Tranexamic Acid in Melasma: Comparative Evaluation of Therapeutic Efficacy of Oral Tranexamic Acid versus Its Transepidermal Administration
Address for correspondence: Dr. Jayati Batra, Department of Dermatology, GMC, Patiala, Punjab, India. E-mail: jayati.batra13@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Melasma is a common disorder of hyperpigmentation mainly affecting the face. It is difficult to treat and is a cause for great cosmetic concern for the patient. So, we did this research to compare the therapeutic efficacy of tranexamic acid (TXA) through oral route versus its transepidermal administration with a dermaroller in melasma.
Materials and Methods:
This was a hospital-based, interventional study carried over a span of 1 year on 40 patients with facial melasma. The enrolled patients were randomly divided into 2 groups of 20 patients each. Group A patients received oral tablet TXA in a dose of 250 mg twice daily for 12 weeks and Group B patients were given TXA solution transepidermally into the melasma lesions using a dermaroller at 2 weekly interval for 12 weeks. Thereafter, all patients were followed up for improvement in Melasma Area and Severity Index (MASI) score, adverse effects and relapse at 4 weekly intervals till 24 weeks. All the patients were evaluated for therapeutic outcome both objectively (by MASI) and photographically at 4, 8, 12, 18, and 24 weeks. Grading of improvement was done by assessing percentage reduction in MASI score.
Results:
Majority of the patients, 24 (60%) in our study had centrofacial pattern of melasma followed by malar pattern seen in 16 (40%) patients. The baseline MASI score was 11.98 (± 5.47) in Group A and 12.13 (± 3.16) in Group B. The mean MASI score in Group A at the end of 4, 8, 12, 18, and 24 weeks was 9.75 (±4.83), 6.09 (±3.64), 4.21 (±2.48), 2.56 (±1.95), and 3.10 (±3.38) while these values were 9.73 (±3.32), 6.06 (±2.75), 4.55 (±1.89), 2.94 (±1.36), and 3.09 (±1.32) for Group B. At the end of 24 weeks follow-up period, good (51%-75% improvement) and very good (>75% improvement) response occurred in 5 (25%) and 14 (70%) patients in Group A and 11 (55%) and 9 (45%) patients in Group B, respectively. However, the final reduction in MASI score was similar in both the groups. Relapse was uncommon and occurred at 24 weeks in 1 patient from Group A.
Limitation:
The major limitation was small sample size due to time limitation and long follow-up period.
Conclusions:
Oral and transepidermal TXA appear equally effective suggesting that the efficacy of TXA is perhaps independent of its route of administration. Oral therapy is convenient for the patient. Transepidermal therapy of TXA has the advantage of local action, hence systemic side effects are avoided, but it is slightly painful and the time period between consecutive microneedling sessions is left to the prerogative of the treating doctor, hence a proper quantification is lacking. Moreover, scheduling of maintenance sessions is necessary as melasma is prone for recurrence.
Keywords
Dermaroller
melasma
tranexamic acid
transepidermal
INTRODUCTION
Melasma is acquired, symmetrical hyperpigmentation of sun-exposed areas of the body, most commonly occurring on the face and only occasionally affecting the forearms. Though benign, it can be extremely psychologically distressing and has shown to have a significant impact on quality of life and social and emotional well-being.[12345] The exact etiology is yet to be established, but various implicating factors include sun exposure and hormonal, genetic, and toxic ingredients in cosmetics and drugs such as oral contraceptives (OCPs) and phenytoin.[6]
Both melanocytosis and increased melanogenesis are responsible for the increased pigmentation in melasma. Other factors involved in the pathogenesis include various vascular growth factors (mainly vascular endothelial growth factor [VEGF]) and genetic factors (role of H19, iNOS, and Wnt pathway modulator genes). Identification of these factors could lead to the development of newer treatment options for melasma.[789]
According to the area of distribution, three clinical patterns of melasma are recognized, namely the centrofacial, malar, and mandibular patterns. Among these, the centrofacial pattern is most common.[101112]
The existing treatment modalities such as hydroquinone-based, triple-combination creams, kojic acid, azelaic acid, chemical peels, and lasers have their inherent side effects, especially on prolonged usage and limited efficacy. Moreover, the condition often relapses on discontinuation of therapy. Because there is no satisfactory treatment for melasma, research is ongoing to develop a newer, safer, and innovative treatment for this disorder that causes profound cosmetic disfigurement, significant psychological stress and embarrassment to patients.[213]
The use of tranexamic acid (TXA) for the treatment of melasma is a novel concept. It is a plasmin inhibitor and works on melasma by decreasing melanin synthesis through its antiplasmin activity. It can be used orally, topically, intradermally, and transepidermally through a microneedle. The results of recent clinical trials for the use of TXA in melasma are promising, and it can serve as a ray of hope for treating this challenging condition in future.
MATERIALS and METHODS
This study was carried out in the department of dermatology at a tertiary care hospital of North India by enrolling forty consecutive patients with facial melasma presenting in the outpatient department between February 2016 and March 2017. The diagnosis was based on history and clinical examination.
Inclusion criteria
Subjects aged 18–50 years with melasma
Subjects who were willing to sign a written consent form prior to participation in the study.
Exclusion criteria
Subjects aged <18 or >50 years
Pregnant and lactating women
Patients taking OCPs
Patients with a history of thromboembolism, bleeding disorders, and abnormal coagulogram
Patients who had taken any other melasma therapy within the last three months
Patients with a history of keloidal tendency or post-inflammatory hyperpigmentation (PIH).
The enrolled patients were randomly divided into two groups of 20 patients each. Written informed consent was taken from each patient before starting the study. A detailed history and general examination was conducted in each case. Each patient was subjected to routine blood investigations at the time of reporting along with bleeding time (BT), computed tomography, and International Normalized Ratio (INR).
Treatment protocol
TXA is available as 250-mg tablets and as 500-mg/5-mL injection ampules. To prepare the solution for the transepidermal application, 0.4 mL of sterile water was taken out from a 10-mL sterile water ampule and 0.4-mL of TXA (100 mg/mL) was injected into the sterile water ampule, thus preparing 10 mL of 4 mg/mL solution of TXA.
Group A patients received oral tablet TXA at a dose of 250 mg twice daily for 12 weeks, and Group B patients were given TXA solution transepidermally into the melasma lesions using a dermaroller.
Procedure
After gentle cleansing with normal saline, topical anesthetic cream was applied over the area to be treated and was left for about 60 min. After clearing extra anesthetic cream with sterile gauze, a dermaroller of 1 mm was passed three times over each area in each direction, vertically, horizontally, and in both diagonals. A volume of 0.5–1.0 mL (depending on the area to be covered) of 4 mg/mL of TXA was applied over the area to be treated, after which the dermaroller was passed once again over each area in each direction as done previously. After the procedure, ice packs were placed over the treated area for 60 s. The patients were sent home after applying Tegaderm™ film dressing and were instructed to remove it after 1 h. The procedure was repeated every two weeks for 12 weeks.
The patients in both groups were advised to avoid excessive sun exposure and to apply a broad-spectrum sunscreen in the morning and to reapply at every 3-h interval thereafter till evening, every day.
Patient evaluation
All the patients were evaluated for therapeutic outcome both objectively (by Melasma Area and Severity Index [MASI], which was the primary outcome measure) [Table 1] and photographically at 4, 8, 12, 18, and 24 weeks. Side effects known to be associated with TXA as well as other side effects that seem relevant to the treatment were evaluated on every visit.
| Grades of improvement | Reduction in MASI score |
|---|---|
| 0: No response | No improvement |
| 1: Mild response | <25% improvement |
| 2: Moderate response | 25%–50% improvement |
| 3: Good response | 51%–75% improvement |
| 4: Very good response | >75% improvement |
Statistical analysis
Response to treatment at all the follow-up visits among different subjects within a group was compared and analyzed using related-samples Friedman’s test considering P < 0.05 as statistically significant. The difference of response to treatment among the two treatment groups was compared using independent samples t-test or Mann–Whitney U test, wherever applicable. The categorical variables were analyzed statistically using Pearson’s Chi-square test or Fisher’s exact test, wherever applicable. The data were entered in the form of a data matrix in Microsoft® Excel 2007 and were analyzed statistically employing appropriate tests of significance using IBM® SPSS version 20.0.0®.
RESULTS
A total of forty participants (31 females, 77.5%) with a mean age of 30.25 years (range, 20–48 years) were enrolled. The majority of the patients, 24 (60%), had a centrofacial pattern followed by a malar pattern in 16 (40%) patients. These 40 patients were randomly assigned to Group A (N = 20) for oral TXA 250 mg, twice daily, and Group B (N = 20) for transepidermal administration of TXA (4 mg/mL) through microneedling given at an interval of two weeks for 12 weeks. Thereafter, they were followed up for improvement in MASI score, adverse effects, and relapse at a four-week interval for 24 weeks. Both the groups were statistically similar with regard to the age of patients, duration of melasma, and baseline MASI score [Table 2]. The baseline MASI score was 11.98 (±5.47) in Group A and 12.13 (±3.16) in Group B.
| Characteristics | Number of patients (N = 40) | |
|---|---|---|
| Gender | Female | 31 |
| Male | 9 | |
| M:F | 1:3.4 | |
| Age | Range (years) | 20–48 |
| Mean (years) | 30.25 ± 6.95 | |
| Duration of melasma | Range (years) | 0.5–9 |
| Mean (years) | 2.98 ± 2.24 | |
| MASI score | Range | 4.50–23.20 |
| Mean | 12.06 ± 4.32 |
The mean MASI scores in Group A at the end of 4, 8, 12, 18, and 24 weeks were 9.75 (±4.83), 6.09 (±3.64), 4.21 (±2.48), 2.56 (±1.95), and 3.10 (±3.38), whereas the values were 9.73 (±3.32), 6.06 (±2.75), 4.55 (±1.89), 2.94 (±1.36), and 3.09 (±1.32) for Group B, respectively.
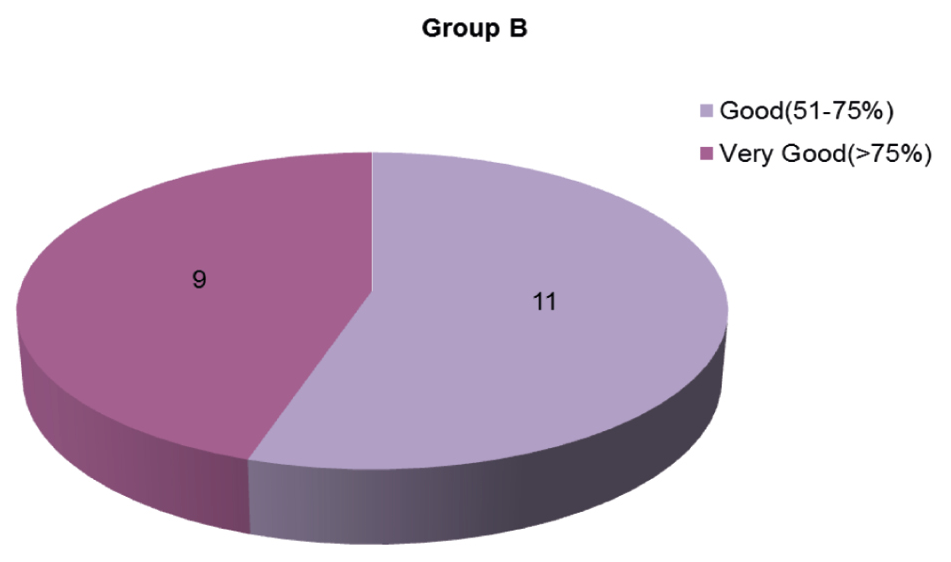
At the end of a 24-week follow-up period, a good and very good response occurred in five (25%) and 14 (70%) patients in Group A [Figures 1,2 and Graph 1] and 11 (55%) and nine (45%) patients in Group B [Figures 3, 4 and Graph 2], respectively. However, the final reduction in MASI score was similar in both the groups [Tables 3, 4 and Graph 3]. Relapse was uncommon and occurred at 24 weeks in one patient from Group A.
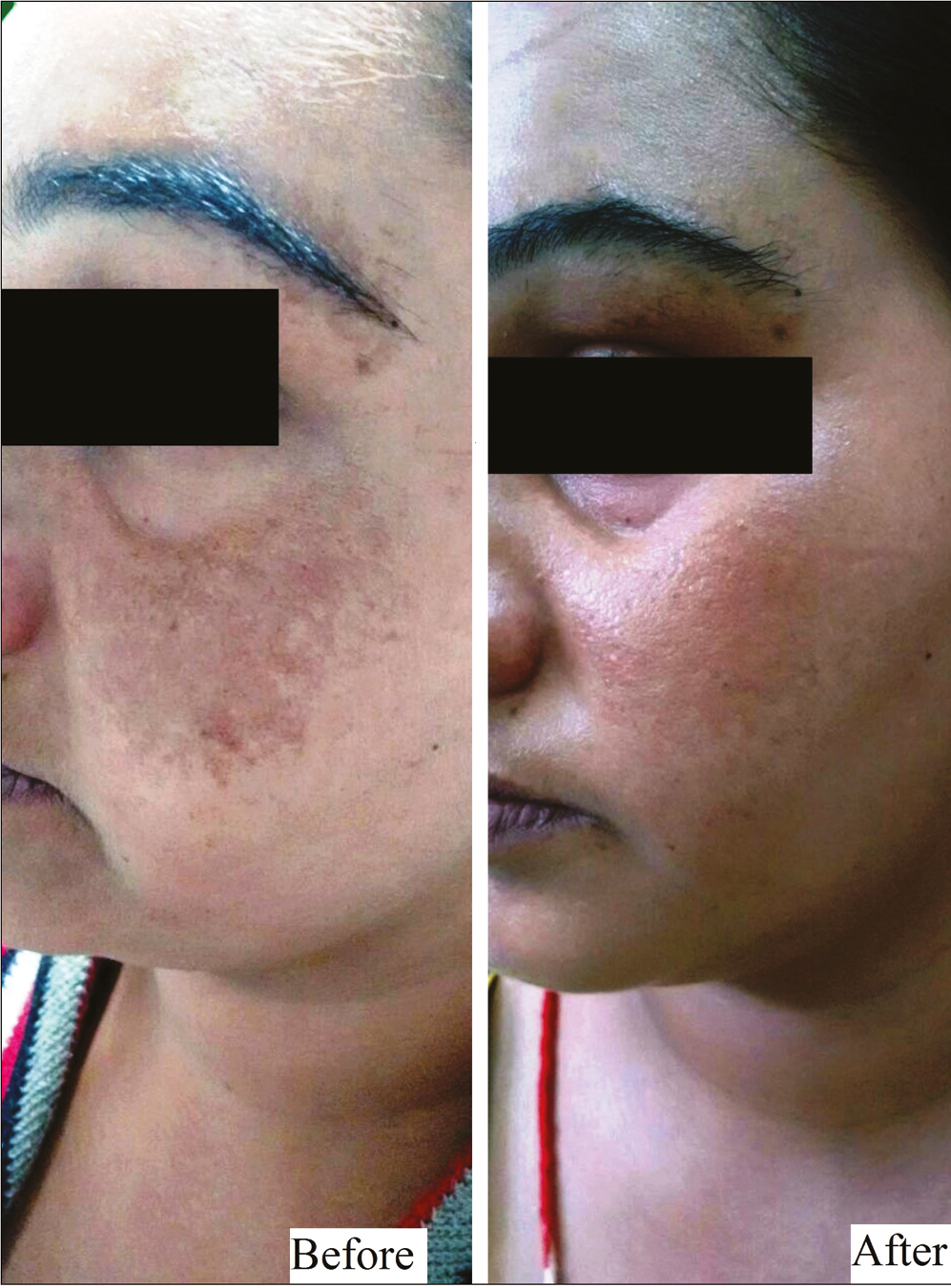
- Group A (oral TXA) – patient 1
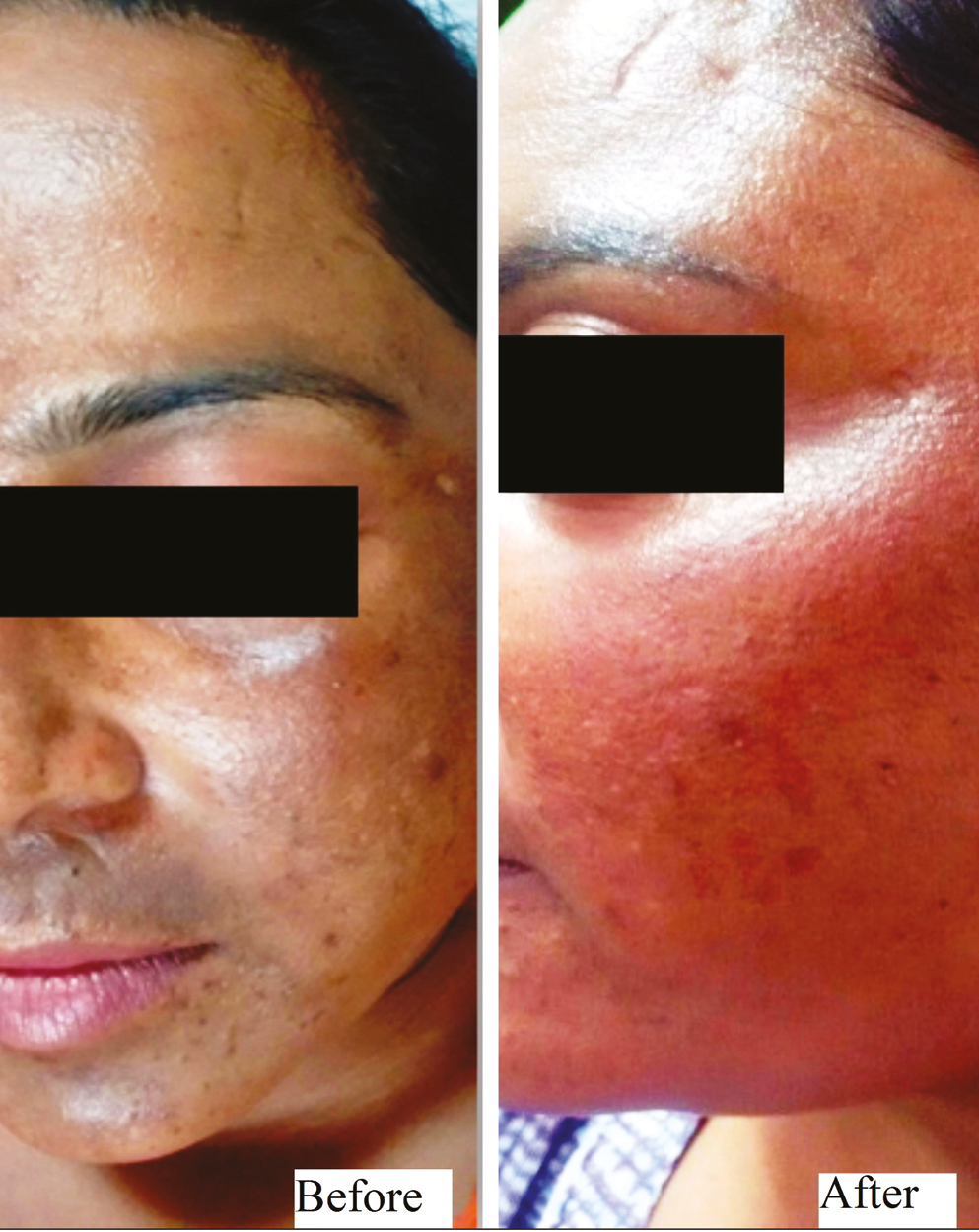
- Group A (oral TXA) – patient 2
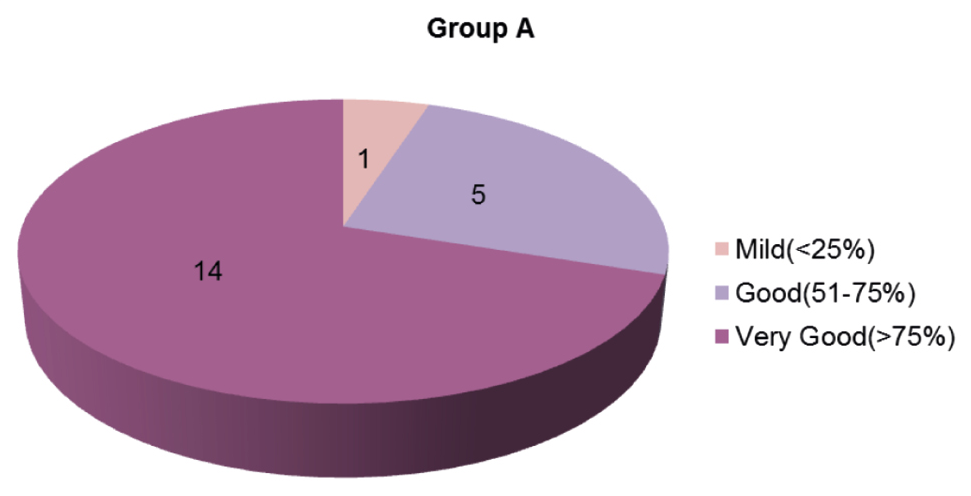
- Grading of improvement in Group A
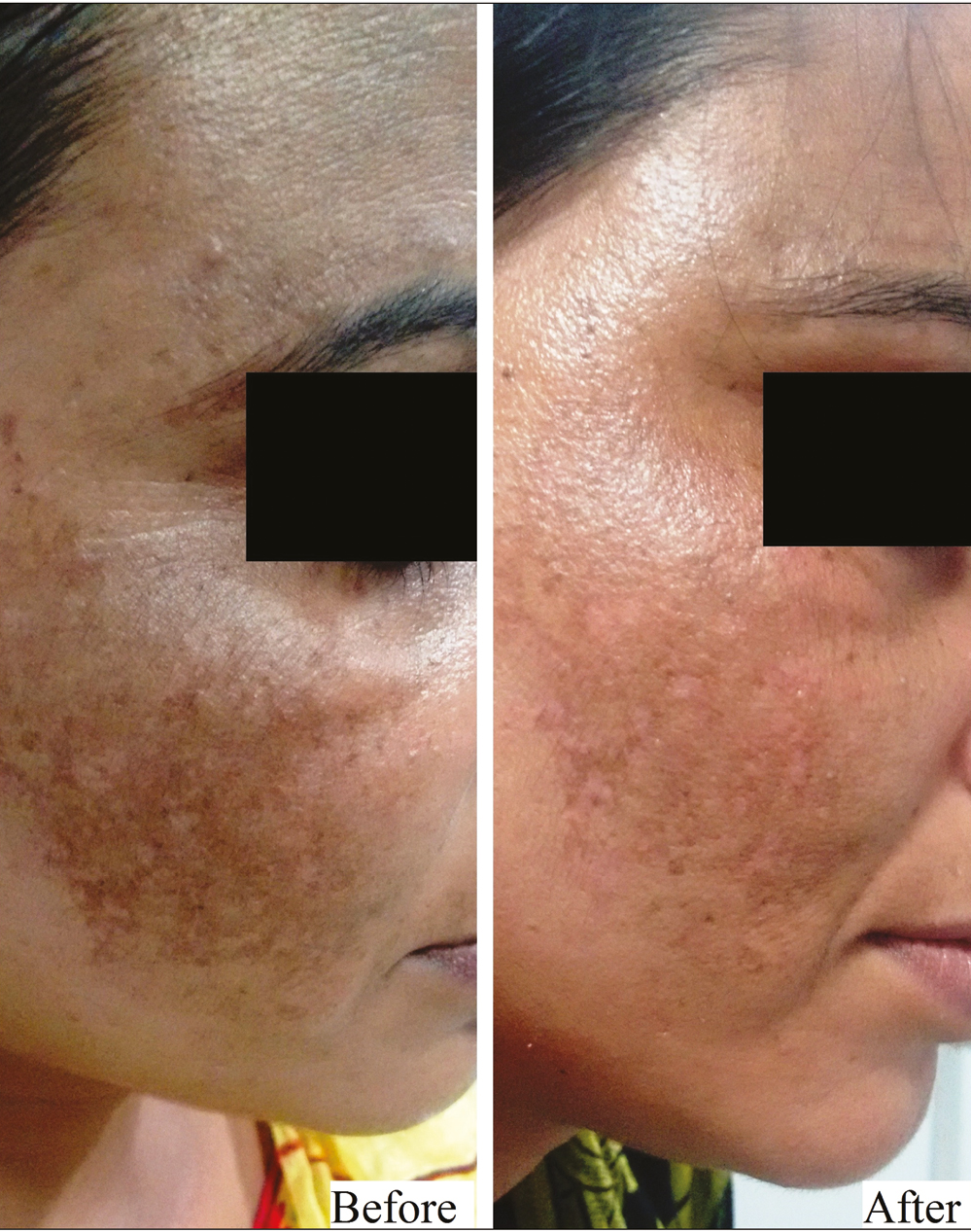
- Group B (Dermaroller) – patient 1
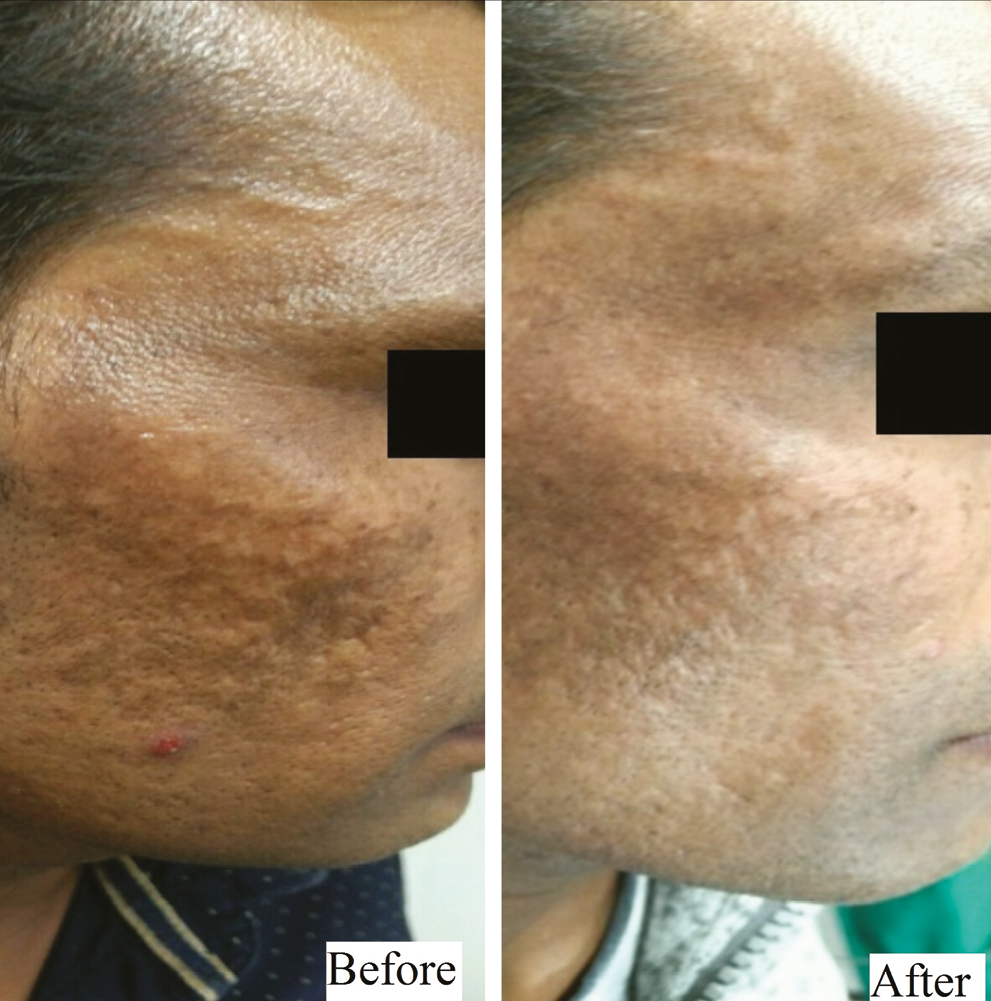
- Group B (Dermaroller) – patient 2
- Grading of improvement in Group B
| Time (weeks) | Percentage reduction in MASI score | |
|---|---|---|
| Group A (oral), N = 20 | Group B (microneedling), N = 20 | |
| 4 | 18.61 | 19.78 |
| 8 | 49.16 | 50.04 |
| 12 | 64.85 | 62.48 |
| 18 | 78.63 | 75.76 |
| 24 | 74.12 | 74.52 |
| Grades of improvement | Group A (oral), N = 20 | Group B (microneedling), N = 20 | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Mild (<25%) | 1 | 5.00 | - | - |
| Moderate (25%–50%) | - | - | - | - |
| Good (50%–75%) | 5 | 25.00 | 11 | 55.00 |
| Very good (>75%) | 14 | 70.00 | 9 | 45.00 |
| P | 0.105 | |||
P ≥ 0.05 not significant
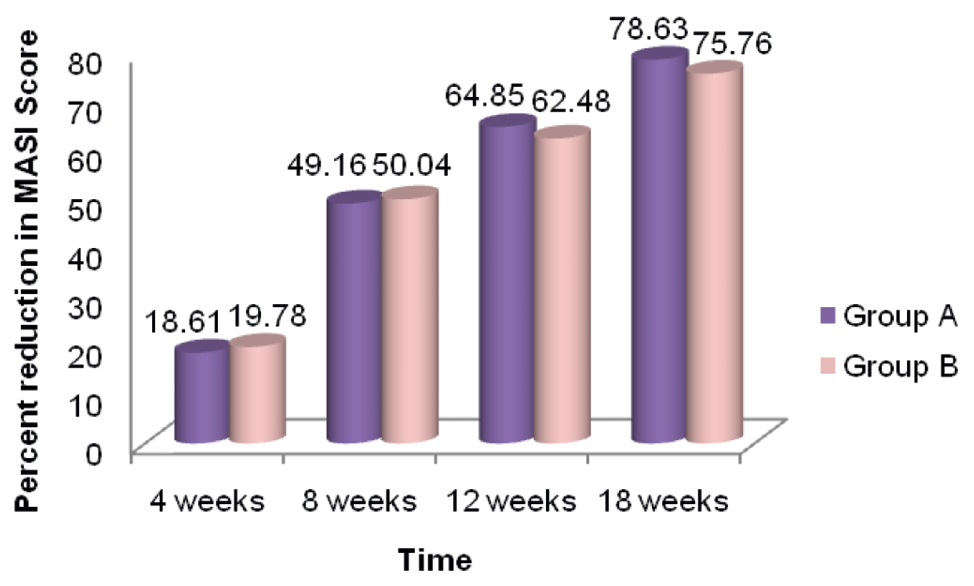
- Comparison of percentage reduction in MASI score with time in both groups
TXA was found to be effective by both routes of administration in our study. When compared, highly significant improvement was obtained in both the groups, but there was no statistically significant difference between the two groups with regard to the overall response seen in the subjects.
In this study, only mild adverse effects were noted in the form of epigastric discomfort (two patients) with oral TXA and pain at injection site (seven patients) with transepidermal administration of TXA with microneedling, which did not warrant its discontinuation. Transient reversible hypomenorrhea, which has been commonly seen with oral TXA, was not observed in our study.
DISCUSSION
Management of cutaneous disorders of hyperpigmentation generally requires a long duration of treatment, and so does melasma. Historically, the treatment of melasma has been challenging because of the refractory and recurrent nature of the condition. The treatment for melasma is aimed to slow down the proliferation of melanocytes, inhibit the formation of melanosomes, and promote their degradation.[13]
The most tried topical therapy is hydroquinone and its triple combination with tretinoin and corticosteroids, which is considered the gold standard for melasma. Besides that, a whole range of drugs that have been tried in the past with limited success includes azelaic acid, kojic acid, arbutin, ascorbic acid, glycolic acid, and salicylic acid peels.[14] Multiple novel drugs are being investigated for their potential as hypopigmenting agents, out of which TXA appears to deliver promising results. Its usage for the treatment of melasma was initially reported in 1979 by Nijo in Japan.[15] It was discovered accidentally in a patient with chronic urticaria being treated with TXA.
The pathology of melasma includes epidermal and dermal hyperpigmentation, basement membrane weakening, vascular proliferation, and increased number of mast cells. TXA works on melasma by a variety of mechanisms. It inhibits ultraviolet-induced pigmentation through its antiplasmin activity. It decreases angiogenesis and neovascularization by its action on VEGF and basic Fibroblast Growth Factor (bFGF). Mast cell tryptase is responsible for the degradation of collagen, and hence weak basement membrane and solar elastosis are seen in melasma. The effect of TXA on melasma can be partly due to its inhibitory effect on mast cells as well.[16]
In a split-face controlled trial done by Saki, et al. on 37 patients with melasma, topical hydroquinone cream was compared with intradermal injections of TXA, and TXA was found to have a better therapeutic outcome.[17]
Our study showed a statistically significant reduction in MASI score as compared to that of baseline after treatment with both oral and transepidermal administration of TXA (P < 0.0001). Significant improvement was noted from eight weeks onward at each follow-up visit.
These results are comparable with those of a study conducted by Christina, et al. enrolling 10 patients in a prospective split-face trial lasting 12 weeks. One side of the face received 4 mg/mL of TXA, whereas the other side received placebo (normal saline solution). The patients were given weekly treatment with a dermaroller. The MASI score showed a decreasing trend as the treatment continued. In another study conducted in Korea on melasma patients, TXA was directly administered intradermally (4 mg/mL) weekly for a period of 12 weeks. More than 75% of the patients experienced a statistically significant improvement.[18]
Zhu, et al. reported that increasing the treatment duration was more effective than increasing the dose of TXA. The duration of therapy has varied in different studies, ranging from six weeks to a maximum of six months. The onset of effect was mostly in four weeks compared with eight weeks in our study. The dosage has also varied from 500 mg/day to 1.5 g/day, but consensus has evolved around 500 mg/day.[19]
Budamakuntla, et al. compared the efficacy of TXA given through microinjections with through microneedling. There was 35.72% improvement in the MASI score in the microinjection group compared with 44.41% in the microneedling group, at the end of a follow-up period of six months.[20] However, in our study, we observed a greater degree of reduction in MASI score in both the treatment groups.
CONCLUSION
Oral and transepidermal TXA appear equally effective, suggesting that the efficacy of TXA is perhaps independent of its route of administration. Oral therapy is convenient for patients. The duration of therapy is perhaps more important than the doses, higher than 500 mg/day for better therapeutic effect in the long term, but because of potential adverse effects such as coagulation abnormalities, it is given up to a maximum of six months. Transepidermal therapy of TXA has the advantage of local action; hence, systemic side effects are avoided, but it is slightly painful and requires a special instrument (dermaroller), and the time period between consecutive microneedling sessions is left to the prerogative of the treating doctor; hence, a proper quantification is lacking. Moreover, scheduling of maintenance sessions is necessary as melasma is prone to recurrence.
Limitation
The major limitation was a small sample size because of time limitation and the long follow-up period.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Effect of melasma on quality of life in a sample of women living in southern Brazil. J Eur Acad Dermatol Venereol. 2008;22:655-62.
- [Google Scholar]
- The prevalence of melasma and its association with quality of life in adult male Latino migrant workers. Int J Dermatol. 2009;48:22-6.
- [Google Scholar]
- Prevalence of self-diagnosed melasma among premenopausal Latino women in Dallas and Fort Worth, Tex. Arch Dermatol. 2007;143:424-5.
- [Google Scholar]
- Hypomelanoses and hypermelanoses. In: IM Freedburg, Az Eisen, K Woeff, eds. Dermatology in General Medicine. New York: McGraw-Hill; 1999. p. :945-1016.
- [Google Scholar]
- Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-7.
- [Google Scholar]
- Pigmentary disorders. In: RG Valia, ed. IADVL Textbook of Dermatology (3rd ed). Mumbai: Bhalani Publishing House; 2008. p. :781-2.
- [Google Scholar]
- Transcriptional profiling shows altered expression of Wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J Invest Dermatol. 2011;131:1692-700.
- [Google Scholar]
- H19 RNA downregulation stimulated melanogenesis in melasma. Pigment Cell Melanoma Res. 2010;23:84-92.
- [Google Scholar]
- Melasma. In: AD Katsambas, TM Lotti, eds. European Handbook of Dermatology Treatments. Berlin: Springer; 2003. p. :336-41.
- [Google Scholar]
- Origin, clinical presentation, and diagnosis of facial hypermelanoses. Dermatol Clin. 2007;25:321-6, viii.
- [Google Scholar]
- Melasma and its impact on health-related quality of life in Hispanic women. J Dermatolog Treat. 2007;18:5-9.
- [Google Scholar]
- Newer and upcoming therapies for melasma. Indian J Dermatol Venereol Leprol. 2012;78:417-28.
- [Google Scholar]
- Effect of tranexamic acid on melasma: A clinical trial with histological evaluation. J Eur Acad Dermatol Venereol. 2013;27:1035-9.
- [Google Scholar]
- Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: A split-face controlled trial. J Dermatolog Treat. 2018;29:405-10.
- [Google Scholar]
- Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: A preliminary clinical trial. Dermatol Surg. 2006;32:626-31.
- [Google Scholar]
- A randomised, open-label, comparative study of tranexamic acid microinjections and tranexamic acid with microneedling in patients with melasma. J Cutan Aesthet Surg. 2013;6:139-43.
- [Google Scholar]






