Translate this page into:
Dressing Materials: A Comprehensive Review
Address for correspondence: Dr. Karan Malhotra, 480 Parnasree, Kolkata 700 060, West Bengal, India. E-mail: dr.karan2015@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Injury to the skin provides a difficult challenge, as wound healing is a complex and dynamic process. Wound healing process recruits three different phases: inflammation, proliferation, and maturation. The sequence of events involved in wound healing can be affected by numerous disease processes, resulting in chronic, non-healing wounds that give significant discomfort and distress to the patients while draining the medical fraternity of enormous resources. Wound tissue never reaches its pre-injured strength and multiple aberrant healing states can result in chronic non-healing wounds. There is a growing concern about the usage of correct materials for wound dressings. The development of new and effective treatments in wound care still remains an area of intense research. There are a number of wound dressings available in the market. The objective of the article is to enhance knowledge about characteristics of an ideal wound dressing and guide in finding the correct dressing material. It also provides a detailed classification of traditional and modern wound dressings.
Keywords
Dressing
healing
inflammation
regeneration
wound
Dressing is an important component of wound care. Basic and practical knowledge of dressing material is imperative for faster wound healing. Choice of dressing depends upon the type of wound, infection, discharge, and safety

INTRODUCTION
The skin being the largest organ in the human body with a surface area of around 2 square meters, and skin performs a wide range of intricate tasks that are vital to our existence.[123] The skin is made up of three layers: the epidermis, dermis, and hypodermis, all of which are naturally self-renewable and serve a variety of purposes.[4] The primary role of the skin is to act as a barrier, guarding inner organs against microbial invasion and ultraviolet radiation while also controlling the body temperature.[5] The immune system and the body’s sensory detection process are also aided by the skin.[6] Skin injuries present a special challenge since wound healing is a difficult and sophisticated process.
WOUND
A wound is defined as a break in the continuity of the epithelial lining of the skin or mucosa as a result of thermal or physical trauma. Acute and chronic wounds are distinguished by the length and kind of the healing process.[78] An injury to the skin caused by an accident or surgical procedure is referred to as an acute wound. Depending on the size, depth, and degree of the injury to the epidermis and dermis layers of the skin, it normally recovers between 8 and 12 weeks.[910] On the other hand, chronic wounds are unable to heal in an ordered and timely manner and fail to move through the typical stages of healing.[1112] Burns, leg ulcers, and decubitus ulcers are the three main causes of chronic wounds.
WOUND HEALING
One of the most important, challenging, and dynamic processes in a human life is healing of wounds of varying thickness and intensity.[1314] To restore the integrity of the damaged tissue and regenerate the missing one, various components of the human body (such as extracellular matrix (ECM) molecules, mediators, fibroblasts and keratinocytes, and infiltrating leukocyte subtypes) work in a complicated interplay.[15] Inflammation, proliferation, and remodeling are the three consecutive and overlapping phases that can be used to characterize the fundamental components of wound healing.[16]
Four distinct phases of tissue development and regeneration make up the dynamic and complex process of wound healing Figure 1:[171819202122]
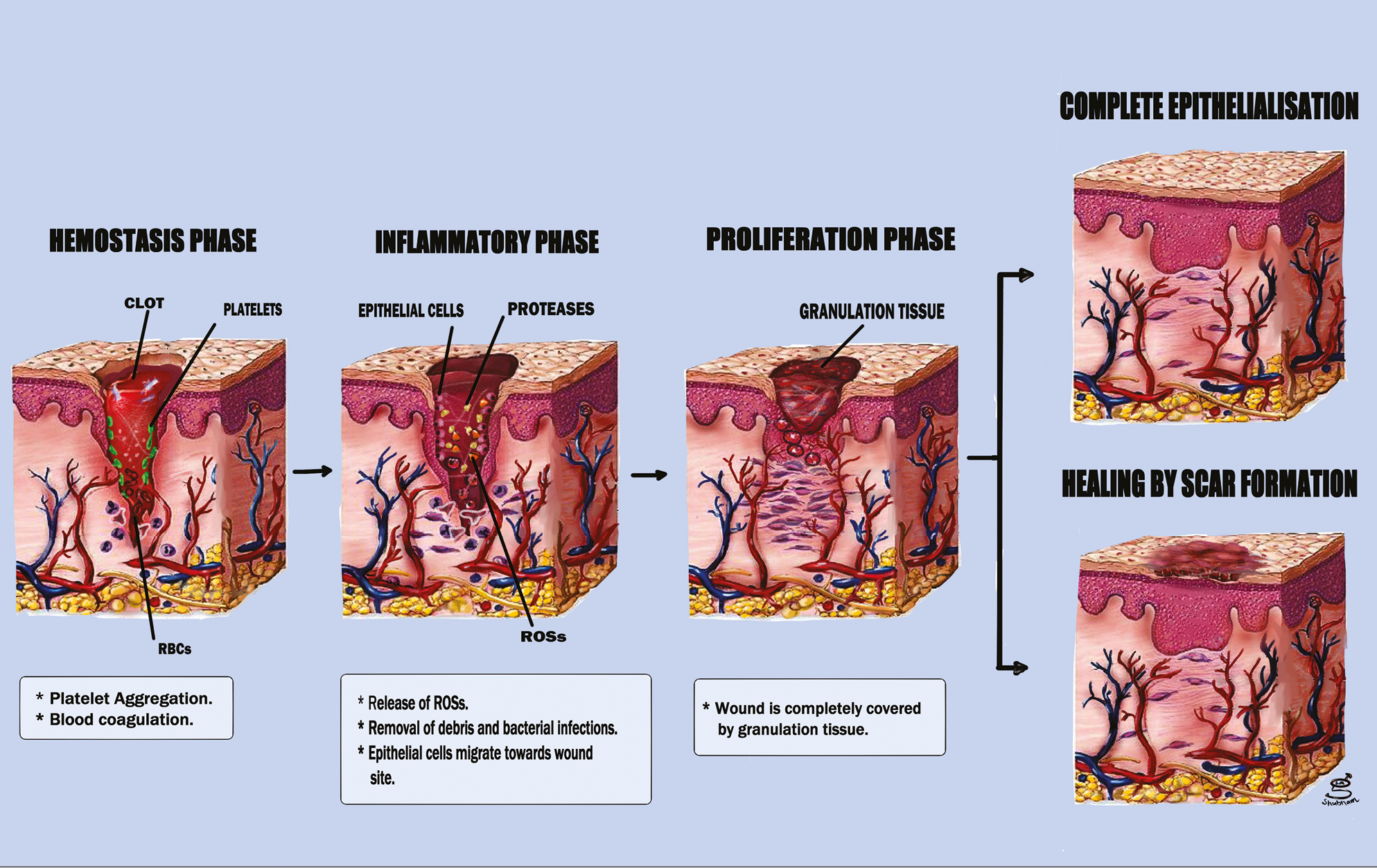
- Illustrates the sequence of events in various phases of wound healing
-
(i)
the coagulation and hemostasis phase (immediately following injury);
-
(ii)
the inflammatory phase, which occurs shortly after tissue injury and is characterized by swelling;
-
(iii)
the period of proliferation, during which new blood vessels and tissues are produced, and
-
(iv)
the maturation stage, during which the remodeling of new tissues occurs.
PHASES OF WOUND HEALING
The sequence of events in various phases of wound healing have been summarized in Table 1.[23]
| 1. Hemostasis phase | a. Histamine, serotonin with other vasoactive substances is released as a result of blood factor leakage. b. Through platelet activation and the release of growth factors, including platelet derived growth factor (PDGF), and pro-inflammatory cytokines, fibrin clot is formed to achieve hemostasis. |
| 2. Inflammatory phase | a. Recruitment of cells such as keratinocytes, monocytes, and polymorphonuclear leukocytes, which are crucial for the development of wound healing. |
| 3. Proliferative phase | a. Epithelialization and neovascularization are present, as well as fibroblast activity and granulation of extracellular matrix components. b. A number of cytokines and growth factors, such as vascular endothelial growth factor, are crucial. |
| 4. Maturation phase | a. Scar tissue’s newly laid down collagen is eliminated by releasing, activating, or inhibiting numerous preteolytic and degradative enzymes, such as matrix metalloproteinase. b. In order to reach the closest attainable tensile strength (80%) of the original structure, the scar mutation continues for a considerable amount of time. |
FACTORS AFFECTING WOUND HEALING
The various factors affecting wound healing have been summarized in Table 2.[2425]
| Local factors | Genetic factors | ||
|---|---|---|---|
| Presence of foreign bodies, local radiation, and occlusion | Occlusion promotes wound healing | Venous insufficiency | Varicose veins |
| Local infection | Necrotic tissue, poor tissue blood supply | Systemic disease | For example, jaundice, uremia, anemia, diabetes, and malignancies, etc. |
| Site | Wounds of the head, neck and hand always heal well in contrast to those below the knee | Age | Protein turnover reduces with age, leading to slower healing |
| Rest | Enough rest ensures faster healing | Cytotoxic drugs and immunosuppressives e.g., steroids | Depress the proliferation of fibroblasts, keratinocytes, and other cells involved in wound healing |
| Tension of the tissue | Inhibits blood supply leading to delayed healing | Malnutrition | Leads to defective synthesis of collagen and ground substance |
WOUND DRESSING
The history of medicine and wound care has been extensively documented. According to English literature, the world’s oldest medical document and the first written historical record were both discovered on Sumerian clay. In this manuscript, the “three healing gestures” were defined as: washing the wound, applying dressings, and bandaging.[26]
Since the beginning of time, a variety of substances have been applied to wounds to halt bleeding, absorb exudates, and speed up healing. Honey, animal oils or fat, cobwebs, mud, leaves, sphagnum moss, and animal excrement were a few of these items.[27]
It is crucial to properly care for any wound, regardless of how severe it is, which involves applying wound dressing. A bandage holds the dressing in place, whereas a dressing is intended to be in contact with the wound. Wet-to-dry dressings have long been a popular choice for wounds that need to be debrided. In 1600 Before Christ, linen strips wrapped in plaster and saturated in oil or grease were used to patch wounds. Beginning around 2500 Before Common Era, clay tablets of Mesopotamian origin were used to cure wounds. Before applying a honey or resin bandage, they cleansed the wounds with water or milk. Hippocrates of ancient Greece practiced the use of wine or vinegar for washing the wounds with honey, oil, and wine as further treatment from 460 to 370 Before Common Era. They used wine or water-boiled wool as a bandage.[28] Antibiotics were developed in the 19th century, marking a significant advance in the antiseptic technique. The 20th century saw the introduction of modern wound dressings.[29]
CHARACTERISTICS OF AN IDEAL WOUND DRESSING
The characteristics of an ideal wound dressing have been summarized in Figure 2.[30]
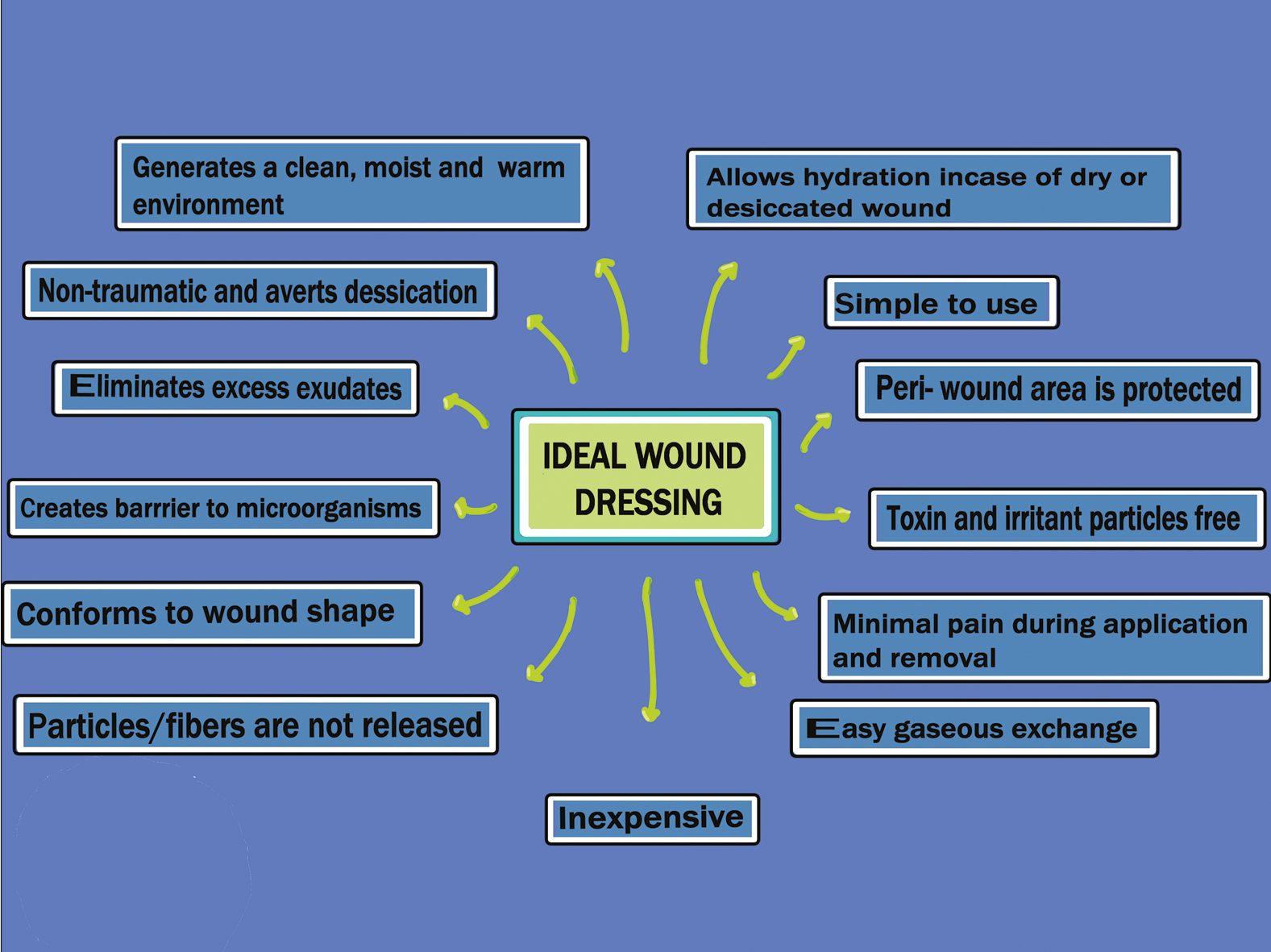
- The characteristics of an ideal wound dressing
TRADITIONAL WOUND DRESSINGS[14]
Gauze, lint, plasters, bandages (natural or synthetic), and cotton wool are common dry items used as primary or secondary dressings for wounds to prevent contamination.
Gauze
Gauze dressings composed of cotton, rayon, and polyester woven and nonwoven fibers offer some level of defense against bacterial infection. With the aid of their fibers, some sterile gauze pads can be utilized to absorb liquids and exudates from an open wound. The frequent replacement of these dressings is necessary to prevent the maceration of healthy tissues. Gauze bandages are less economical. Excessive wound drainage causes dressings to get wet and stick to the wound, making removal uncomfortable. Wet wraps using gauze are commonly used in maintaining remission of atopic eczema and dry skin conditions.
Bandages
Different purposes are served by bandages made of natural cotton wool, cellulose, or synthetic bandages made of polyamide materials. For instance, short stretch compression bandages and high compression bandages give prolonged compression in the event of venous ulcers, whereas cotton bandages are used to retain light dressings.
Tulle dressings
Examples of tulle dressings ideal for clean, superficial wounds that are commercially available include Bactigras, Jelonet, and Paratulle. Traditional dressings should typically be used as a secondary dressing or for clean, dry wounds with low exudate levels. Modern dressings with more sophisticated formulations have taken the place of conventional dressings because they cannot provide a moist environment for the wound.
MODERN WOUND DRESSINGS
Films
These dressings are made of translucent, adherent polyurethane, which permits gaseous exchange from the wound for oxygen, carbon dioxide, and water.[31] Films were initially occlusive since they were made of nylon derivatives and supported by an adhesive polyethylene frame. Nylon-derived film dressings were initially avoided for significantly exuding wounds due to their inadequate absorption capacity and propensity to cause maceration of the wound and the healthy tissues surrounding it.[32] However, these dressings are so elastic and flexible that additional tapping is not required for them to conform to any shape. For venous access locations, more advanced films are produced with different moisture/vapor permeabilities. They can be used to create a sterile field. Since films are transparent, monitoring the wound is made straightforward. Due to their film structure, they are semi-occlusive and retain moisture, allowing for autolytic debridement of necrotic wounds and the creation of a moist healing environment for granulating wounds. It is therefore recommended to use dressings such as Opsite™, Tegaderm™, and Biooclusive™ for epithelializing wounds, shallow wounds, and wounds with little exudates. Variations can be seen in the extensibility, conformability, and vapor permeability of commercially available film dressings.[33]
Hydrocolloids
Hydrocolloid dressings, which feature two layers—an outer, water-impermeable layer and an inner, colloidal layer—are the most often used interactive dressings.[14] They contain gelatine, pectin, alginate, sodium carboxymethylcellulose, and other ingredients that gel. When these polymers come into contact with wound exudate, they swell and absorb it.[34] Hydrocolloids can absorb wound exudates, debride wounds, and are impermeable to germs but porous to water vapor.[35] Hydrocolloid dressings are occlusive, which means they keep water, oxygen, and germs from getting into the wound. Granulation and angiogenesis may be facilitated by this. Additionally, hydrocolloids reduce the pH of the wound surface because bacteria cannot grow in an acidic environment. Another key benefit is the hydrocolloid wound dressings’ capacity to conform to the anatomy of the body and adhere effectively to high-friction areas such as the sacrum and heels. They are used in lightly to moderately exuding lesions such pressure sores, minor burns, and traumatic wounds. Because they do not cause pain on removal, these dressings are also recommended for managing pediatric wound care.[36] When these hydrocolloids come into contact with the wound exudate, they form gels and a moist environment, preserving the granulation tissue by absorbing and holding onto exudates. Granuflex™, Comfeel™, and Tegasorb™ are all widely available in thin films or sheets. The drawback of hydrocolloids is that they are not suggested for wounds with excessive exudate or neuropathic ulcers. They frequently serve as an additional dressing as well.[14]
Hydrogels
Utilizing synthetic polymers such as polymethacrylates and polyvinyl pyrrolidine, hydrogels—insoluble hydrophilic materials—are produced. In a wet environment, the high water content (70%–90%) of hydrogels benefits granulation tissues and epithelium. carboxymethyl cellulose (CMC), agar, glycerol, and pectin can all be used in the production of hydrogel wound dressings. The cooling and soothing properties of hydrogels help to bring down the warmth of skin sores. Hydrogels are used to treat burn wounds, necrotic wounds, pressure ulcers, and dry chronic wounds. The hydrogel dressings can be applied to dermabrasions, minor burns, and skin donation sites. Use hydrogels to keep a clean, healthy, granulating lesion moist and to perform autolytic debridement in wounds with necrotic tissue like slough or eschar. Hydrogel dressings, with the exception of infected and severe drainage wounds, are suitable for all four stages of wound healing, according to Morgan.[37] Hydrogel dressings are non-irritating, metabolite-permeable, and insensitive to living tissue. Numerous studies have shown that hydrogel dressings can be used to heal chronic leg ulcers. Hydrogel dressings have the drawback of accumulating exudate, which leads to maceration and bacterial development that fouls up wound odors. Additionally, handling hydrogels is difficult due to their weak mechanical strength. Hydrogels include things like Intrasite™, Nu-gel™, Aquaform™ polymers, sheet dressings, impregnated gauze, and water-based gels.[38]
Foams
Foam dressings may have foam that is hydrophilic or hydrophobic and have edges that stick.[39] The hydrophobic properties of the outer layer shield against liquid while enabling gaseous exchange and water vapor. The porous foams are flexible materials with a high capacity for absorption. With a silicone basis, rubber foam (silastic) adapts to the contour of wounds. Foam can absorb a variety of wound drainage, depending on the thickness of the lesion. There are foam treatments that are both adhesive and non-adhesive. Foam dressings, which are also advised for granulating wounds, can help lower leg ulcers and mild to severely oozing wounds. They are frequently used as primary dressings for absorption because of their high absorption capacity and moisture vapor permeability, eliminating the need for additional dressings.[3840] Inadequate for low-exuding wounds, dry wounds, and dry scars, which depend on exudates for healing, foam dressing is one of its drawbacks. It also requires frequent applications. Foam dressings available for use in wound care include Silastic™, Lyofoam™, Tegaderm™, HyperFoam™, Shingna™, Allevyn™, and Tielle™, to name a few.[40]
Alginates
Alginate dressings are made using the sodium and calcium salts of mannuronic and guluronic acid units. Alginates that are biodegradable and absorbent come from seaweed. Absorption is made possible by a robust hydrophilic gel formation that lowers bacterial contamination and wound exudates. In certain studies, alginate has been demonstrated to stop keratinocyte migration but a study by Thomas et al.[41] suggested that alginates can hasten the healing process by triggering macrophage activation to produce tumor necrosis factor alpha, which initiates inflammatory signals. When alginate dressings are applied to a wound, blood ions interact with the ions in the alginate to form a protective layer. It acts as the primary dressing for the treatment of significantly oozing wounds, including acute wounds such as abrasions, lacerations, and post-surgical wounds, as well as chronic leg ulcers, pressure ulcers, and fungating carcinomas. It is not suggested for severe wounds with exposed bone, third-degree burns, or dry wounds. Additional dressings are also required because these dressings run the risk of drying up the wound and delaying recovery. High levels of absorption, biodegradability, gelling, and biocompatibility are few of the excellent intrinsic properties that alginate fibers possess. The dressings’ painless removal is facilitated by alginate’s tendency to gel. Since alginate can absorb 15–20 times its weight in fluid, it is utilized as dressings to treat moderate to heavy exudates.[42] Alginate dressings have been demonstrated to induce human macrophages to generate pro-inflammatory cytokines that have been linked to accelerated healing.[41] Alginate is extremely biocompatible and has a variety of fascinating properties, such as improved absorbency, swelling, and being non-toxic, non-irritating, and easy to use. Alginate, however, has some disadvantages, such as poor dimensional stability and untidy handling.[43] Alginate dressings such as Sorbsan™, Kaltostat™, CarraSorb™, AlgiDERO™, and Algisite™ are commercially available.[14]
Hydrofibers
The well-known hydrofiber dressing Aquacel™ was developed by American company ConvaTec Ltd. in 1997. Sodium carboxymethyl cellulose is an ingredient in the sauces. This particular hydrocolloid can inflate significantly and absorb up to 25 times its initial weight, making it a more effective absorbent. These are the characteristics that are most sought-after for use in wound dressings. When the skin is punctured, many germs may grow on and/or inside the wound. In such circumstances, CMC wound dressings can absorb significant amounts of body fluids, hence removing a significant amount of the exudate. This may lead to a decrease in the number of germs on the wound surface.[4445] The CMC-containing non-woven dressing gives the patient a soft and comfortable feeling while providing the greatest outcomes for wound healing due to its conformability and high water absorption. CMC promotes wound healing and resembles alginates in appearance and gel-forming capacity. The CMC non-woven dressing absorbs liquid when it comes into intimate contact with the liquid rather than through the conventional capillary action procedure. Fluid that has been absorbed remains in the fiber’s structure even when crushed. There is not much fluid wicking across the dressing on the side. As it absorbs exudates, the dressing swiftly changes from a dry dressing to a soft cohesive gel sheet.
The CMC fibers maintain a wet environment for the greatest wound healing. It is very simple to remove with little to no damage to newly produced tissue, and it aids autolysis debridement. Abrasions, lacerations, incisions, donor sites, first- and second-degree burns, as well as chronic wounds like leg ulcers and pressure ulcers, can all be treated with CMC dressings. The dressing can also be used on surgical or traumatic wounds that have been purposefully neglected to heal.
Silver-containing hydrofiber dressings (Aquacel™) have been preferred as an effective adjunct for treatment of immunobullous disorders.[46] It is recommended for the local treatment of bleeding wounds, including donor sites, traumatic wounds, and wounds that have been degraded by mechanical or surgical processes.[47]
Bioactive wound dressings
The creation of bioactive dressings requires the use of biomaterials, which are essential to the healing process. These dressings are made from natural and synthetic materials[48] such as collagen,[49] hyaluronic acid (HA),[50] chitosan,[51] alginate and elastin, and are prized for their biocompatibility, biodegradability, and non-toxic properties. One or more of these materials’ polymers may be used, depending on the kind of wound. Growth factors and antimicrobials are occasionally added to biological dressings to hasten the healing of wounds.
Collagen is a crucial structural protein that has been extensively studied for its active role in the body’s natural healing process.[4952,53] Collagen begins to create fibroblasts and speeds up endothelial migration when it comes into contact with wounded tissue.[54] HA, a glycosaminoglycan that is a part of the ECM, has unique biological and physical characteristics. HA is naturally immune-suppressive, biocompatible, and biodegradable, just like collagen.[55] Chitosan promotes the growth of granulation tissue during the proliferative phase of wound healing.[56]
Tissue engineered skin substitutes
There are two types of tissue-engineered substitutes for human skin, or dermal equivalent (HSE); one replicates the layer of skin made up of keratinocytes and fibroblasts on collagen matrix (cell containing matrix). Only the dermal components with fibroblasts on collagen matrix are present in the second (acellular matrix). HSE’s main mechanism is to release and promote wound growth factor, which results in epithelialization. Bioengineered organisms have the ability to adjust to their surroundings and release the growth factors and cytokines used in dressings. Both venous leg ulcers and diabetic foot ulcers can be treated using bioengineered dressings.[57] Apligraf, a skin substitute for venous ulcers made of keratinocytes and fibroblast-seeded collagen, has received food and drug administration approval. Commercially available skin substitutes include Integra™ artificial skin, which is made of collagen and chondroitin six sulphate matrix and is covered with a thin silicone sheet; and Alloderm™, which is made of normal human fibroblasts with all cellular components removed. Laserskin™, Biobrane™, Bioseed™, and Hyalograft3-D™ are a few further alternatives.[58]
Medicated dressings
By removing necrotic tissues, medicated dressings with built-in medications contribute significantly to the healing process. Cleaning or debriding chemicals for necrotic tissue and antimicrobials that fight infection and encourage tissue regeneration have been used to achieve this. Compounds including growth hormones, enzymes, and antibacterial agents are frequently added. Cutisorb™ is a commercially marketed antimicrobial dressings. There are three types of accessible silver-impregnated dressings: silicone gels, fibrous hydrocolloid, and polyurethane foam film. Antiseptic Iodine treatment, which is extremely powerful against pathogen, operates on bacterial cells by interfering with protein activity and oxidatively degrading cell components. Iodine use for a long period of time causes skin irritation and discoloration.[59] Antimicrobials are mostly used to prevent or treat infections, particularly in diabetic foot ulcers.
Cellular activity brought on by growth factors that are present in our bodies automatically regulate the normal tissue repair process in the body. Growth factors and cells are stopped in the wound bed within the clots in cases of chronic wounds, which impairs the healing process. The most often used growth factor among the several growth factors is platelet derived growth factor (PDGF), which encourages chemotactic cell recruitment, proliferation, and increased angiogenesis. Additionally, PDGF, fibroblast growth factor, epidermal growth factor, and autologous platelet thrombin are all extensively researched for their potential applications in the healing process. Among them, PDGF and epidermal Growth Factor have food and drug administration approval for use in humans.
Another essential component to encourage a typical healing process is the enzymatic debridement of necrotic areas without hurting healthy tissue. Currently, ointments containing collagenase and papain are utilized to break down necrotic tissue. Papain destroys cystein residue and is linked to an inflammatory response, while collagenase affects native collagen and is mild on living collagen by gradually breaking down tissue. A commercially available dressing called Debridace™ boosts proteolytic activity.[60]
Composite dressing
Both partial and full thickness wounds can benefit from the versatility and convenience of composite dressings. Each layer of a composite or combination dressing is biologically unique and has numerous layers. The majority of composite dressings include three layers. Composite dressings may also have a transparent film or non-woven fabric tape border that adheres to the skin. They may be used in conjunction with topical drugs and can serve as a main or secondary dressing for a variety of wounds. The topmost layer shields the wound from infection, the middle layer is typically made of absorbent material to help keep the environment wet and aid with autolytic debridement, and the bottom layer is made of non-adherent material to avoid sticking to newly granulating tissues. Composite dressings are more expensive and less flexible.[61]
3-dimensional printed wound dressings
Applying an external dressing to a wound could be necessary to temporarily make up for the compromised barrier and promote healing. According to this viewpoint, the wound area must be covered with a dressing as much as possible to keep the injury site isolated from the outside environment; nevertheless, particular body parts, such as the ear, nose, and chin, among others, require customized shapes to provide an ideal environment. To create 3D models of these bodily parts using 3D scanning, it is possible to tailor the shape and size of a patient’s wound dressing.[62]
The following methods of 3D printing are available: electrohydrodynamic jet (E-jet), roller extrusion Fused Deposition Modelling (FDM), pneumatic FDM, piston-based FDM, modified FDM, and stereolithography (SLA).
-
i.
ROLLER EXTRUSION FDM stands for traditional FDM, which fabricates 3D parts by feeding a filament through a heated element using a stepper motor fitted with a roller gear.[63]
-
ii.
PNEUMATIC FDM denotes that pneumatic air pressure was used to achieve the material deposition.[64]
-
iii.
PISTON BASED FDM uses a piston to feed bioink onto the printing bed in order to build 3D objects. The piston-based FDM utilized a stepper motor to maintain the piston movement, just like the roller-extrusion FDM does.[65]
-
iv.
MODIFIED FDM: Prusa’s open-source 3D printing platform (roller extrusion FDM) has helped businesses and research organizations improve their results. By customizing a RepRap Prusa i3 3D printer from Prusa Research in Prague, Czech Republic, Streifel et al., Cereceres et al., and Mirani et al. also utilized this platform to create their individual works on wound dressing research.[666768]
-
v.
ELECTROHYDRODYNAMIC JET (E-JET) PRINTING is a micro-to-nano size liquid deposition (droplet creation) technique which uses micro capillary nozzles to create fluid flows by electrohydrodynamic force.[69]
-
vi.
STEREOLITHOGRAPHY (SLA) is the first 3D printing method ever created, which was invented by 3D Systems [46]. SLA relies on the spatially controlled photo-polymerization of a photosensitive resin to solidify the 3D objects.[70]
A summary of various dressing materials, their description and commercially available products have been outlined in Table 3.
| Type of dressing | Description | Commercially available material |
|---|---|---|
| Gauze | • Composed of cotton, rayon, and polyester woven and non-woven fiber • Inexpensive, drying, may cause further injury on changing |
Xeroform, Curity, Vaseline gauze |
| Hydrocolloid | • Hydrogel mixed with synthetic rubber and sticky material • Long time between changes, fluid trapping, occlusive, not for infected wounds |
Comfeel, Tegasord, Aquacel, DuoDERM, Granuflex |
| Hydrogel | • Three-dimensional network of hydrophilic polymers • Rehydrates dry wounds, easy/removal changes may cause over hydration |
Nu-gel, SAF-gel, X-cell, Purilon, Curagel, Restore, Carrasyn |
| Foam | • Consists of polyurethane or is silicon based • Moderate absorbent, insulating |
Allevyn, Tielle, 3M adhesive foam, Lyofoam |
| Film | • Consist, of adhesive, porous, and thin transparent polyurethane • Occlusive, retains moisture, only for non-exudative wounds |
Flexigrid, Tegaderm, Bioclusive, Cutifilm, OpSite, Blisterfilm |
| Alginate | • Consists of polysaccharides derived from brown seaweed • Highly absorbent and hemostatic |
Kaltostate, Tegagen, Algisite, Sorbsan |
| Hydrofibers | • Made up of sodium carboxin methane cellulose • Highly absorbent, can absorb 25 times its initial weight |
Aquacel Hydrofiber |
| Tissue Engineered Skin Substitutes | • Replicate layer of the skin made up of keratinocytes and fibroblasts on collagen matrix • Addresses deficient growth factor and cytokines, expensive, risk of infections, antigenicity |
Integra Omnigraft, Apligraf, Dermagraft, TransCyte, Epicel, Alloderm, Bioseed, Myskin, Hyalograft, Biobrane, EZ Derm, Laserskin |
An overview of various wounds and appropriate dressings have been outlined in Table 4.
| Type of wound | Appropriate dressing |
|---|---|
| Pressure injury | Multi-layered soft silicone foam dressing, polyurethane foam dressing, hydrocolloids dressings, polyurethane film, foam dressing |
| Diabetic foot ulcer | Non-adhesive dressing, silver ion foam dressing, UrgoStart contact dressing, hyaluromic acid, hydrofiber dressing |
| Burn and Scald | Moist occlusive dressing |
| Chronic venous leg ulcer | Alginate dressing, hydrocellular foam dressings |
| Split-Thickness skin grafting | Alginate Silver, calcium alginate, Polyurethane foam |
| Radiation dermatitis | Film dressing, Silver containing hydrofiber |
CONCLUSION
Currently thousands of dressings are available in the market. This causes a lot of confusion to the treating physician to choose the correct dressing material for wound care. No product is superior to the other. Choosing the correct dressing material should be based on the type of wound. Cost of the dressing needs to be addressed and local innovations need to be encouraged so that use of dressing material becomes cost-effective and easily available.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Nanofibrous scaffolds with biomimetic structure. J Biomed Mater Res Part A. 2018;106:370-6.
- [Google Scholar]
- MicroRNAs and the skin: Tiny players in the body’s largest organ. J Dermatol Sci. 2009;53:169-75.
- [Google Scholar]
- Trehalose-based hydrogel potentially useful for the skin burn treatment. J Appl Polym Sci. 2017;134:1-6.
- [Google Scholar]
- Wound healing: Biological features and approaches to maximize healing trajectories. Curr Prob Surg. 2001;38:77-89.
- [Google Scholar]
- Modern wound dressings: A systemic approach to wound healing. J Biomater Appl. 1992;7:142-213.
- [Google Scholar]
- Hi-tech textiles for interactive wound therapies. In: Bartels VT, ed. Handbook of medical textiles. Cambridge: Elsevier; 2011. p. :38-79.
- [Google Scholar]
- Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130:489-93.
- [Google Scholar]
- Wound healing dressings and drug delivery systems: A review. J Pharm Sci. 2008;97:2892-923.
- [Google Scholar]
- Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514-25.
- [Google Scholar]
- Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg. 2004;187:S6565S-S70.
- [Google Scholar]
- Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4:321-5.
- [Google Scholar]
- Wound repair: Overview and general considerations. In: Clark RAF, ed. The molecular and cellular biology of wound repair. New York, NY: Plenum Press; 1996. p. :3-50.
- [Google Scholar]
- Basics of cutaneous surgery. In: Sacchidanand S, Oberai C, Inamadar AC, eds. IADVL textbook of dermatology (4th ed.). Mumbai: Bhalani; 2015. p. :2346-7.
- [Google Scholar]
- Wound healing. In: Savant SS, ed. Textbook and atlas of dermatosurgery and cosmetology (2nd ed.). Mumbai: ASCAD; 2005. p. :12-7.
- [Google Scholar]
- Biology of wound healing. In: Bolgnia JL, Jorizzo JL, Schaffer JV, eds. Dermatology (3rd ed.). Philadelphia, PA: Elsevier; 2012. p. :2313-25.
- [Google Scholar]
- The healing hand: Man and wound in the ancient world. Cambridge: Harvard University Press; 1975.
- A history of materials and practices for wound management. Wound Pract Res. 2012;20:174-86.
- [Google Scholar]
- Wound dressings: Current advances and future directions. J Appl Polym Sci. 2019;136:47738.
- [Google Scholar]
- Comparative review of the properties of six semipermeable film dressings. Pharm J. 1988;240:785-7.
- [Google Scholar]
- A comparative study of twelve hydrocolloid dressings. World Wide Wounds. 1997;1:1-12.
- [Google Scholar]
- New dressings, including tissue engineered living skin. Clin Dermatol. 2002;20:715-23.
- [Google Scholar]
- 2006. Foam composite. US Patent 7048966.. Available at: https://patentimages.storage.googleapis.com/8d/63/83/fe275a1c37aac0/US7048966.pdf Last accessed on 5th July 2022
- The release of model macromolecules may be controlled by the hydrophobicity of palmitoyl glycol chitosan hydrogels. J Control Release. 2002;80:87-100.
- [Google Scholar]
- Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-a. Biomaterials. 2000;21:1797-802.
- [Google Scholar]
- Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011;25:251-6.
- [Google Scholar]
- Medical textiles and bio-materials for healthcare. Cambridge: Woodhead Publishing; 2006.
- A phase II prospective, non-comparative assessment of a new silver sodium carboxymethylcellulose (AQUACEL(®) Ag BURN) glove in the management of partial thickness hand burns. Burns. 2012;38:1041-50.
- [Google Scholar]
- Silver-containing hydrofiber dressing is an effective adjunct in the treatment of pemphigus vulgaris. Kaohsiung J Med Sci. 2009;25:622-7.
- [Google Scholar]
- Algosteril calcium alginate dressing for moderate/high exudate. Br J Nurs. 1999;8:313-7.
- [Google Scholar]
- Collagen-based wound dressing: Effect of hyaluronic acid and fibronectin on wound healing. Biomaterials. 1986;7:3-8.
- [Google Scholar]
- Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials. 2002;23:833-40.
- [Google Scholar]
- Recent developments of collagen based materials for medical applications and drug delivery. J Biomater Sci Polym Ed. 1995;7:623-45.
- [Google Scholar]
- Collagen as a pharmacological approach in wound healing. Int J Tissue React. 1992;14:1-9.
- [Google Scholar]
- Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105-15.
- [Google Scholar]
- Engineered skin substitutes: Practices and potentials. Clin Dermatol. 2005;23:403-12.
- [Google Scholar]
- Mesenchymal stem cells’ seeded amniotic membrane as a tissue-engineered dressing for wound healing. Drug Deliv Transl Res. 2022;12:538-49.
- [Google Scholar]
- Antimicrobial wound dressings: Mechanism and function. 2009. Symposium on advanced wound care. Available at http://content.stockpr.com/qmdt/media/40675b8d6e97bd8a7412648f4c5e29d3.pdf Last accessed on 15th July 2022
- [Google Scholar]
- Composite membrane dressings system with metallic nanoparticles as an antibacterial factor in wound healing. Membranes. 2022;12:215.
- [Google Scholar]
- Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharm. 2017;527:161-70.
- [Google Scholar]
- FDM 3D printing technology in manufacturing composite elements. Arch Metal Mater. 2013;58:1415-8.
- [Google Scholar]
- A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater Sci Eng C. 2019;104:109873.
- [Google Scholar]
- Biomimicry of oil infused layer on 3D printed poly(dimethylsiloxane): Non-fouling, antibacterial and promoting infected wound healing. Mater Sci Eng C. 2019;100:915-27.
- [Google Scholar]
- An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. Adv Healthc Mater. 2017;6:10.
- [Google Scholar]
- Bactericidal activity of 3D-printed hydrogel dressing loaded with gallium maltolate. APL Bioeng. 2019;3:026102.
- [Google Scholar]
- Hemostatic and absorbent PolyHIPE-Kaolin composites for 3D printable wound dressing materials. Macromol Biosci. 2018;18:1700414e1700414.
- [Google Scholar]
- A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121-30.
- [Google Scholar]





