse cutaneous subsets and leads to difficulty in opening the mouth. Approximately 70% of cases of progressive SSc show involvement of the head and neck region.[9] Limited mouth opening (LMO) occurs as a result of fibrosis and interferes with mastication, phonation, and dental care. Basic oral hygienic care becomes difficult.[1011] Severe LMO is defined by an interincisal distance (IID) <30 mm.[12] There are a few options available for the treatment of LMO in SSc. In some studies, autologous fat transplantation and autologous adipose-derived stromal cells (ADSCs) injection into the perioral region have been used.[13] Various forms of laser therapy have been tried. Intense pulsed laser (IPL) has also been shown to help improve microstomia that is associated with scleroderma.[14] It has been observed that IPL leads to increase dermal mobility around the mouth area and leads to a significant posttreatment improvement in the IID. Few studies have shown the role of fractional CO2 laser in LMO in SSc. In view of the paucity of literature on this effective treatment in SSc, we designed this study to find out the role of fractional CO2 in LMO associated with SSc.
MATERIALS and METHODS
This was a hospital-based prospective study in which diagnosed cases of SSc were recruited and studied over a period of 2 years from March 2018 to February 2020. Ethical clearance from the institutional ethical committee was taken before starting the study. The diagnosis was made based on clinical features, skin biopsy, dermoscopy, and other relevant tests. The patients were classified as cases of SSc based on ACR/EULAR criteria.[15] Modified Rodnan skin score (MRSS) of each patient was calculated.[16] Patients who had significant LMO and who gave written consent after they were explained the whole treatment protocol were included in the study. Patients having a past or present history of photosensitivity, pregnant and lactating females, and patients receiving other anti-fibrotic treatment modalities were excluded from the study.
Fractional CO2 laser ([Cis F1 Sellas Primium CO2 laser manufactured by DINONA, South Korea], with 600 W of power and a wavelength of 10,600 nm) treatment was performed in all patients in the perioral area. Treatment was carried out under topical local anesthesia (combination of 7% lidocaine and 7% tetracaine). Saline-soaked gauze was placed in the mouth between teeth and lips to prevent accidental teeth burns. Fractional CO2 laser in the moving mode with F100 handpiece was used. Spot density taken was 81–100, in scattered form. Fluence used was from 8 mJ/cm2, increased by 1–2 mJ/cm2 at each session. The patients were given two to three passes, each pass without overlapping the spot [Figure 1]. Six laser sessions were performed at intervals of 4 weeks between sessions. Patients were assessed at baseline, after three and six sessions, and 3 months after the last session. Assessment was performed by measurement of the IID using a ruler and calculation of the mouth handicap in SSc (MHISS) scale [Table 1].
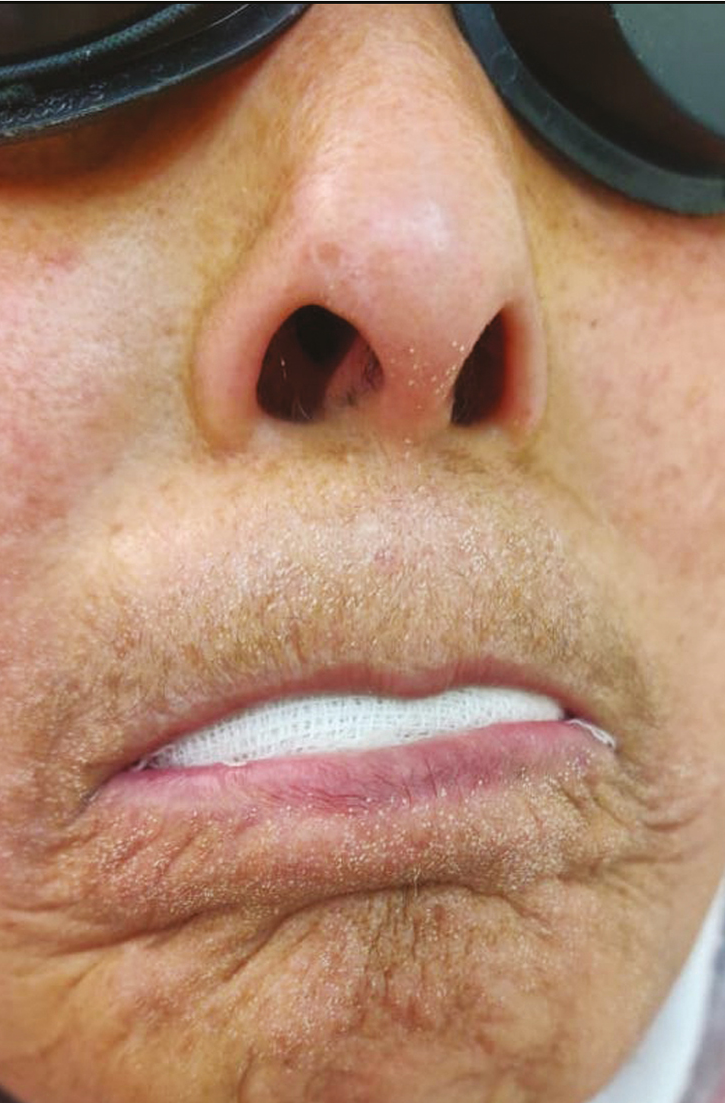
- Fractionated grid imprints immediately postprocedure
| Never | Rarely | Occasionally | Often | Always | |
|---|---|---|---|---|---|
| 1. I have difficulties opening my mouth | 0 | 1 | 2 | 3 | 4 |
| 2. I have to avoid certain drinks (sparkling, alcohol, acidic) | 0 | 1 | 2 | 3 | 4 |
| 3. I have difficulties chewing | 0 | 1 | 2 | 3 | 4 |
| 4. My dentist has difficulties taking care of my teeth | 0 | 1 | 2 | 3 | 4 |
| 5. My dentition has become altered | 0 | 1 | 2 | 3 | 4 |
| 6. My lips are retracted and/or my cheeks are sunken | 0 | 1 | 2 | 3 | 4 |
| 7. My mouth is dry | 0 | 1 | 2 | 3 | 4 |
| 8. I must drink often | 0 | 1 | 2 | 3 | 4 |
| 9. My meals consist of what I can eat and not what I would like to eat | 0 | 1 | 2 | 3 | 4 |
| 10. I have difficulties speaking clearly | 0 | 1 | 2 | 3 | 4 |
| 11. The appearance of my face is modified | 0 | 1 | 2 | 3 | 4 |
| 12. I have trouble with the way my face looks | 0 | 1 | 2 | 3 | 4 |
Statistical analysis
It was done using Student’s t test. For comparison of categorical data such as sex and residence, chi-square test was used. A value of P < 0.05 was considered statistically significant.
RESULTS
Fifteen female SSc patients received laser treatment for LMO. The mean age was 52.8 years (range 40–65). The Fitzpatrick skin types varied from III to V. The duration of disease was at least 10 years in all patients. The demographic and clinical characteristics of patients are summarized in Table 2. The patients did not receive more than one immunosuppressant therapy during or in the 12 months prior to laser treatment. Other modalities such as physical therapy or stretching exercises to improve mouth opening were also not performed during the follow-up period or in the 12 months prior to treatment. The initial mean IID and MHISS scores were 24.06 mm and 25, respectively [Figures 2 and 3].
| Patient characteristic | Mean value |
|---|---|
| Age | 52.8 years |
| Phototype | III–IV |
| Duration of disease | >10 years |
| Modified Rodnan score (0–51) | 15 (range 10–20) |
| Concurrent systemic disease | Hypertensive, diabetes (two patients) |
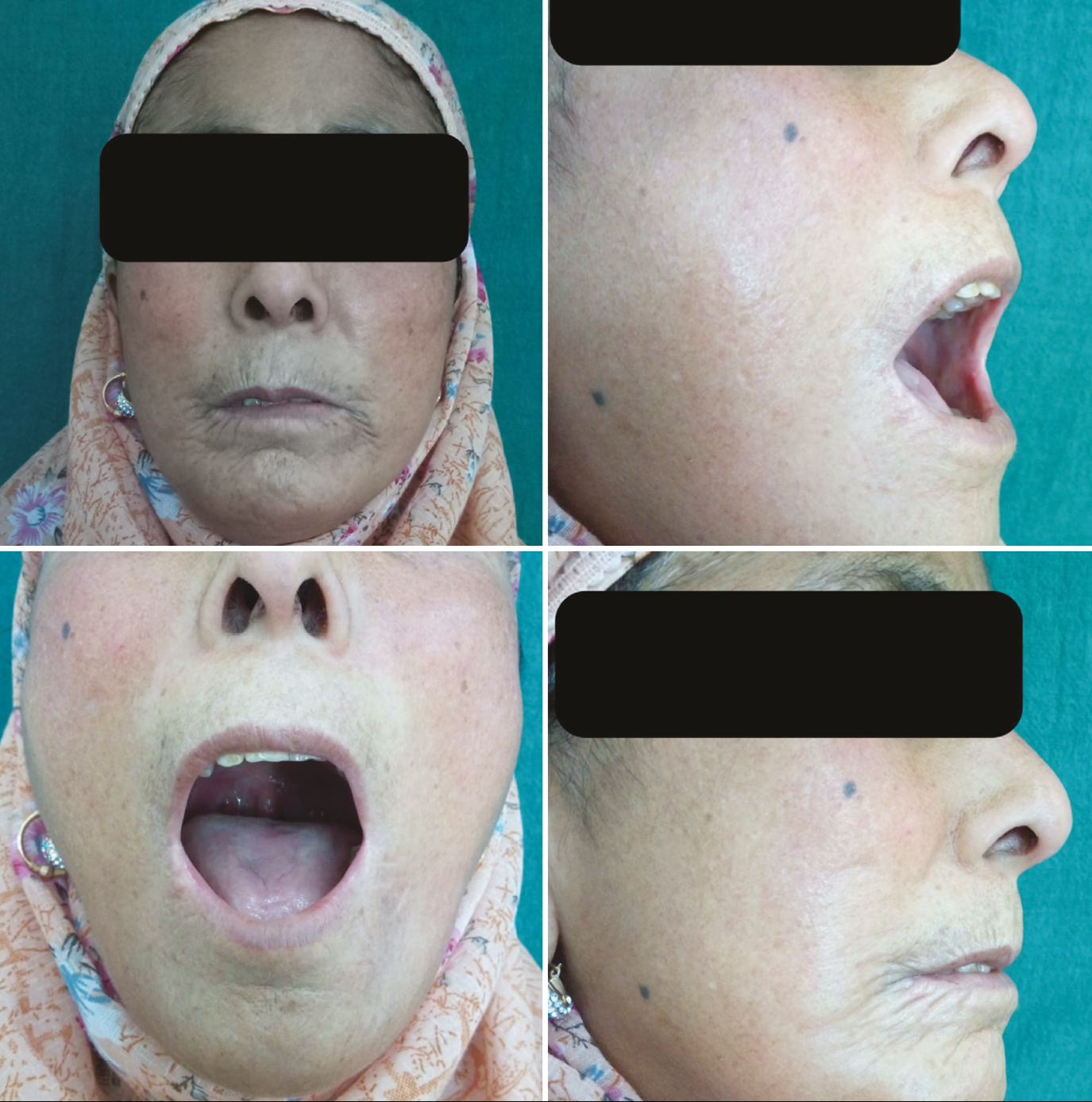
- Limited mouth opening and lip retraction at baseline
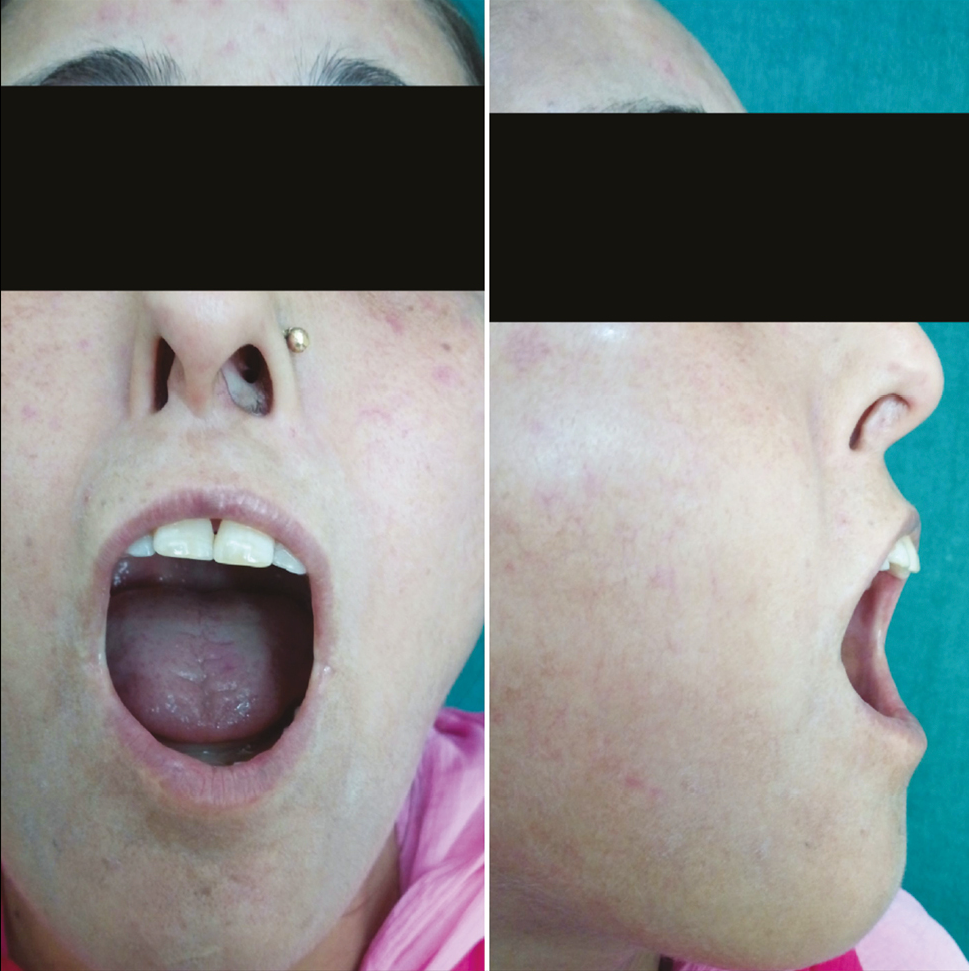
- Difficulty in opening the mouth at baseline
In all patients, an improvement in IID was observed 3 months following the first CO2 laser session with a mean gain of +4.8 mm [Figures 4 and 5]. At 6 months, the mean gain in IID was +2.7 mm, and at 9 months the gain was +2.2 (P < 0.001). The MHISS score was evaluated in patients and improved by a mean of –8 at 3 months and –6 at 6 months (P < 0.001). All patients showed an improvement in mouth opening, lip flexibility, better phonation and mastication, and easier dental care. There was an improvement in radial furrowing posttreatment [Figures 6 and 7]. The MHISS and IID of patients at various follow-ups are given in Tables 3 and 4.
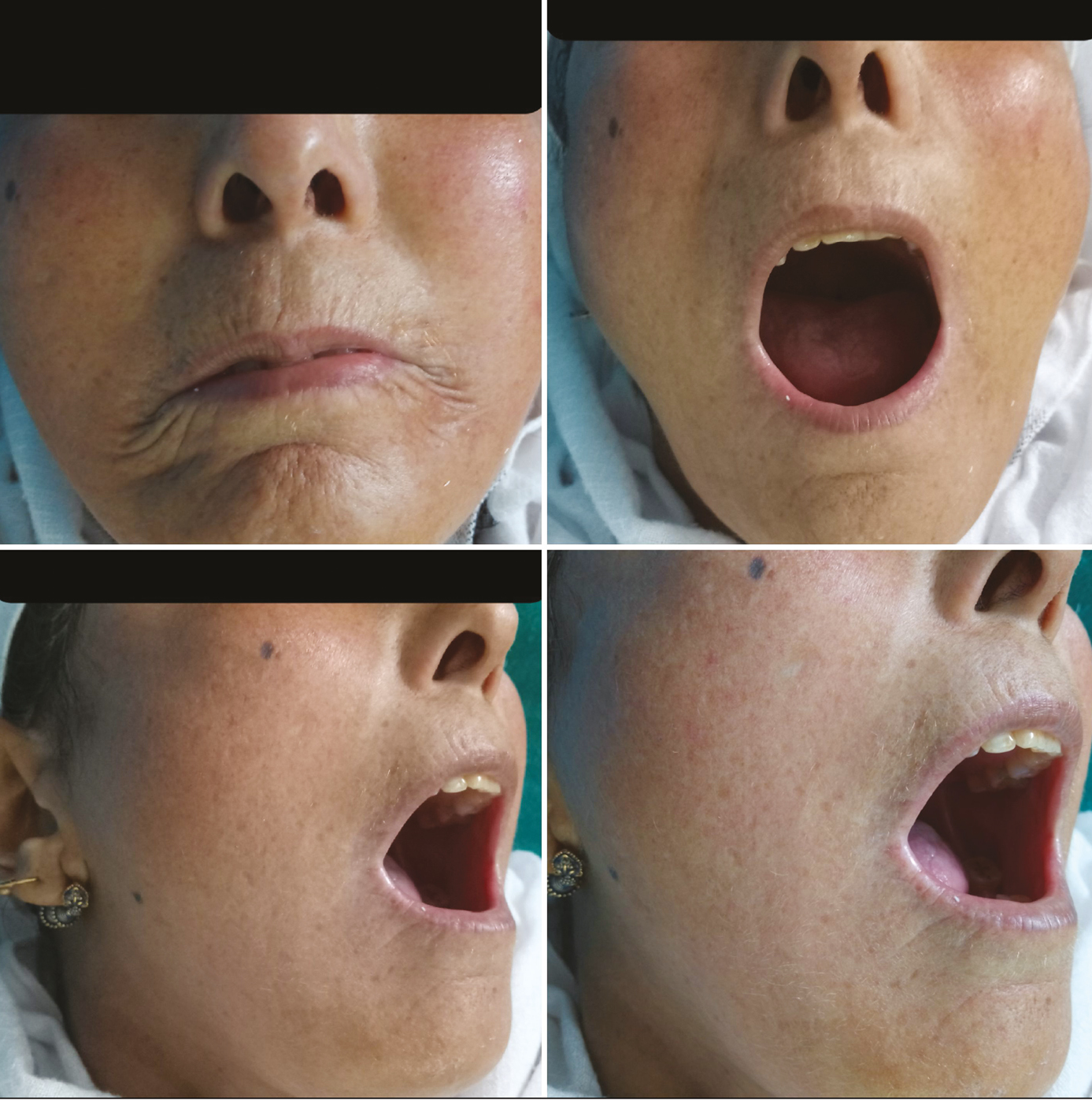
- Increase in mouth opening, reduction in lip retraction, and radial furrowing after three sessions
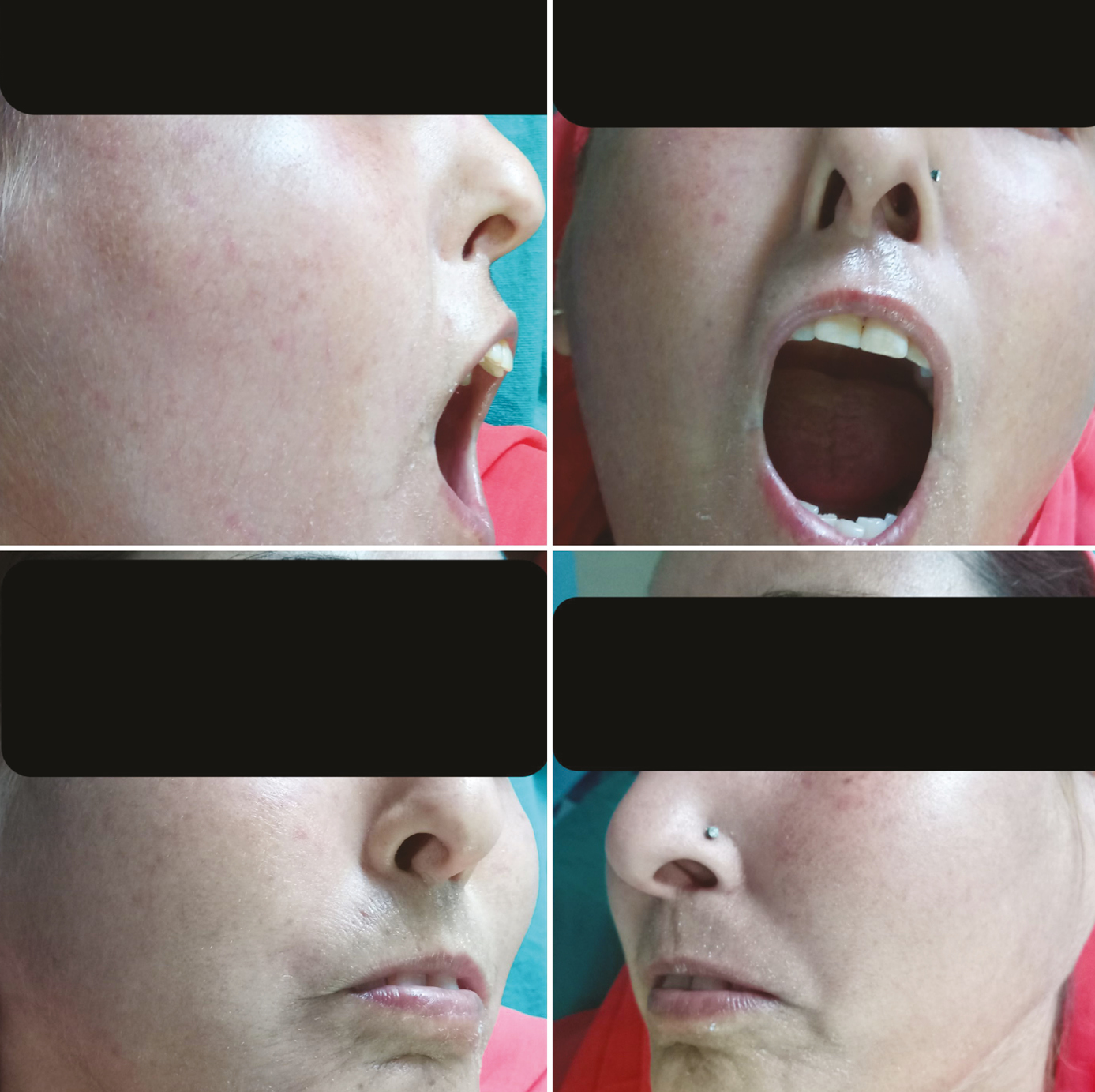
- Improvement in mouth opening and interincisor distance after three sessions
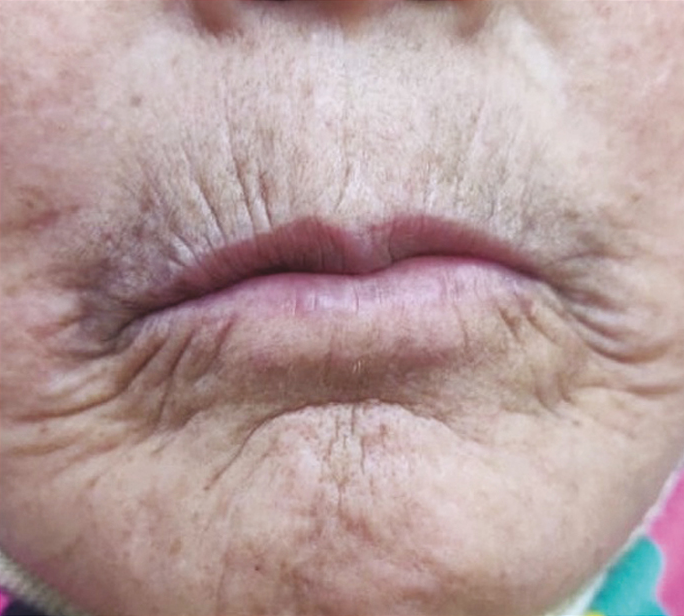
- Radial farrowing at baseline
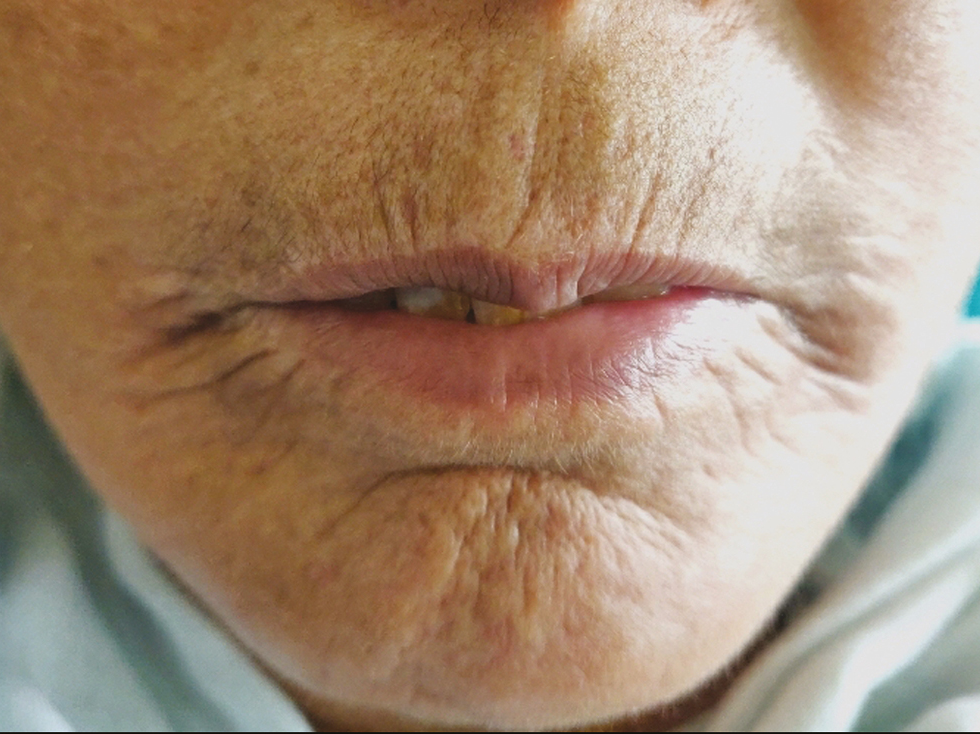
- Significant improvement after laser treatment
| MHISS at baseline | MHISS at 3 months | MHISS at 6 months | MHISS at 9 months | |
|---|---|---|---|---|
| Mean | 24.7 | 17.9 | 12.3 | 11.5 |
| SD | 3.92 | 5.03 | 5.30 | 4.98 |
| P Value* | <0.001 | |||
| P Value (vs. previous time)$ | – | <0.001 | <0.001 | <0.001 |
*Repeated-measures ANOVA
$Bonferroni corrected
| IID at baseline | IID at 3 months | IID at 6 months | IID at 9 months | |
|---|---|---|---|---|
| Mean | 2.56 | 2.95 | 3.54 | 3.63 |
| SD | 0.434 | 0.479 | 0.578 | 0.641 |
| P Value* | <0.001 | |||
| P Value (vs. previous time)$ | – | <0.001 | <0.001 | <0.001 |
*Repeated-measures ANOVA
$Bonferroni corrected
DISCUSSION
Our study was designed to determine the role of fractional CO2 laser for improvement of mouth opening in SSc. In our study, we found that the MHISS score improved and IID distance increased in patients post laser treatment. Patients had better phonation and dental care became easier after treatment.
Skin fibrosis develops in almost all patients of SSc. Skin fibrosis leads to typical “mauskopf appearance,” which is characterized by inability to retract the lower eyelids in patients with progressive disease(Ingram’s sign), reduced mouth aperture and sclerosis of the frenulum, radial furrowing around the mouth and atrophy of nasal alae which leads to pinched appearance of the nose.
In SSc, skin fibrosis occurs as a result of fibroblast activation. When the fibroblasts from the lesional skin of SSc patients are explanted in vitro, they show increased synthesis of collagen and fibronectin. Constitutive production of cytokines and chemokines and spontaneous myofibroblast transdifferentiation has also been seen..[171819]
The CO2 laser was one of the earliest of laser systems used in dermatology. It is not pigment-selective and targets both pigmented and non-pigmented skin lesions. The chromophores for this laser are intracellular and extracellular water. The wavelength emitted is 10,600 nm. CO2 laser surgery is a tissue-selective process as only the target tissue is ablated and surrounding normal tissue is minimally involved.[20] The laser delivers high fluence with a small spot diameter that produces pixels of skin damage measuring less than 500 mm in diameter. There is no harm to surrounding tissues and recovery is also rapid, occurring within 24–48 h.[21] There is formation of controlled zones of thermal heating and tissue damage, which are surrounded by spared areas of viable epidermis and dermis. This viable area allows rapid repair of the microthermal zones. High-speed pulse energies deposit microthermal zones in the skin in random patterns.[22]
Comstedt et al.[23] performed a study in which four patients with SSc having LMO were treated with IPL. The patients included in this study experienced softening of the perioral skin with improvement in daily functions. The only side effects seen were transient erythema and edema.
Bennani et al.[24] reported in their study that CO2 laser treatment of severe LMO in SSc patients was connected with significant improvement in the mouth opening. The improvement was seen 3 months after the first laser session and further improvement was observed at 1 year.
In our study, we found a significant improvement in mouth opening in patients after treatment. The adverse effects noted in these patients included erythema that resolved spontaneously or after icing posttreatment. The other adverse effects noted were stinging and burning sensation, which were mild and transient. A significant improvement in mouth opening 3 months after the first laser session (mean +4.8 mm vs. baseline) was observed with further improvement observed at 6 months (mean +2.7 mm vs. baseline) and 9 months (+2.2 vs. baseline). This improvement was clinically significant and resulted in a reduction of mouth handicap as evaluated by the MHISS score.
CONCLUSION
Fractional CO2 laser forms a safe, effective, and well-tolerated treatment modality for improvement of LMO in SSc.
Ethical policy and institutional review board statement
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee. This article does not contain any studies with animals performed by any of the authors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
REFERENCES
- Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437-44.
- [Google Scholar]
- Biomarkers in systemic sclerosis: Their potential to predict clinical courses. J Dermatol. 2016;43:29-38.
- [Google Scholar]
- Pulmonary arterial hypertension in systemic sclerosis: The need for early detection and treatment. Intern Med J. 2007;37:485-94.
- [Google Scholar]
- Scleroderma lung: Pathogenesis, evaluation and current therapy. Drugs. 2007;67:985-96.
- [Google Scholar]
- Oral manifestations in progressive systemic sclerosis: Case report. J Dent Oral Hyg. 2011;3:89-94.
- [Google Scholar]
- Small mouths. Big problems? A review of scleroderma and its oral health implications. J Can Dent Assoc. 2007;73:831-6.
- [Google Scholar]
- Recommendations for the care of oral involvement in patients with systemic sclerosis. Arthritis Care Res (Hoboken). 2011;63:1126-33.
- [Google Scholar]
- Outcome measures in systemic sclerosis: An update on instruments and current research. Curr Rheumatol Rep. 2007;9:151-7.
- [Google Scholar]
- Improvement of mouth functional disability in systemic sclerosis patients over one year in a trial of fat transplantation versus adipose-derived stromal cells. Stem Cells Int. 2016;2016:2416192.
- [Google Scholar]
- Improvement of microstomia in scleroderma after intense pulsed light: A case series of four patients. J Cosmet Laser Ther. 2012;14:102-6.
- [Google Scholar]
- Development of classification and response criteria for rheumatic diseases. Arthritis Rheum. 2006;55:348-52.
- [Google Scholar]
- Development and validation of a scale for mouth handicap in systemic sclerosis: The mouth handicap in systemic sclerosis scale. Ann Rheum Dis. 2007;66:1651-5.
- [Google Scholar]
- Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J Exp Med. 1972;135:1351-62.
- [Google Scholar]
- Increased collagen synthesis by scleroderma skin fibroblasts in vitro: A possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest. 1974;54:880-9.
- [Google Scholar]
- Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-7.
- [Google Scholar]
- In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007;39:96-107.
- [Google Scholar]
- Laser fractional photothermolysis of the skin: Numerical simulation of microthermal zones. J Cosmet Laser Ther. 2014;16:57-65.
- [Google Scholar]
- Improvement of microstomia in scleroderma after intense pulsed light: A case series of four patients. J Cosmet Laser Ther. 2012;14:102-6.
- [Google Scholar]
- Improvement of microstomia in scleroderma after carbon dioxide laser treatment. Case Rep Dermatol. 2016;8:142-50.
- [Google Scholar]






