Translate this page into:
A Randomized Controlled Study Comparing the Efficacy of Autologous Smashed Follicular Dermal Graft and Epidermal Cell Suspension versus Normal Saline Dressing in the Treatment of Chronic Nonhealing Trophic Ulcers in Patients with Hansen’s Disease
Address for correspondence: Dr. M. Dheemant, Department of Dermatology, Rajarajeswari Medical College & Hospital, 88/A, 2nd Cross, 4th Main, 1st Block, Banashankari 3rd Stage, Bengaluru 560 085, Karnataka, India. E-mail: dheemantgowda@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Trophic ulcers remain the most common reason for hospitalization in patients with Hansen’s disease. With the introduction of new therapeutic regimens, leprosy can now be cured. However, complications of the disease, such as sensory loss, muscle palsy, absorption of extremities, and recurrent ulcers, still lead to substantial morbidity. The management of patients with trophic ulcers and their consequences is difficult, because it is a recurrent and recalcitrant problem.
Aims:
To evaluate the efficacy of autologous smashed follicular dermal graft and epidermal cell suspension (ECS) in the treatment of chronic nonhealing trophic ulcers in patients with Hansen’s disease and to compare its efficacy with normal saline dressing.
Materials and Methods:
A total of 46 chronic nonhealing trophic ulcers were randomized into two groups (23 ulcers in each): Ulcers in Group A were treated with autologous smashed follicular dermal graft and ECS; ulcers in Group B were treated with normal saline dressings. Ulcers were assessed based on the rate of ulcer size reduction at every week till 12 weeks and then once a month till the sixth month.
Results:
All 23 (100%) ulcers in Group A had healed within the study period of six months, whereas only 14 (60.9%) ulcers had healed in Group B. Nine (39.1%) ulcers in Group B had not healed even at the end of six months. All 23 (100%) ulcers in Group A had healed within eight weeks, which was statistically significant, P value <0.05.
Conclusion:
Trophic ulcers heal faster by autologous smashed follicular dermal graft and ECS, with good results of re-epithelialization of the ulcer bed than by normal saline dressing.
Keywords
Dermatologic surgery
leprosy
ulcer
INTRODUCTION
Leprosy is a chronic granulomatous disease caused by Mycobacterium leprae, affecting the peripheral nervous system, the skin, and certain other tissues such as the reticuloendothelial system, bones, joints, mucous membrane, eyes, testes, muscles, adrenals, and so on.[1] An ulcer is defined as a disruption in the continuity of the epithelial lining of the skin or mucosa resulting from physical or thermal damage. Leg ulcers that do not respond to treatment and persisting for more than six weeks are called chronic nonhealing leg ulcers.[2] Nonhealing trophic ulcers in patients with Hansen’s disease are one of the major causes for disability.[3]
Trophic ulceration, also termed plantar ulceration, is the most common foot problem. A plantar ulcer is defined as a chronic ulceration of the anesthetic foot.[4] The abhorrent images often seen in the pictures and depictions about leprosy-affected individuals with eaten away fingers and toes are no longer a trend of the disease. However, we cannot ignore the fact that among us, we have more than a million individuals with leprosy-related deformities and disabilities living in homes and colonies surrounding us.[5]
Managing chronic trophic ulcers is challenging. When all the conservative and medical methods fail, the surgical management of ulcers may be the only ray of hope. Many patients with Hansen’s disease are unable to cope with the expenses involved in the treatment of nonhealing trophic ulcers. There is a need for an economical as well as an effective mode to treat such ulcers. Cutaneous wound repair is a multifaceted process involving clot formation, cell migration, and, finally, dermal and epidermal reconstitution.[6]
Autologous non-cultured ECS has already gained popularity in stable vitiligo because of superior results, the requirement of only a smaller donor area, and the suitability to treat difficult areas. Similar to cultured keratinocyte allografts, autologous ECS stimulates the growth factors and extracellular matrix proteins.[7] Thus, if used in ulcers, it may promote migration or multiplication of acceptor keratinocytes and the formation of extracellular matrix proteins. To prepare cultured keratinocytes for grafting, a minimum of three to four weeks is required,[8] whereas ECS is easily trypsinized and economically prepared in a few hours.[7]
Hair follicles (HFs) act as ulcer promoters.[9] The restoration of the epithelium after injury takes place by the migration of epithelial cells from the old epithelium adjoining an ulcer or by centrifugal migration from any HFs remaining within the ulcer.[10] Thus, follicular unit grafting into ulcer beds is feasible and represents a promising therapeutic alternative for managing nonhealing chronic leg ulcers.[2]
As per the review of literature, in all published studies till date, the authors have used either ECS or autologous smashed follicular dermal graft for the treatment of chronic nonhealing ulcers. Hence, we sought to combine both of these methods to achieve a cumulative effect for better results in difficult-to-treat trophic ulcers in patients with Hansen’s disease.
MATERIALS AND METHODS
We conducted an open-label, randomized controlled study after obtaining approval from the Institutional Ethics Committee, no.: XXMCH-IEC/72/2018–19. Patients with Hansen’s disease with trophic ulcers attending the Dermatology outpatient department, fulfilling the inclusion and exclusion criteria were included in the study from December 2018 to May 2020.
Male patients between 18 and 60 years of age with chronic nonhealing trophic ulcers of more than six weeks duration (with an area not more than 5 cm × 5 cm) secondary to Hansen’s disease who had already been released from treatment were included in the study.
Cases with bleeding disorders, anemia and other hematological disorders, uncontrolled diabetes, and malignant or venous ulcers were excluded from the study.
All patients were explained about the method, expected outcome, variable results, and relapses and informed consent was taken from each subject. The ulcer size in terms of length, breadth, and depth was measured. Basic investigations to find out the primary cause of ulcer were done, including random blood sugar, pus culture and sensitivity, and skin biopsy as and when indicated. Primary infection, if any, was treated using antibiotics and/or surgical debridement as and when required before including them in the study. Overall, 46 nonhealing trophic ulcers of patients with Hansen’s disease were randomized into two groups of 23 ulcers each with the help of a computer-generated random number table [Figure 1]. Group A ulcers were subjected to autologous smashed follicular dermal graft and ECS. Group B ulcers were subjected to normal saline dressings. Photographic evidence was taken using Nikon Coolpix L120 14.1 High Definition Digital Camera.
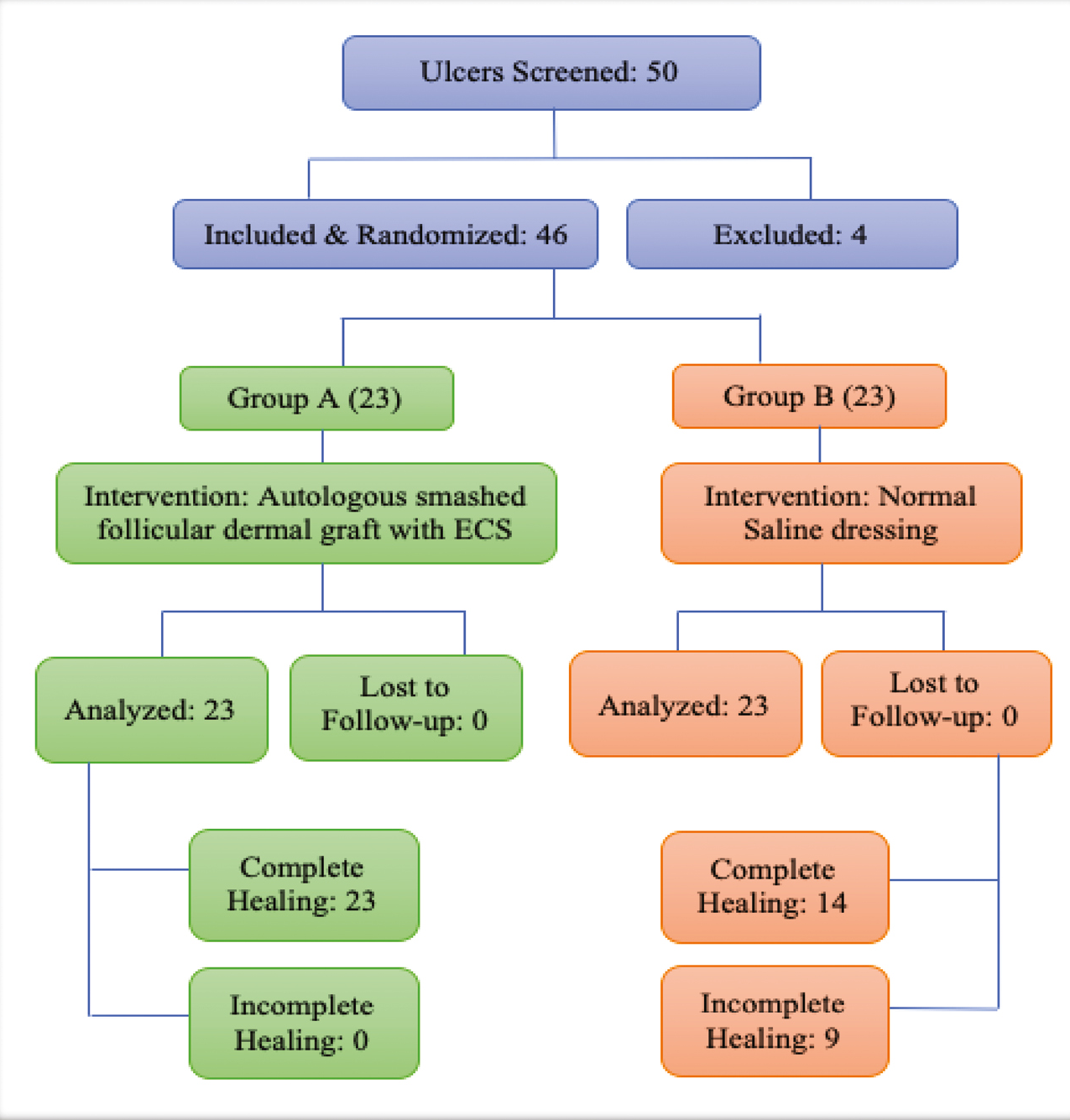
- Flowchart depicting the randomization of the study
Group A
Step 1: Harvesting epidermal cell suspension (ECS) [Figures 2 and 3]
The anterolateral aspect of the thigh was chosen as the donor site, and it was anesthetized using topical anesthesia; a mixture of Lidocaine 25 mg and Prilocaine 25 mg under occlusion was applied for 1 h. An ultra-split thickness graft measuring 5 × 5 cm2 was then harvested from the donor site using a razor blade holding dermatome. The graft harvested was placed in 10ml Trypsin-Ethylenediaminetetraacetic acid (Trypsin-EDTA) solution with the epidermis facing upward in a petri dish and incubated at 37°C for 50 min. After incubation, the graft was transferred to Dulbecco’s Modified Eagle’s Medium (DMEM) in another petri dish. Using blunt forceps and agitation, the epidermis was separated from the dermis. The cell suspension obtained was then transferred to a 15ml centrifuge tube.
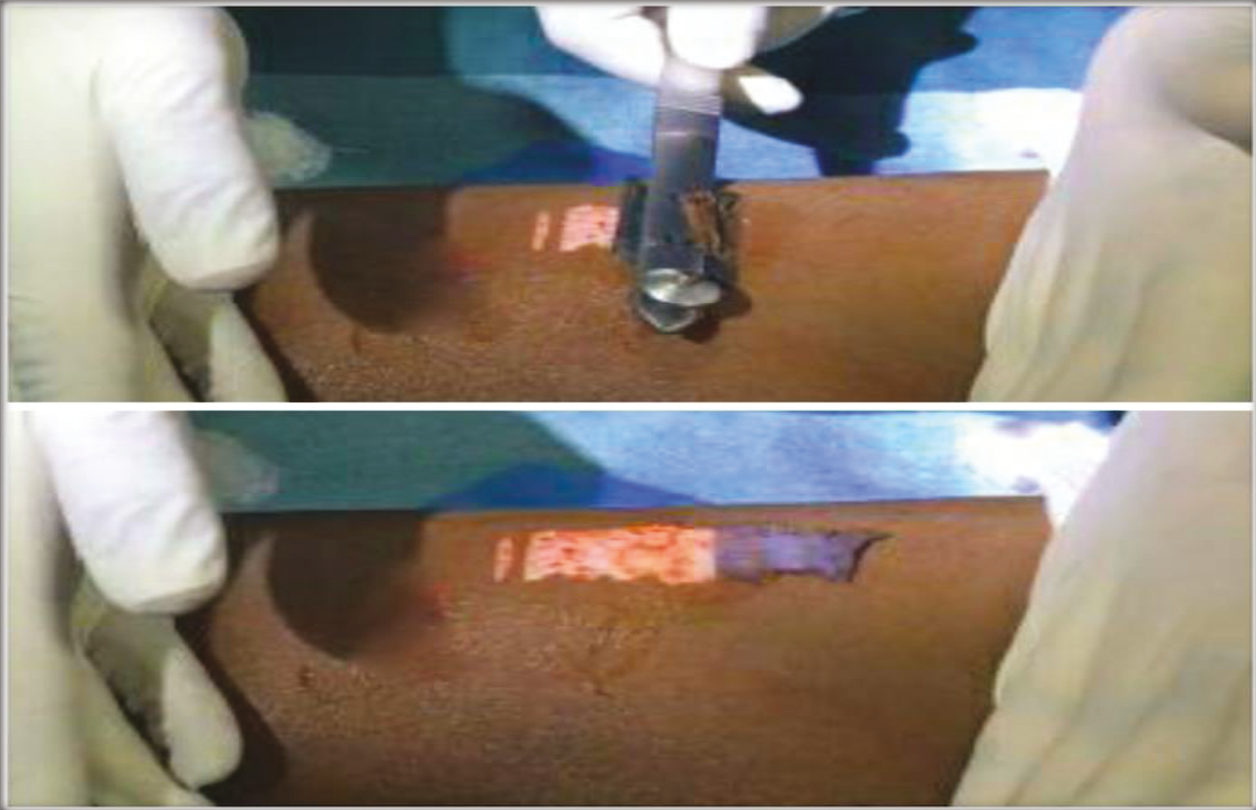
- Harvesting ultra-split thickness graft
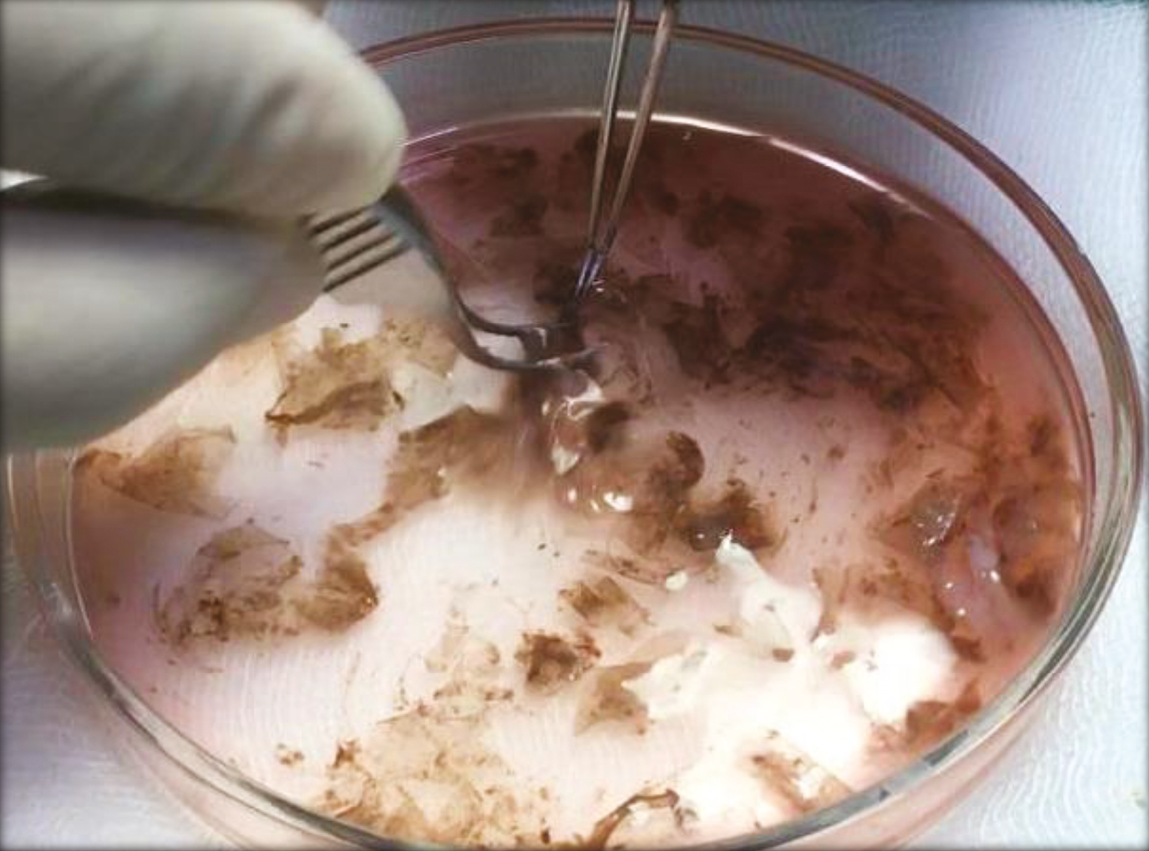
- Graft is transferred to a petri dish containing Trypsin-EDTA and then incubated. The incubated specimen is then subjected vigorous agitation to separate epidermis and dermis
Step 2: Harvesting dermal grafts [Figure 4]
Follicular dermal grafts were harvested using punches of size 2.5 mm from the donor site, spaced at least 5 mm from each other, ensuring that each graft consisted of an HF. The number of grafts required was to be approximately 2 grafts per cm2 of ulcer area. The grafts was then transferred to a petri dish containing normal saline. Dermal grafts were minced with surgical blade number 15 to form smashed dermal grafts. The smashed dermal graft was transferred into the same 15ml centrifuge tube that contained ECS from step 1.
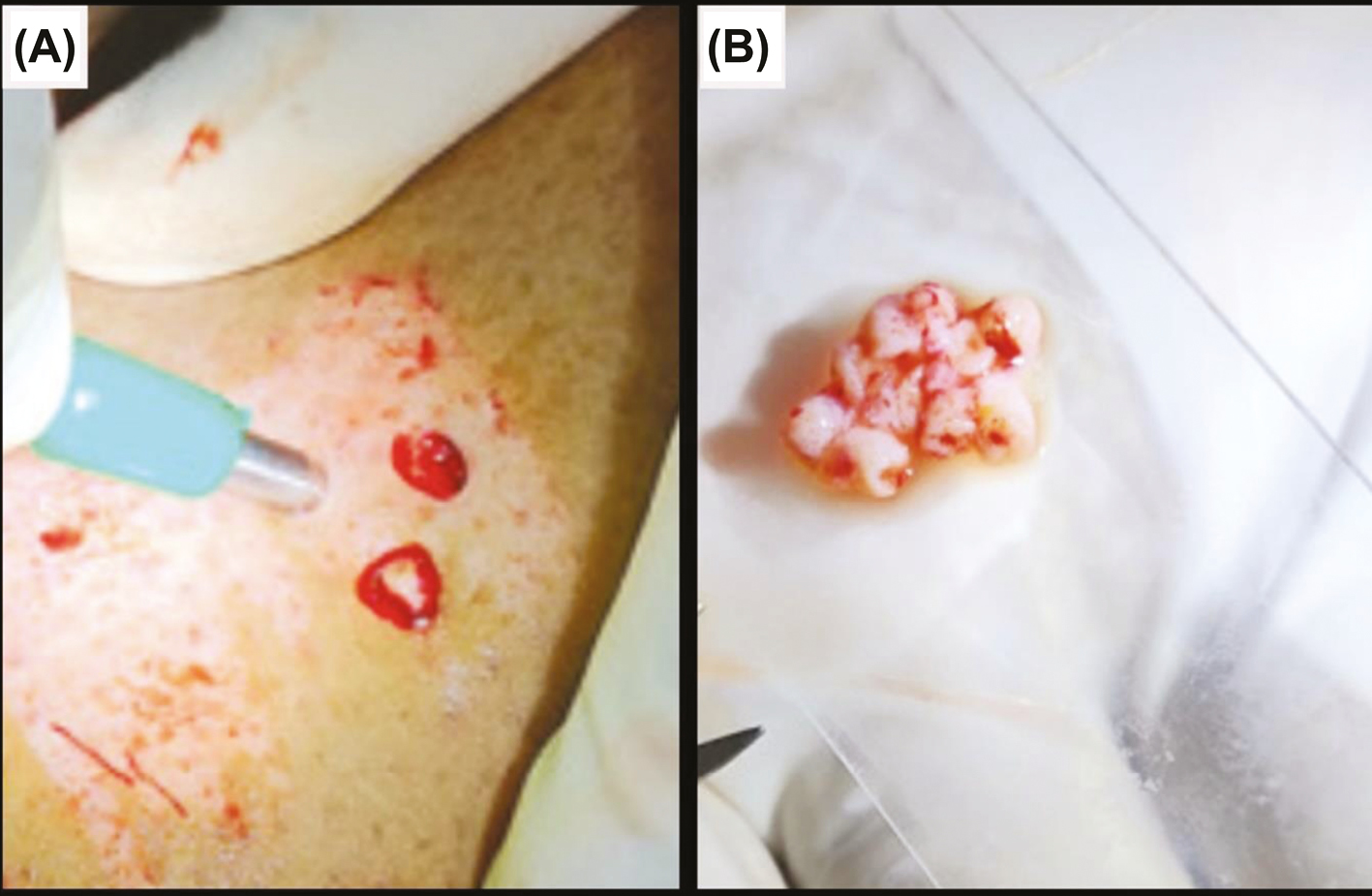
- (A) Harvesting follicular dermal grafts with biopsy punch. (B) Follicular grafts obtained after smashing
Step 3: Dressing of donor area
Firm compression with dressing paraffin gauze dressing was applied over the donor area.
Step 4: Application of dermal graft and ECS to ulcer bed [Figure 5]
The mixture of smashed dermal grafts and ECS harvested above in the 15ml centrifuge tube was centrifuged at 3000 rpm for 10 min. The pellet thus obtained was spread evenly over the ulcer bed. The ulcer bed was then covered with paraffin gauze and Johnson and Johnson 3M Dynaplast. In case of ulcers in the vicinity of joints such as the ankle, immobilization was required with a below-knee plaster of Paris slab.
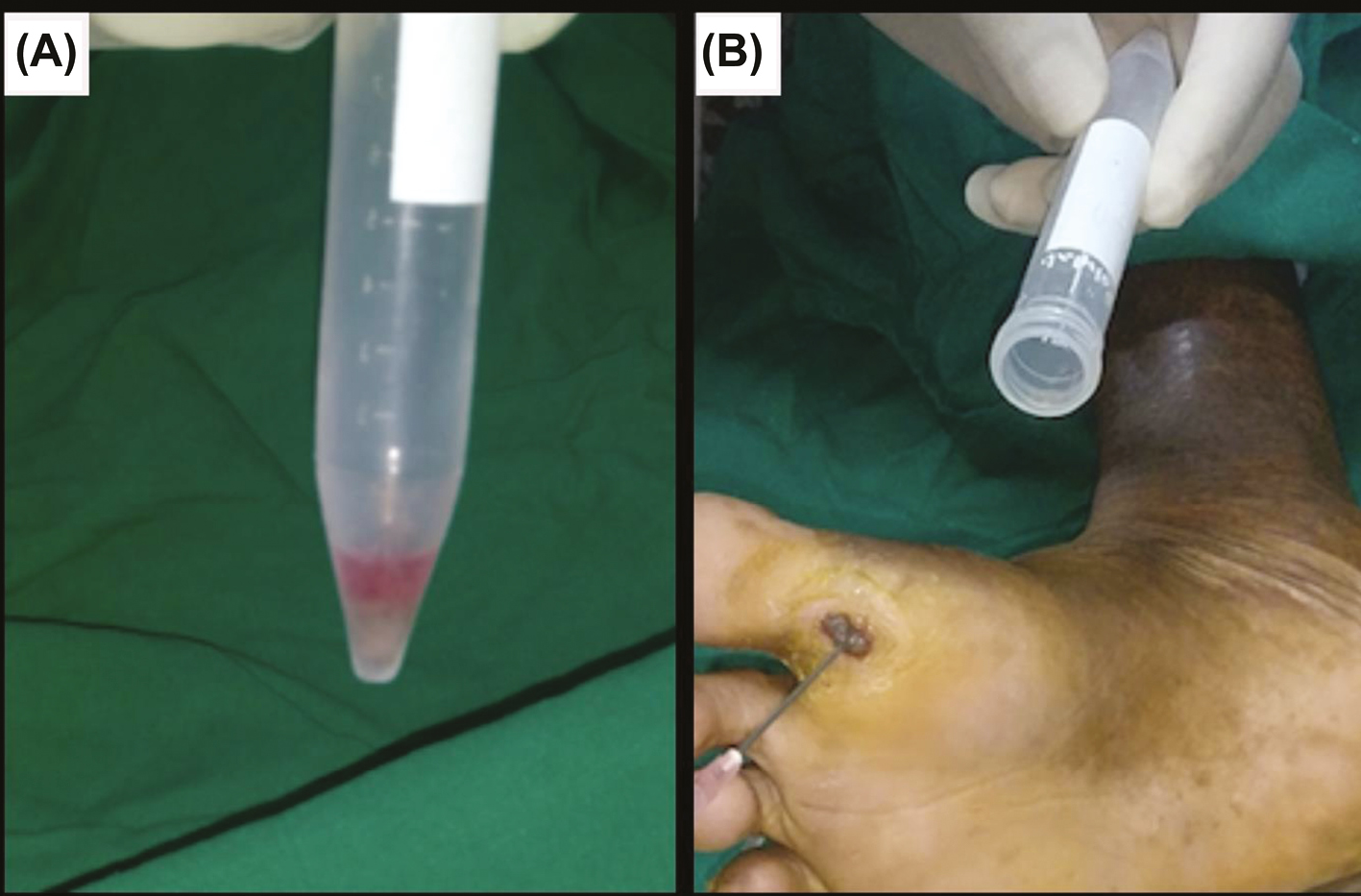
- (A) Pellet thus obtained after centrifugation. (B) Application of smashed follicular dermal graft and ECS mixture to the ulcer bed
Group B
After taking aseptic precautions, the ulcer bed was washed with normal saline and the surrounding skin of the ulcer was cleaned with surgical spirit. The ulcer was then dressed with gauze and later with roller gauze.
Post-procedure instructions
All subjects were instructed to take complete rest and to avoid all vigorous physical activities for seven days. They were prescribed oral antibacterial agents for five days.
Follow-up
Below-knee plaster of Paris cast, if any, was removed after seven days post-surgery. Dressing on both donor and recipient was removed; regular follow-up was done with regular paraffin gauze and saline dressing at every week till 12 weeks and then once a month till the sixth month or complete healing, whichever was earlier in both the groups. Photographic assessment was done at every follow-up visit. Baseline and at every follow-up visit, wound area was measured by the formula Length x Width x 0.7854 (an ellipse is closer to a wound shape than a square or rectangle); volume was measured by Length x Width x Depth x 0.7854.[11] A percentage reduction of 100% in the size of the ulcer was defined as complete healing, and anything less than that was considered as incomplete healing.
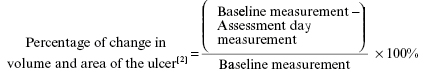
Statistical analysis
Statistical data were entered in an excel sheet and analyzed using SPSS 26 software. Continuous data were presented as proportion and percentage. Independent t-test was used as the test of significance with a 95% confidence interval; a P-value of <0.05 was considered statistically significant.
RESULTS
We included all ulcers that were less than or equal to 5 x 5 cm size in our study as inclusion criteria. The smallest ulcer size in the study was 2.1 x 1.9 cm, and it belonged to Group B. Among the 46 ulcers studied, eight (34.78%) out of 23 ulcers in Group A and nine (39.13%) out of 23 ulcers in Group B were situated on the base of the great toe, suggesting that the majority (73.91%) of trophic ulcers in the study were found on the base of the great toe [Table 1]. Even though the ball of the foot is the most common affected site in trophic ulcers, it was found to be the least affected site in our study. Out of the 46 ulcers that we studied, the average duration of ulcers before inclusion in the study was 8.68 months in Group A and 8.78 months in Group B.
| Site of ulcer | Group A | Group B | ||
|---|---|---|---|---|
| Number of ulcers | Percentage (%) | Number of ulcers | Percentage (%) | |
| Base of great toe | 8 | 34.78 | 9 | 39.13 |
| Tip of 2nd toe | 5 | 21.74 | 4 | 17.39 |
| Ball of foot | 3 | 13.04 | 4 | 17.39 |
| Heel of foot | 7 | 30.44 | 6 | 26.09 |
| Total | 23 | 100 | 23 | 100 |
The mean area of ulcers in Group A with respect to baseline was 6.78 ± 2.33 cm2 after one week of treatment, which reduced to 1.04 ± 0.94 cm2 in four weeks and 0.01 ± 0.03 cm2 in seven weeks, which later healed completely in eight weeks [Table 2]. When compared with the mean area of ulcers in Group B from baseline, this showed 9.23 ± 2.11 cm2 after one week of treatment, which reduced to 8.78 ± 2.00 cm2 in four weeks and 8.53 ± 2.40 cm2 in seven weeks, which later did not heal completely even after six months. The mean area of Group A was more statistically significant (P < 0.05) at two weeks with respect to baseline when compared with Group B.
| Time of follow-up | Mean area of ulcer (Mean ± SD) (cm2) | P value | |
|---|---|---|---|
| Group A | Group B | ||
| Baseline | 8.06 ± 2.77 | 9.45 ± 2.21 | 0.302 |
| 1st week | 6.78 ± 2.33 | 9.23 ± 2.11 | 0.060 |
| 2nd week | 3.48 ± 2.04 | 9.19 ± 2.16 | 0.024** |
| 3rd week | 2.40 ± 1.64 | 9.00 ± 2.18 | 0.005** |
| 4th week | 1.04 ± 0.94 | 8.78 ± 2.00 | <0.001** |
| 5th week | 0.30 ± 0.31 | 8.65 ± 2.01 | <0.001** |
| 6th week | 0.09 ± 0.12 | 7.71 ± 1.84 | <0.001** |
| 7th week | 0.01 ± 0.03 | 7.60 ± 1.77 | <0.001** |
| 8th week | 0.00 ± 0.00 | 6.86 ± 1.65 | — |
| 9th week | 0.00 ± 0.00 | 6.73 ± 1.59 | — |
| 10th week | 0.00 ± 0.00 | 5.97 ± 1.59 | — |
| 11th week | 0.00 ± 0.00 | 5.04 ± 1.54 | — |
| 12th week | 0.00 ± 0.00 | 3.83 ± 1.95 | — |
| 4th month | 0.00 ± 0.00 | 3.38 ± 2.07 | — |
| 5th month | 0.00 ± 0.00 | 2.53 ± 1.98 | — |
| 6th month | 0.00 ± 0.00 | 2.36 ± 1.83 | — |
**P value < 0.05 = significant
The mean volume of ulcers in Group A with respect to baseline was 6.70 ± 4.68 cm3 after one week of treatment, which reduced to 0.30 ± 0.45 cm3 in four weeks and 0.01 ± 0.00 cm3 in seven weeks, which later healed completely in eight weeks [Table 3]. When compared with the mean area of ulcers in Group B from baseline, this showed 9.93 ± 5.75 cm3 after one week of treatment, which reduced to 11.43 ± 5.37 cm3 in four weeks and 8.89 ± 4.25 cm3 in seven weeks, which later did not heal completely even after six months. The mean volume of Group A was more statistically significant (P < 0.05) at two weeks with respect to baseline when compared with Group B.
| Time of follow-up | Mean volume of ulcer (Mean ± SD) (cm3) | P value | |
|---|---|---|---|
| Group A | Group B | ||
| Baseline | 6.65 ± 7.16 | 10.63 ± 5.83 | 0.107 |
| 1st week | 6.70 ± 4.68 | 9.93 ± 5.75 | 0.073 |
| 2nd week | 4.16 ± 2.79 | 9.02 ± 5.60 | 0.048** |
| 3rd week | 2.08 ± 1.46 | 8.92 ± 5.68 | 0.002** |
| 4th week | 0.30 ± 0.45 | 7.43 ± 5.37 | <0.001** |
| 5th week | 0.06 ± 0.08 | 7.22 ± 5.48 | <0.001** |
| 6th week | 0.03 ± 0.02 | 6.17 ± 4.25 | <0.001** |
| 7th week | 0.01 ± 0.00 | 5.89 ± 4.25 | <0.001** |
| 8th week | 0.00 ± 0.00 | 5.64 ± 4.21 | — |
| 9th week | 0.00 ± 0.00 | 5.45 ± 3.61 | — |
| 10th week | 0.00 ± 0.00 | 4.16 ± 3.05 | — |
| 11th week | 0.00 ± 0.00 | 4.08 ± 3.18 | — |
| 12th week | 0.00 ± 0.00 | 3.82 ± 2.48 | — |
| 4th month | 0.00 ± 0.00 | 3.63 ± 2.07 | — |
| 5th month | 0.00 ± 0.00 | 2.56 ± 1.59 | — |
| 6th month | 0.00 ± 0.00 | 2.26 ± 1.14 | — |
**P value < 0.05 = significant
The mean percentage of change in area of the ulcers in Group A with respect to baseline showed 15.88% improvement after one week of treatment, which improved to 87.10% at four weeks and 99.88% at seven weeks, which later completely healed with 100% improvement at eight weeks [Figure 6]. When compared with the mean percentage of change in area of the ulcers in Group B from baseline, this showed 2.33% improvement after one week of treatment, which improved to 7.09% at four weeks and 19.58% improvement at seven weeks, with poor improvement even after six months. The mean percentage of change in volume of the ulcers in Group A with respect to baseline showed 30.57% improvement after one week of treatment, which improved to 96.89% at four weeks and 99.90% at seven weeks, which later completely healed with 100% improvement at eight weeks [Figure 7]. When compared with the mean percentage of change in area of the ulcers in Group B from baseline, this showed 6.59% improvement after one week of treatment, which improved to 30.10% in four weeks and 44.59% improvement in 7 weeks, with poor improvement even after six months. This showed that Group A regimen had better results when compared with Group B in terms of mean percentage of change in area and volume of the ulcers with respect to baseline, suggesting that Group A treatment regimen was superior to Group B treatment regimen.
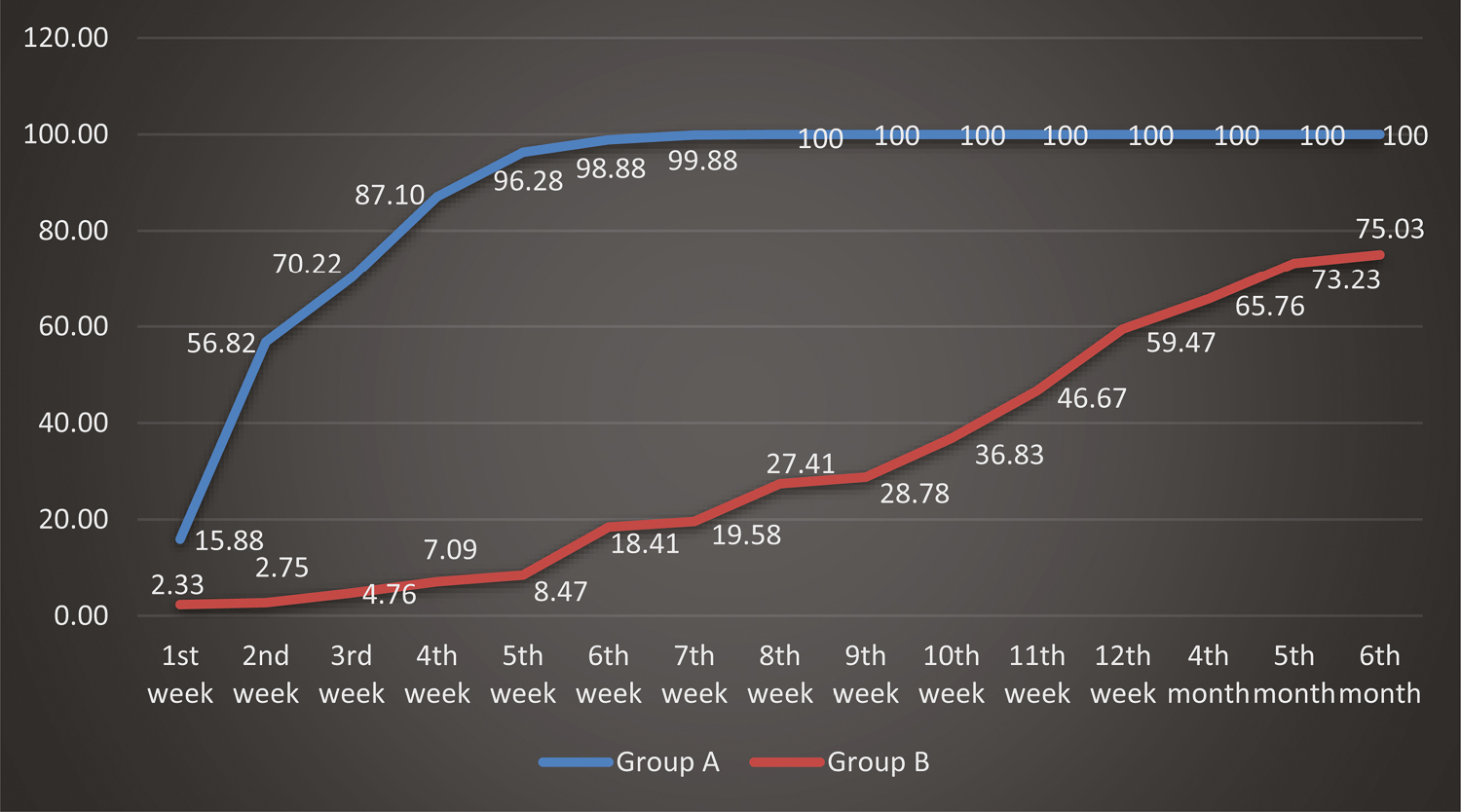
- Comparison of percentage of change in area in the two groups of ulcers studied with respect to baseline at different study points
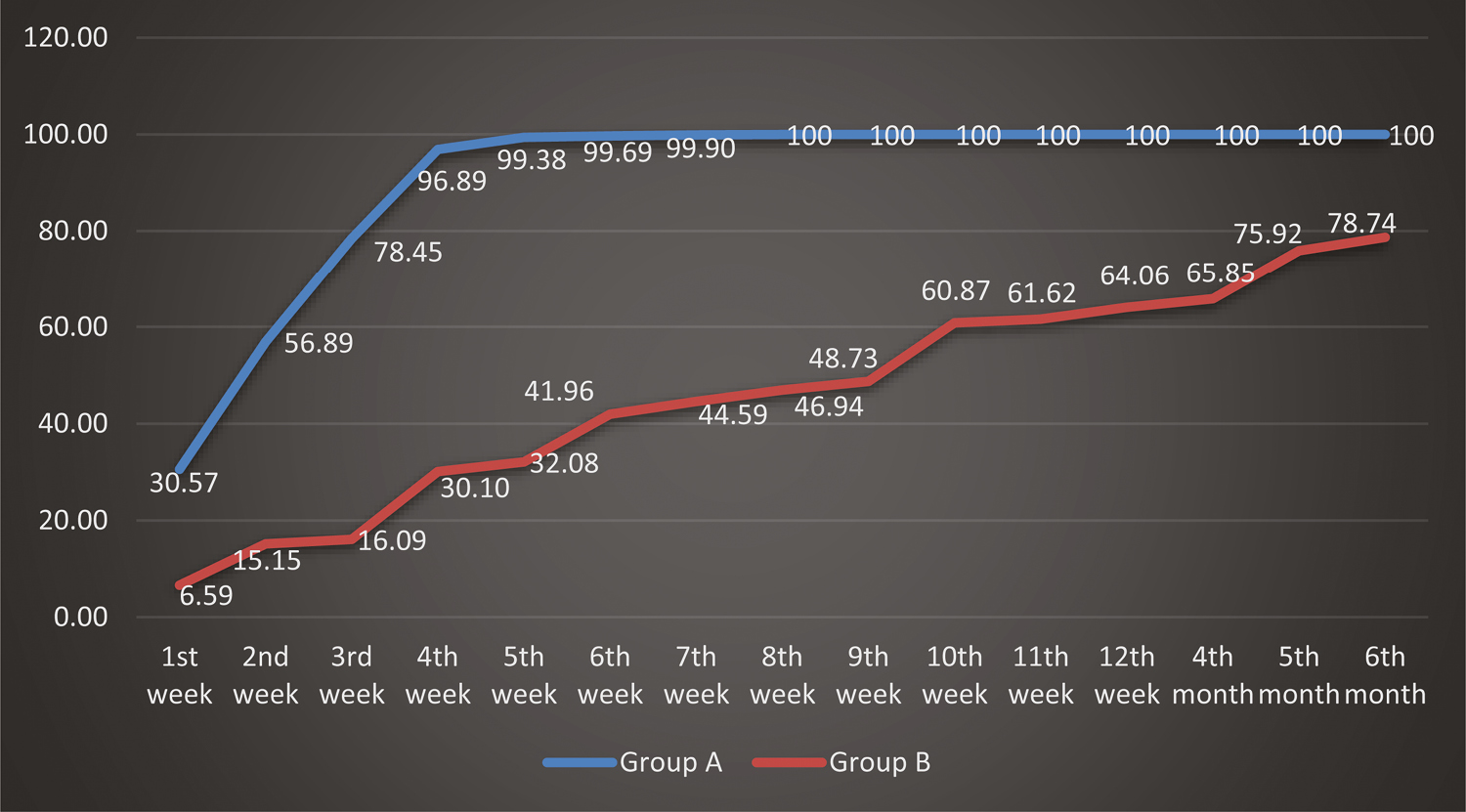
- Comparison of percentage of change in volume in the two groups of ulcers studied with respect to baseline at different study points
In Group A, out of 23 trophic ulcers, three (13%) ulcers healed within five weeks; eight (34.8%) ulcers in six weeks; 10 (43.5%) ulcers in seven weeks; and two (8.7%) ulcers in eight weeks [Figure 8] when compared with 23 trophic ulcers in Group B, where three (13%) ulcers took 12 weeks to heal; three (13%) ulcers took four months; four (17.4%) ulcers took five months; and four (17.4%) ulcers took six months to heal [Figure 9 and Table 4]. All 23 (100%) ulcers in Group A had healed within the study period of six months, whereas only 14 (60.9%) ulcers in Group B had healed. Nine (39.1%) ulcers in Group B had not healed even at the end of six months. All 23 (100%) ulcers in Group A had healed within eight weeks. All these results show that there was a commendable difference between Group A and Group B in terms of time of complete healing, suggesting that Group A regimen shows better treatment response than Group B regimen.
| Time of healing | Group A | Group B | |||
|---|---|---|---|---|---|
| Number of ulcers | Percentage (%) | Number of ulcers | Percentage (%) | ||
| Healed | 5th week | 3 | 13 | 0 | 0 |
| 6th week | 8 | 34.8 | 0 | 0 | |
| 7th week | 10 | 43.5 | 0 | 0 | |
| 8th week | 2 | 8.7 | 0 | 0 | |
| 12th week | 0 | 0 | 3 | 13 | |
| 4th month | 0 | 0 | 3 | 13 | |
| 5th month | 0 | 0 | 4 | 17.4 | |
| 6th month | 0 | 0 | 4 | 17.4 | |
| Not healed | 0 | 0 | 9 | 39.1 | |
| Total | 23 | 100 | 23 | 100 | |
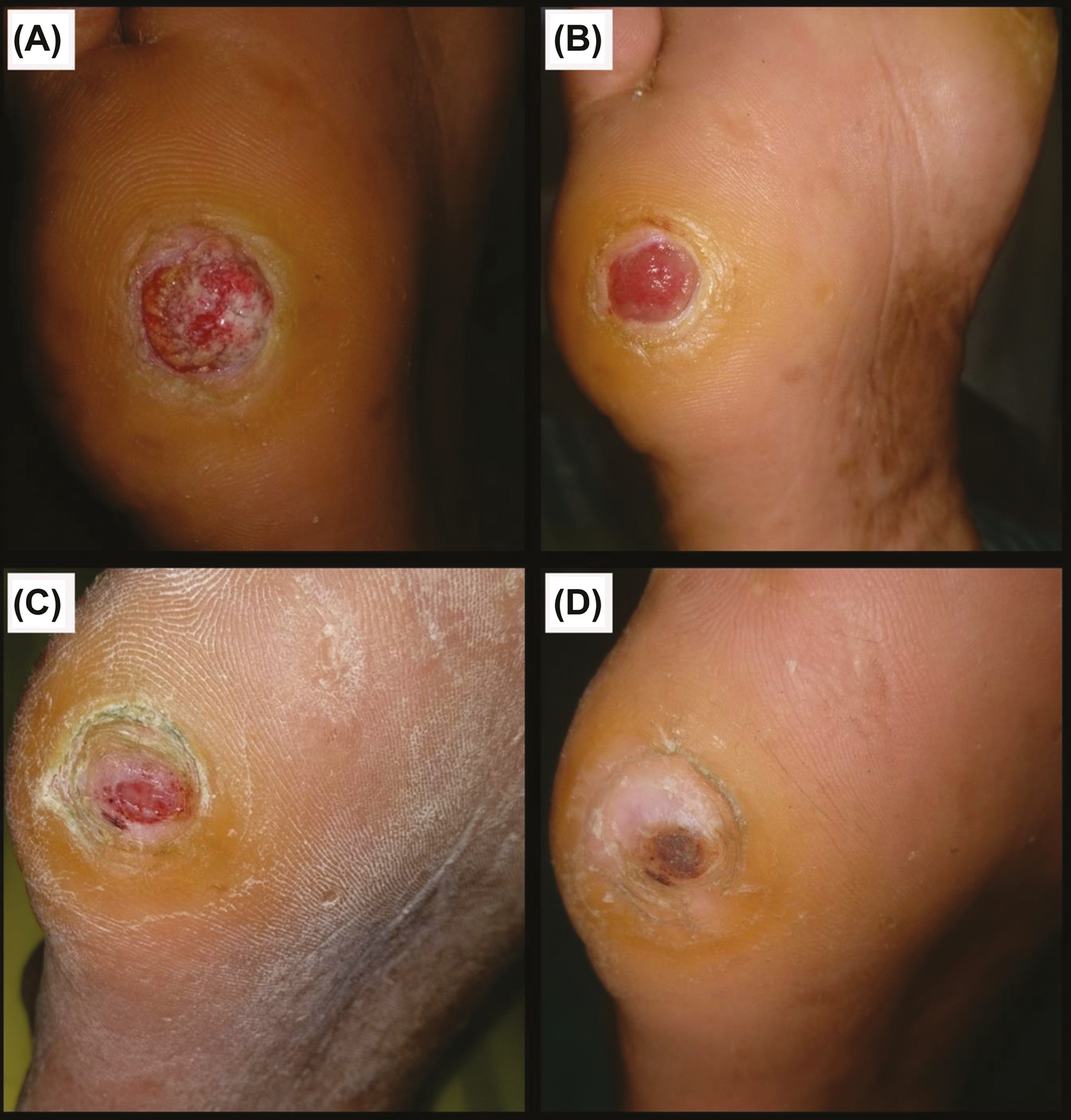
- Group A: (A) Before procedure. (B) 1 week post-procedure. (C) 3 weeks post-procedure. (D) 5 weeks post-procedure
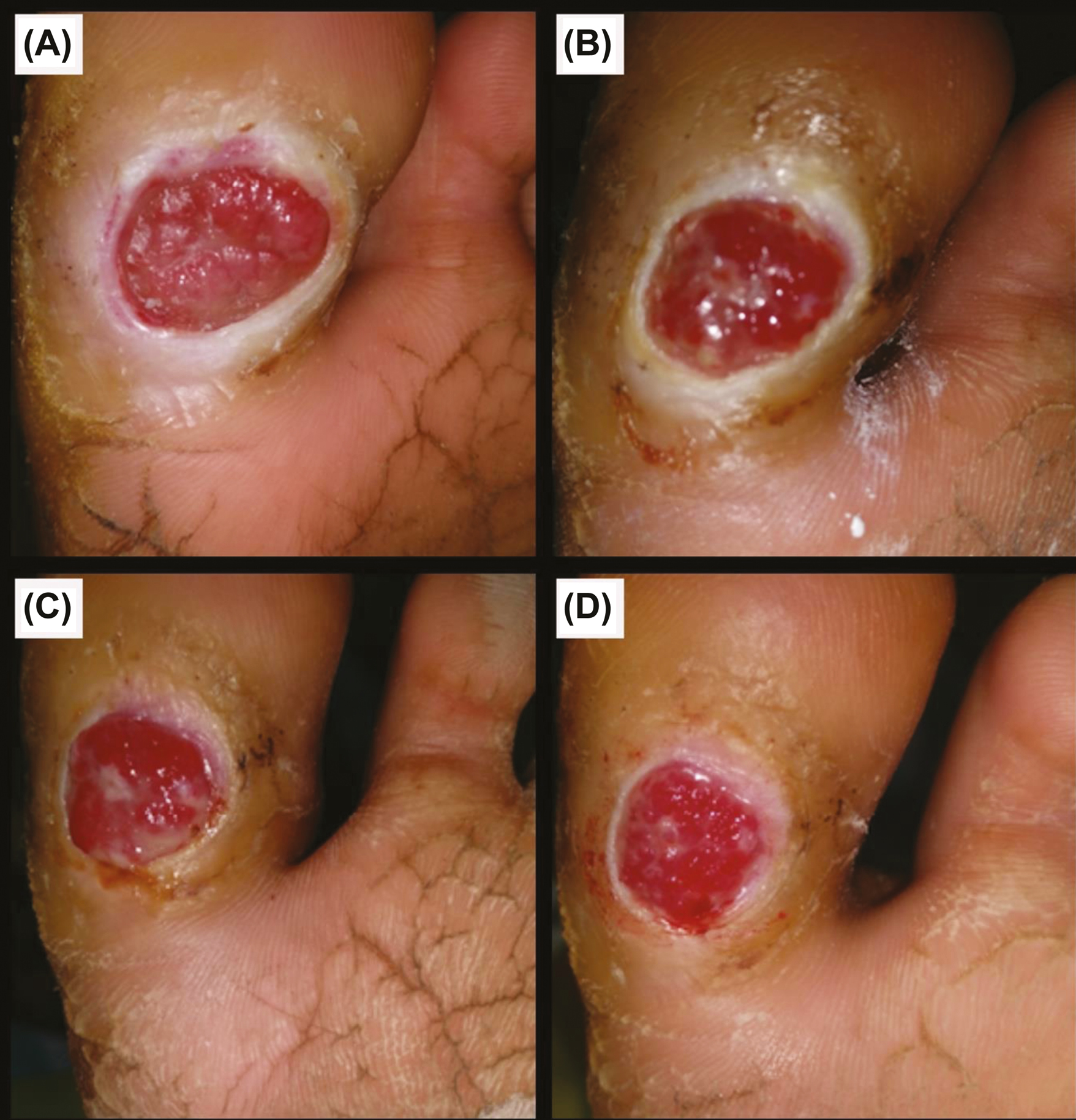
- Group B: (A) Before procedure. (B) 1 week post-procedure. (C) 12 weeks post-procedure. (D) 6 months post-procedure
DISCUSSION
The various treatment modalities available for trophic ulcers are moist wound dressings, vacuum-assisted closure, hyperbaric oxygen therapy, reconstruction surgeries, and topical applications of growth factors,[12] pinch graft, split-thickness graft, cultured epidermal autograft, and cultured keratinocyte allograft.[131415]
The technique involving ECS was invented to enable the possibility of treating larger wounds with a small piece of donor skin.[16] Autologous ECS was used for wound healing for the first time in rabbits,[17] and a comparative study was performed in pigs. In his study on pigs, Svensjo T et al,[6] concluded that there were significantly fewer keratinocyte colonies associated with non-cultured keratinocyte transplantation than with cultured keratinocyte transplantation. An experimental study using cultured and non-cultured keratinocytes in a porcine model revealed that ulcers transplanted with keratinocyte suspension showed accelerated re-epithelialization and rapid healing.[7] In a pilot study on the effect of autologous ECS in chronic nonhealing wounds by Shukla VK et al,[7] six (40%) out of 15 patients had completely healed at 12 weeks, one patient (7%) at 16 weeks, and two patients (13%) at 20 weeks post-procedure. In a study by B De Angelis et al,[18] on the use of a non-cultured autologous cell suspension in chronic ulcers, complete ulcer healing, defined as 100% re-epithelialization, was observed between 40 and 60 days in 14 patients (70%) depending on the type of ulcer and comorbidity. At 60 days post-procedure, 80% re-epithelialization was present in five patients (25%), whereas 50% re-epithelialization was observed in one patient with concomitant psoriasis.
In a study on follicular unit grafting in chronic nonhealing leg ulcers by Budamakuntala L et al,[2] a total of 15 patients with 17 ulcers were treated with follicular unit graft. Of these 17 ulcers, 11 were venous ulcers, two were pyoderma gangrenosum associated with varicose veins, two were traumatic ulcers, and two were trophic ulcers. The baseline mean area of the ulcer was 6.72 cm2, and the baseline volume was 2.87 cm.[3] The final area of the ulcer at the end of 18 weeks after the procedure was 3.84 cm[2] and the final volume was 1.21 cm,[3] which was statistically significant. The mean percentage improvement in the area and volume of the ulcer was 48.8% and 71.98%, respectively. In a randomized controlled study on HF-containing punch grafts in chronic ulcers by Martinez M et al,[19] ulcer healing, measured as the average percentage reduction, 18 weeks post-intervention was significantly increased (P = 0.002) in the HF group with a 75.15% ulcer area reduction compared with 33.07% in the control group (non-hairy grafts).
In a study by Nagaraju et al.,[20] on the technique of autologous smashed follicular dermal graft with ECS in 12 cases of chronic nonhealing ulcers, it was noted that 41.6% of the ulcers healed within four weeks post-procedure, and 100% ulcers completely healed by eighth week. At the end of four weeks, the mean percentage of improvement in the ulcer area was 98.33% and that of the ulcer volume was 99.26%. Ulcers healed both from the periphery and in a centrifugal manner, which gradually merged to cover the ulcer bed completely. Hypertrophic granulation tissue was seen in two of the ulcers, which subsided spontaneously within a week with regular normal saline dressing. The donor site in all cases showed complete healing with mild pigmentation by seven days post-procedure. No other adverse effect was noted in any case.
The results from all the studies just cited [Table 5] infer that non-cultured ECS is one of the better surgical modalities in treating chronic nonhealing ulcers, as it seems to be an effective, simple, economical, and time-saving method and a larger area can be treated with a smaller donor area. Similarly, HF dermal grafting is a minimally invasive surgical procedure that appears to be effective as a therapeutic tool for chronic leg ulcers. Hence, epithelial cells from ECS, HFs and collagen from the dermis may enhance the ulcer-healing process and reduce the duration of ulcer healing. Thus, by shortening the wound-healing phase, the quality of life of patients with Hansen’s disease can be improved, thereby reducing their financial burden and rehabilitating them at the earliest.
| Parameters | Other studies | Our study (smashed follicular dermal graft ± ECS) |
|---|---|---|
| Mean area of ulcer at baseline | Budamakuntala L et al.[2]: (Follicular dermal graft) The mean area of ulcers at baseline was 6.72 cm2. Shukla VK et al.[7]: (ECS) The mean area of ulcers at baseline was 80.96 cm2. |
The mean area of ulcers at baseline in our study was 7.06 cm2. |
| Time for complete healing | Shukla VK et al.[7]: (ECS) 6 (40%) out of 15 patients had completely healed at 12 weeks, 1 patient (7%) at 16 weeks and 2 patients (13%) at 20 weeks after treatment. De Angelis B et al.[18]: (ECS) 100% re-epithelialization was observed between 40 and 60 days in 14 (70%) out of 20 patients. At day 60 post-procedure, 80% re-epithelialization was present in 5 patients (25%), while 1 patient with concomitant psoriasis had 50% re-epithelialization. Nagaraju U et al.[20]: (Smashed follicular dermal graft ± ECS) At the end of 4 weeks, mean percentage of improvement in ulcer area was 98.33% and that in ulcer volume was 99.26%. |
In our study, out of 23 ulcers in Group A, 3 (13%) ulcers had healed within 5 weeks; 8 (34.8%) ulcers in 6 weeks; 10 (43.5%) ulcers in 7 weeks and 2 (8.7%) ulcers in 8 weeks. The mean percentage of change in area of the ulcers with respect to baseline showed 34.13% improvement after 1 week of treatment, which improved to 87.26% in 4 weeks and 99.88% in 7 weeks, that later completely healed with 100% improvement in 8 weeks. All 23 (100%) ulcers had healed within the study period of 6 months. |
| Secondary procedures | Shukla VK et al.[7]: (ECS) 1 patient required skin grafting, and 3 patients were lost to follow-up. |
In our study none of the patients required secondary procedures and none were lost to follow-up. |
We observed no complications in our study. None were lost to follow-up.
CONCLUSION
Trophic skin ulceration in Hansen’s disease is a complex phenomenon, the management of which has always been challenging. The rapid healing of ulcers improves the patient’s quality of life by reducing the morbidity. Hence, there is always a search for viable alternative treatment methods. The study of chronic nonhealing trophic leg ulcers evokes a lot of interest and is spectacular as far as the treatment of these cases is concerned. With improvisation of the surgical graft technique, there is certainly a great improvement in the treatment of chronic nonhealing ulcers. The use of autologous smashed follicular dermal graft with ECS is a simple technique, is manageable even on an outpatient basis, and quickly reduces and hastens the re-epithelialization of ulcers. Thus, we conclude that by using autologous smashed follicular dermal graft and ECS, it is possible to achieve a faster rate of granulation tissue and a faster recovery of the ulcer.
Limitations and recommendations in our study
Only males were included in the study
Large sample sizes are required to standardize the procedure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowlegdments
The authors thank (Late) Dr. Umashankar Nagaraju (Professor of Dermatology, Rajarajeswari Medical College and Hospital, Bengaluru) for his expert guidance and encouragement. He was the pioneer in conceptualizing this study, in a constant endeavor to serve the patients with Hansen’s disease and to reduce their morbidity. They also thank Dr. Naveen S (Professor of Surgery and Principal, Rajarajeswari Medical College and Hospital Bengaluru) for his excellent surgical graft techniques used in the study.
REFERENCES
- Definition, epidemiology and world distribution. In: Jopling W, McDougall A, eds. Handbook of Leprosy (5th ed.). New Delhi: CBS; 2015. p. :1.
- [Google Scholar]
- Follicular unit grafting in chronic nonhealing leg ulcers: A clinical study. J Cutan Aesthet Surg. 2017;10:200-6.
- [Google Scholar]
- Autologous platelet-rich fibrin matrix in non-healing trophic ulcers in patients with Hansen’s disease. J Cutan Aesthet Surg. 2017;10:3-7.
- [Google Scholar]
- Disablement—Its prevention and management. In: Sacchidanand S, Oberai C, Inamadar AC, eds. IADVL Textbook of Dermatology Vol 3. (4th ed.). Mumbai: Bhalani Publishing House; 2015. p. :3181-93.
- [Google Scholar]
- Deformities of face, hands, feet and ulcers and their management. In: Kumar B, Kumar KH, eds. IAL Textbook of Leprosy (2nd ed.). New Delhi: Jaypee Brothers Medical Publisher (P) Ltd; 2017. p. :517-38.
- [Google Scholar]
- Autologous keratinocyte suspensions accelerate epidermal wound healing in pigs. J Surg Res. 2001;99:211-21.
- [Google Scholar]
- Effect of autologous epidermal cell suspension transplantation in chronic nonhealing wounds: A pilot study. Can J Surg. 2010;53:6-10.
- [Google Scholar]
- Permanent skin replacement using chronic epithelial cultured sheets comprising xenogenesic and syngenetic keratinocyte. Transplant. 1996;61:1290-300.
- [Google Scholar]
- Reepithelialization of a full-thickness burn from stem cells of hair follicles micrografted into a tissue-engineered dermal template (integra) Plast Reconstr Surg. 2004;113:978-81.
- [Google Scholar]
- Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: A multicenter randomized controlled trial. Diabetes Care. 2008;31:631-6.
- [Google Scholar]
- Trophic ulcers-practical management guidelines. Indian J Plast Surg. 2012;45:340-51.
- [Google Scholar]
- Pinch grafting of chronic leg ulcers in primary care: Fourteen years’ experience. Acta Derm Venereol. 2002;82:275-8.
- [Google Scholar]
- Treatment of leg ulcers with cultured epithelial autografts: Clinical study and case reports. Ostomy/Wound Manage. 1995;41:48-50,52,54-60.
- [Google Scholar]
- Treatment of chronic venous ulcers with sheets of cultured allogenic keratinocytes. Br J Dermatol. 1987;117:591-7.
- [Google Scholar]
- Autologous epidermal cell suspension: A promising treatment for chronic wounds. J Tissue Viability. 2016;25:50-6.
- [Google Scholar]
- Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspensions. Br J Plast Surg. 1952;5:25-36.
- [Google Scholar]
- The use of a non cultured autologous cell suspension to repair chronic ulcers. Int Wound J. 2015;12:32-9.
- [Google Scholar]
- Hair follicle-containing punch grafts accelerate chronic ulcer healing: A randomized controlled trial. J Am Acad Dermatol. 2016;75:1007-14.
- [Google Scholar]
- Autologous smashed follicular dermal graft with epidermal cell suspension in chronic nonhealing leg ulcers. J Cutan Aesthet Surg. 2020;13:38-42.
- [Google Scholar]






