Translate this page into:
Polydioxanone Bioactive Sutures—Acetyl Hexapeptide-8 (Argireline): An Intelligent System for Controlled Release in Facial Harmonization
Address for correspondence: Dr. Dubraska V. Suárez-Vega, Department of Research, University of Los Andes (ULA), 23rd Street between avenues 2 and 3, Mérida 5101, Venezuela. E-mail: dubraskasuarez.ula@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction:
We propose a new facial lifting protocol using polydioxanone (PDO) threads embedded in acetyl hexapeptide-8 (Argireline [Arg]). We assume that Arg reinforces the effects of PDO threads, as it is a mimetic of botulinum toxin. Because the PDO suture is hydrolyzable, this assumption is analyzed by instrumental analysis.
Objective:
To demonstrate the capacity of the PDO suture as a system for the controlled release of acetyl hexapeptide-8 to apply in deep wrinkles of the upper third.
Materials and Methods:
Three segments of 1-cm long 21G PDO threads immersed in 1 mL of Arg. PDO threads were observed under an optical, electron microscope at 24, 48, and 72 h later. They were also weighed before and after being soaked in Arg, and employing ultraviolet (UV)-visible spectroscopy, the release rate of Arg from the PDO suture was measured. Finally, was insert the thread PDO-Arg following a protocol designed especially for deep static wrinkles in the upper third.
Results:
The electronic weighing revealed that the PDO thread enjoys capillarity by the peptide, doubling its weight every 24 h. UV spectra revealed that PDO thread is a well-controlled release system for Arg, allowing its sustained release for 1 h. Optical and electronic photomicrographs confirm the swelling of the PDO thread by absorbing Arg by its capillarity, but this hydrophilicity does not lead to its premature physical degradation.
Conclusions:
The PDO thread system with Arg is an intelligent bioactive system useful in facial harmonization. It recommend conduct clinical trial to verify his superior lifting effect.
Keywords
Acetyl hexapeptide
Argireline
facial absorbable suspension sutures
polydioxanone
polydioxanone threads
INTRODUCTION
It is well known that polymer-based controlled release drug delivery system are ideal candidates for sustained release of drugs within the body. Therefore, to modulate the release time and control drugs delivery kinetics, several approaches have been adopted.[1] Among these, there is currently the use of fibrous matrices and polymeric sutures, which seem to offer important advantages due to their compatibility with a wide variety of drugs.[2]
These matrices include polydioxanone (PDO), a polymer that has been used in a variety of biomedical devices, scaffolds, and suture materials, due to its excellent biocompatibility, being the PDO thread a highly flexible, stress-resistant, and biodegradable suture.[3]
The usefulness of PDO threads as drug-releasing devices has been analyzed since 2016.[4] It presumed that its effectiveness as a delivery system is because the drug molecules manage to trap between the fibers of the thread since the fibers have an extremely high absorption surface area, which contributes to a subsequent slow and sustained release of the drug.[5]
This phenomenon of drug release from PDO sutures is possible, thanks to the ability of the polymer to absorb water; this property is known as capillarity, which is the ability to absorb fluid along the filament, from the submerged part of the filament to the dry part. Capillarity differs from the ability to absorb fluid; in that fluid, absorption is determined when the suture is fully immersed in it.[6]
Hence, PDO sutures can absorb drugs and substances of a liquid nature, without necessarily starting their immediate biodegradation by hydrolysis, because although they have capillarity, their biodegradation in the organic environment is slow, up to 180–210 days.[7]
For this reason, PDO threads are of choice for facial deflation; a successful therapy indicated to reverse deflation, lipomatosis, rhytids, and deep folds when the degree of aging does not require surgical treatment.[3]
Based on the above, it can be assumed that this physical property of capillarity in facial harmonization could become an ally, for the use of the PDO suture as an osmotically controlled release system, being able to impregnate with active principles, and slowly release them to the environment. This capillarity of the PDO thread turns to the suture into a bioactive scaffold, without its wetting with active principles irremediably leading to its disolution or degradation afecting important physical properties of the pdo thread such as his resistance modulus.
Such assertions were tested in the present investigation. The objective was to demonstrate, the ability of PDO suture as a system for the controlled release of acetyl hexapeptide-8 (Argireline [Arg]), a biomimetic peptide of botulinum toxin, to apply in deep wrinkles of the upper third. A delivery system was proposed for subdermal use in frontal and ocular contour neuromodulation, because the topical penetration of acetyl hexapeptide-8 (Arg) is deficient and a subdermal delivery system would overcome this limitation.
For this, we started from the instrumental analysis of the PDO suture impregnated with the active, evaluating under four types of techniques to check the absorption and release of fluids: a fluid absorption test by electronic weighing, observation by optical microscopy, and scanning electron microscopy and an analysis of the release rate by ultraviolet-visible (UV-Vis) spectroscopy.
MATERIALS AND METHODS
The present descriptive research, with a laboratory design or created environment, longitudinal or contemporary evolution,[8] considered three specimens as study samples: one for the control–control group (PDO without Arg), one for the control group (embedded PDO in distilled water), and one for the experimental group (PDO loaded with Arg) [Figure 1A].
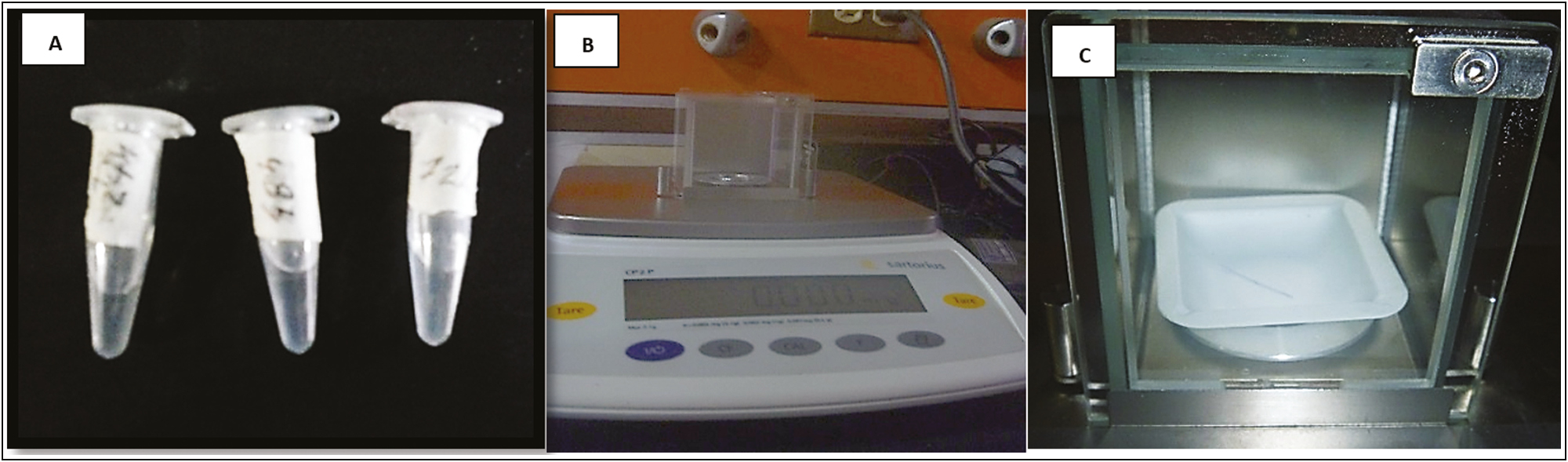
- (A) PDO segments in the middle of immersion. (B) Calibrated electronic balance. (C) Purple segment of the PDO thread in the closed chamber of the electronic balance
The instrumental analysis of the PDO suture impregnated with the peptide was carried out, evaluating under four types of techniques to verify the absorption and release of fluids: a fluid absorption test by electronic weighing, observation by optical reflection microscopy, and observation by electron microscopy. and analysis of the release rate by UV-Vis spectroscopy
Materials
The materials used are as follows: acetyl hexapeptide-8 peptide in solution, Arg brand (Lipotec SAU, Barcelona, Spain) 6% w/v 10-mL vial; PDO monofilament, nonbarbed, JBP V-Lift Premium brand (JBP KOREA CO., LTD, Seoul, South Korea), with a diameter of 23G gauge (4-0) length 150 mm; Ultrapure water 18 MΩ • cm; 1-mL BD Luer Slip hypodermic syringes (BD Plastipak™, New Jersey, United States) with an interchangeable needle to remove peptide; and 3-mL Eppendorf DNA LoBind Polypropylene Microcentrifuge Tubes (Hamburg, Germany).
Fluid absorption test by differential weighing
The suture was sterilized and vacuum-packed without any degree of humidity. The thread was extracted from its guide cannula on a sterile field, and measurements were made with a millimeter metal ruler to cut three segments of 1 cm in length with sterile surgical scissors.
On an electronic balance (Sartorius CPA analytical), the weight of a 1-cm long segment of the PDO suture without impregnation was determined to set the initial reference value [Figure 1B]. Subsequently, a segment of the PDO thread immersed in 1 mL of Arg at 6% (w/v) in sterile tubes of 1.5-mL polypropylene microcentrifuge (Eppendorf Safe-Lock) was labeled for the identification of the samples in three groups. According to the dive time (1 min, 24 h, 48 h, and 72 h), once the tubes were covered, they were stored at a temperature of 37°C.
Once the immersion time had elapsed, each segment of the thread was removed from the medium of the tube with a sterile clamp and it weighed on the scale. Likewise, a PDO suture segment of the same size was immersed for the same time in 1 mL of distilled water, and the respective weight was recorded [Figure 1C].
Observation under the light microscope
At the end of each evaluation (24, 48, and 72 h after immersion), the PDO suture segments were removed from the tube with sterile forceps, dried on a sterile field at room temperature for 5 min, mounted on a slide, and observed to the Nikon reflection optical microscope, at 4× and 10×; a microphotographic scan of each segment was performed.
Scanning electron microscopy
Once the PDO suture had been soaked with Arg for 1 min, it was removed from the medium with the active ingredient and allowed to dry at room temperature for 5 min on the slide. The thread segment is then impregnated on the sheet with a fixative for electron microscopy samples or a 3:3 mixture (3% formaldehyde + 3% glutaraldehyde in 0.1 M cacodylate buffer, pH 6.3).
The coverslip sheet was previously identified with a lateral cut for its spatial location at the time of metallization and its subsequent mounting in the electron microscope. Both control and experimental slides were stored in capped test tubes immersed in a fixative mixture, refrigerated at 4°C, for a minimum of 24 h for immediate fixation.
After fixation, the samples were dehydrated with ethyl alcohol in increasing concentrations. Desiccation was carried out for 48 h, and they were metalized by spraying with gold. They were observed in a Hitachi S-2500 scanning electron microscope (Chiyoda-ku, Tokio, Japón).
Arg release rate from PDO
The PDO-Arg thread was observed indirectly, assisted through software connected to a UV-Vis spectrophotometer. The program outputs data in the form of codes, corresponding to each absorbance signal in nanometers or the wavelength peaks in the UV spectrum.
These signals correspond to the uptake of the drug in water as the photons pass through the cell and the submerged thread. Results were plotted using Origin Lab software (Northampton, Massachusetts, EE. UU). The prolonged release of the drug was assessed by recording the release rate of the Arg from the PDO suture and from this; a concentration curve released over time (at 5, 15, 30, 60, and 2400 min or 24 h) was constructed.
Clinical case
To validate the physicochemical behavior detected in the threads with Argeriline as a cosmeceutical release system and verify its clinical utility, we designed a protocol for its application in deep static wrinkles in the upper third [Figures 2–4]. The effect showed in the results section.
![Configuration suggested polydioxanone threads into deep static wrinkles on the upper third. Source: Kang et al.[8]](/content/173/2023/16/4/img/JCAS-16-325-g002.png)
- Configuration suggested polydioxanone threads into deep static wrinkles on the upper third. Source: Kang et al.[8]
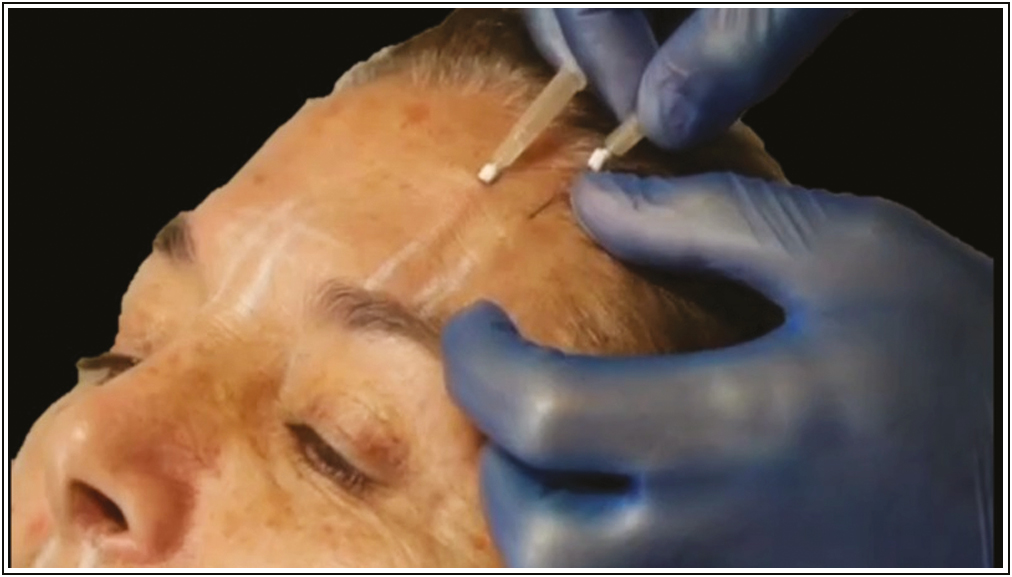
- Suggested application technique for inserting PDO-Arg. Source: Maldonado GJV, Suárez-Vega DV, Miller-Kobisher B, García-Guevara VJ. Bioactive sutures (PDO with Argireline)
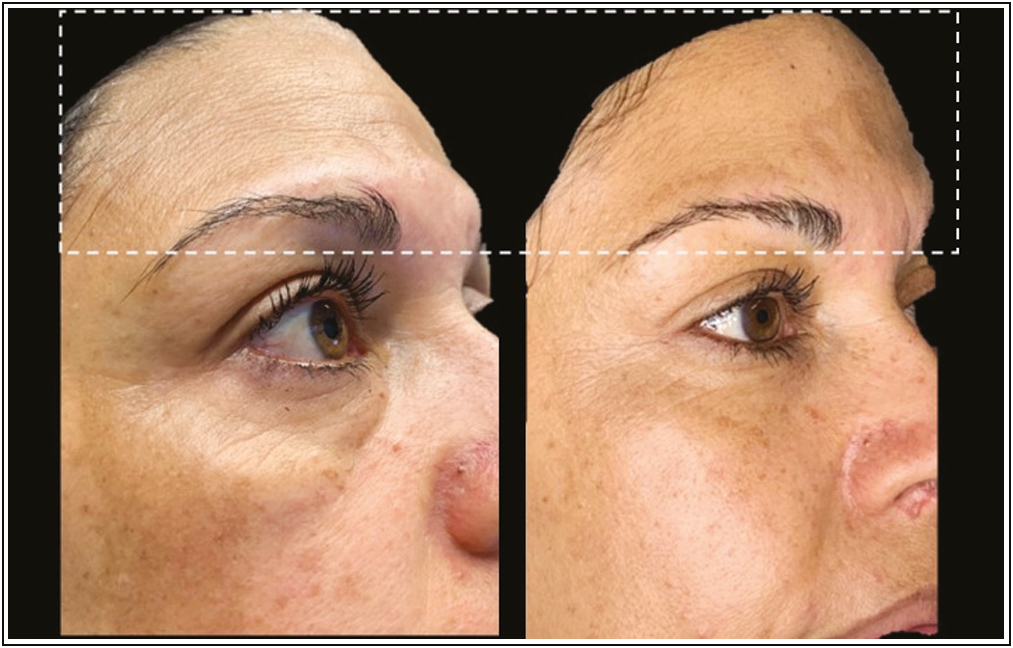
- Before and after (24 h) clinical effects of PDO-Arg into deep static wrinkles. Source: Maldonado GJV, Suárez-Vega DV, Miller-Kobisher B, García-Guevara VJ. Bioactive sutures (PDO with Argireline)
RESULTS
Fluid absorption test by differential weighing
The weight of the PDO thread doubles after 24 h when immersed in distilled water and acetyl hexapeptide-8, exceeding the weight of the thread with water by 10 mg. This weight into the thread increased at 48 and 72 h later of his inmersion in both groups Table 1. But, for the experimental group (thread inmersed in the acetyl hexapeptide) the weight recorded being always greater that the weight of the control group (thread inmersed in water), which suggests a captation of the peptide for the thread (capilarity capacity) and a higher molecular weight of the acetyl hexapeptide than water.
| Sample | 24 h | 48 h | 72 h |
|---|---|---|---|
| PDO single | 0.4965 mg | 0.4965 mg | 0.4965 mg |
| PDO + distilled water | 0.993 mg | 0.955 mg | 0.942 mg |
| PDO + acetyl hexapeptide | 1.09 mg | 1.119 mg | 1.95 mg |
Optical microscopy
The photomicrographs at 24 h already show structural changes in the fibers of the PDO thread with an increase in interlaminar spaces, which are more easily visible in the central column of the thread.
Traces of the violet pigment are also observed moving toward the periphery of the thread (purple line), above a central background of interlaminar and interfibrillar spaces. Both the increased gaps and the pigment outlet correspond to the fluid absorption (Arg) in the fiber [Figure 5].

- PDO thread after 24 h immersion in Arg
Optical photomicrograph of the thread at 4×. Observed in the central column of the thread a interlaminar and interfibrillar spaces (whitish bands vertical and parallel) corresponding to the penetration of the Arg (this are areas where the drug molecules are between the fibers of the thread).
At 48 h, Figure 6 shows how the PDO thread is capable of absorbing fluids. The hygroscopy of the PDO suture was evidenced. (capacity of absorbtion of Arg) being observed this fenomen as a acumulation of aqueous content between the peripheral layers and the central core of the thread, accompanied by an increase of the interlaminar spaces (whitish stripes).
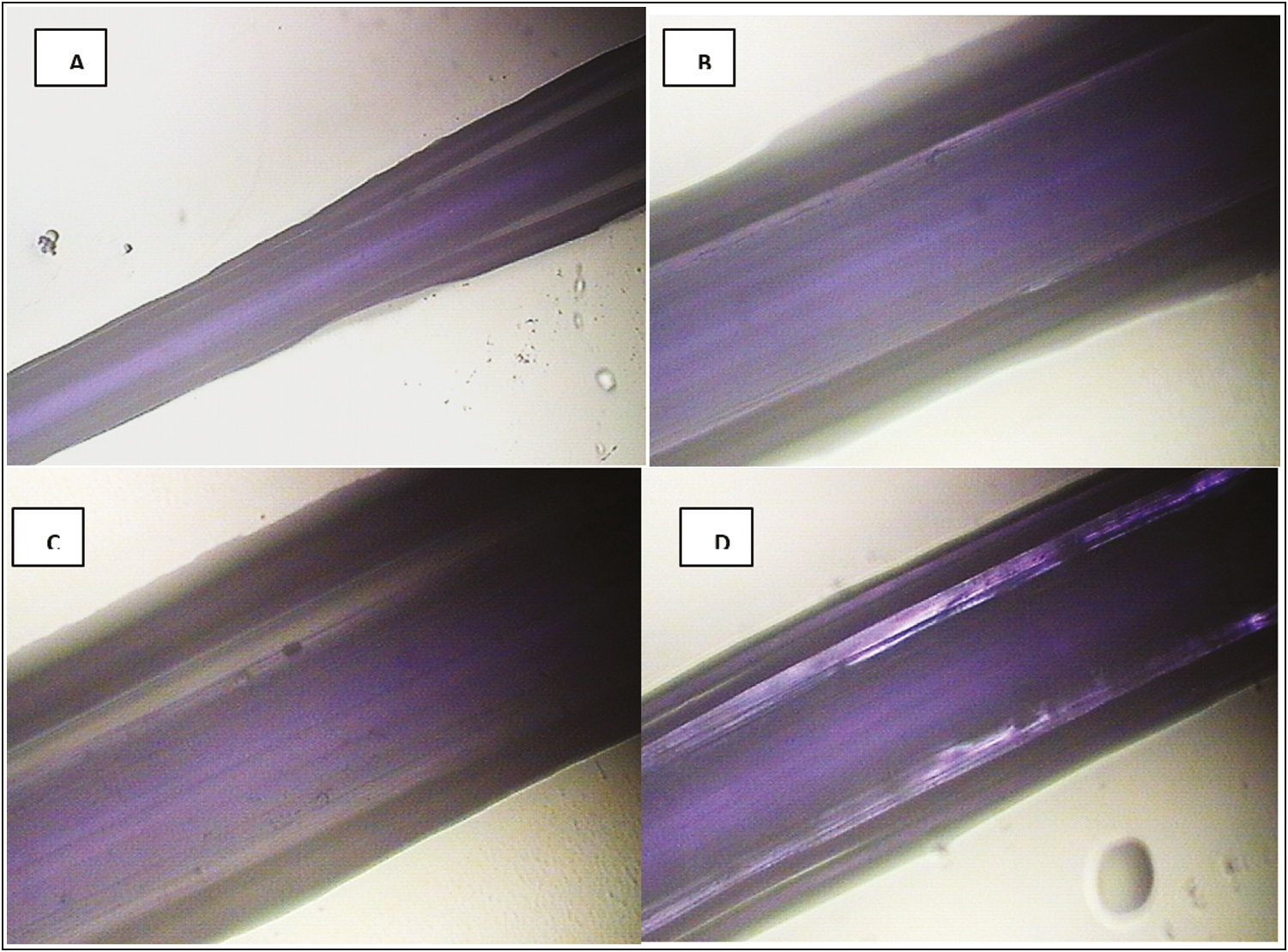
- PDO thread after 48 h of immersion in Arg. (A) 4× optical photomicrograph. Note in the central area of the thread, a bright light purple column corresponding to the central fiber of the PDO thread surrounded by parallel areas in dark light that extend from the central area to the periphery of the thread (increase in interlaminar spaces). (B and C) Thread at 10×. Note the increase in interfibrillar spaces and not only interlaminar spaces, they are observed as bright whitish vertical and parallel bands corresponding to the phenomenon of fluid absorption. (D) Aqueous retention in the thread was observed at 4×
Likewise, it was possible to capture in progress the capillary phenomenon of the PDO suture before its immersion in acetyl hexapeptide. It corresponds to the advancement of a dark purple-pigmented area that interpenetrates the thread [Figure 7].
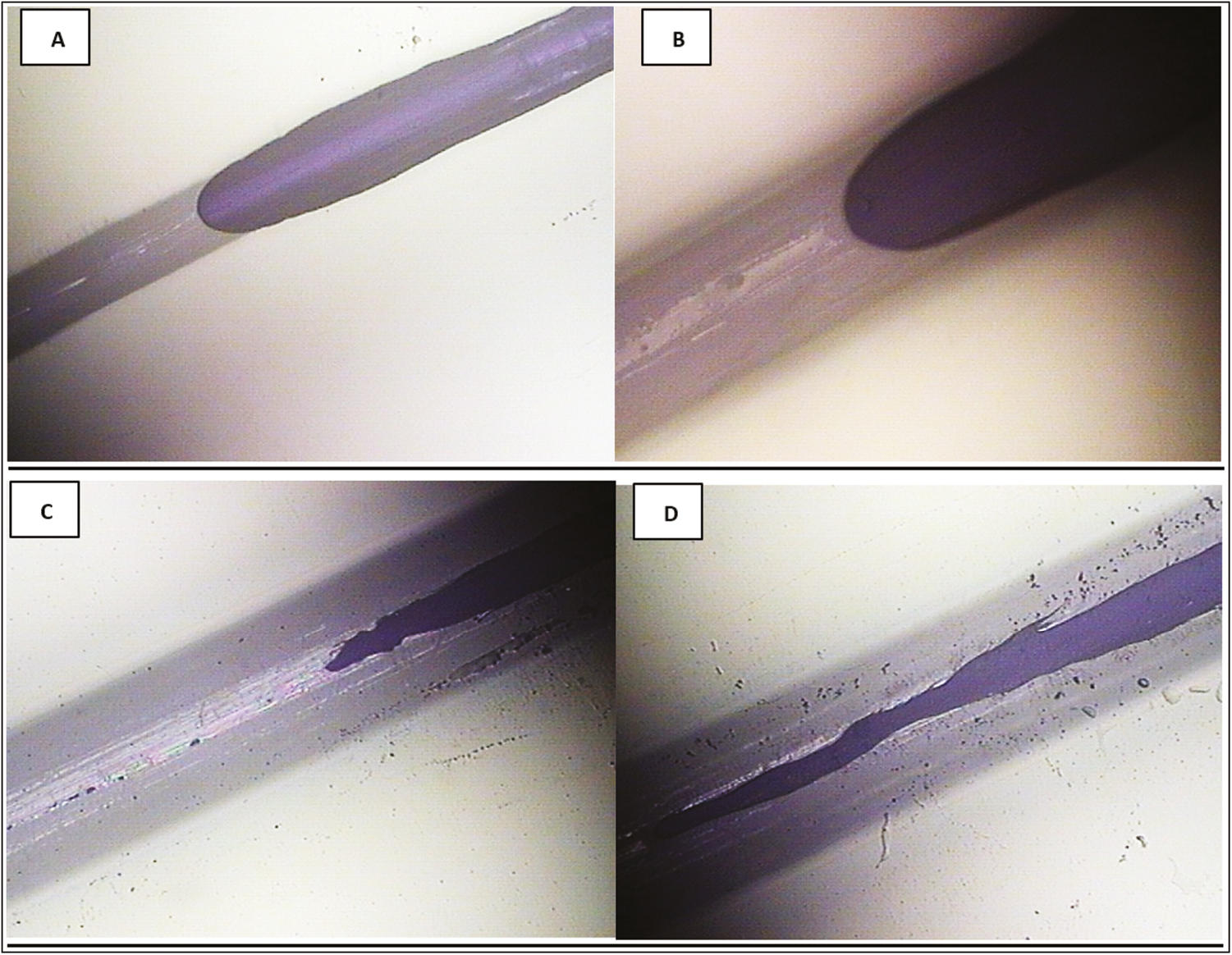
- Capillarity of the PDO thread after 48 h of immersion in Arg. (A and B) Optical photomicrographs of reflection of the thread at 10× and 4×, in which the phenomenon of capillarity is denoted by the effect of fluid absorption along the filament from the embedded part to the dry area of the thread, without completely submerging the thread in the aqueous medium of the active ingredient. (C and D) PDO thread after 72 h of immersion in Arg at 10×. Note the increase in the thickness of the thread and the interpenetration of the fluid in the central column of the suture, as well as the migration of the amorphous zone to the crystalline zone of the polymer in the suture
Scanning electron microscopy
The topography of the smooth PDO threads is seen as homogeneous cylinders with superficial lateral cuts in the form of microspicules in opposite directions, which is possibly part of the factory design for their microanchorage in the subcutaneous tissue [Figure 8A].
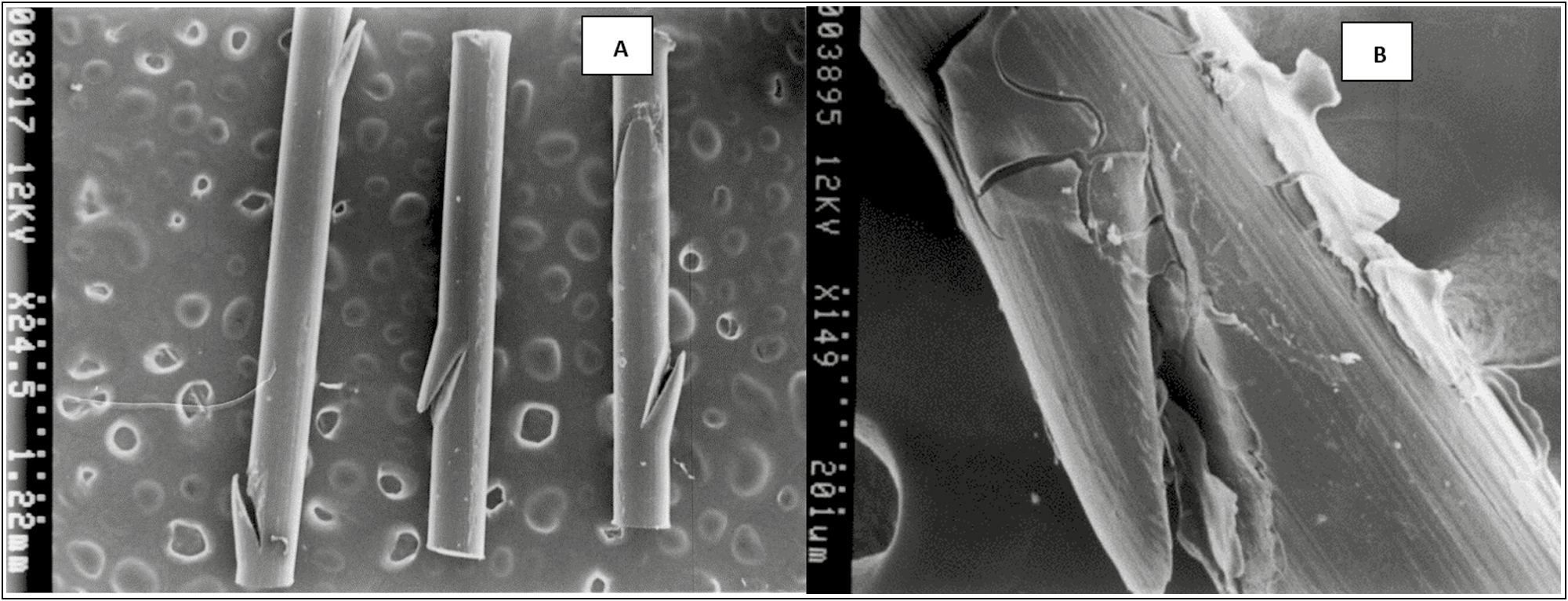
- (A) Topography of the PDO thread without the drug (control) scanning electron microscope photomicrograph at 25×. Spicular fissures are observed at the lateral edges. (B) PDO thread with Arg. Scanning electron microscope photomicrograph at 150×. Note a layer that covers the thread, which interpenetrates the fissure of the fiber and that in certain sections it flakes. It corresponds to the asset fixed on the surface of the PDO thread
When adding the peptide formulation, it was observed that this biomimetic active covers the thread, interpenetrates the fissure of the fiber and in certain sections, it flakes due to the effect of metallization. However, the image shows the indemnity of the PDO thread when the Arg is added to it, the peptide does not seem to alter the structure of the thread, nor does it hydrolyze it; therefore, it is presumed that the PDO + Arg thread is a physically stable system [Figure 8B].
Arg release rate from PDO suture
For mesuring Arg release rate from PDO suture, first it was required to measure the signal UV visible coming from the Arg and the signal of the PDO thread for separate (without Arg).
These signals are a reference for compare, detect when the thread is alone withoth Arg drug of when this suture charged with the Arg, and when this farmacy it is, liberate to the medium.
This parameter called absorbance or UV spectrum of the material, are capted signals by UV-Vis spectrophotometer machine and are corresponding the bounce of the light that pass trhoug a cristal cell in where is the submerged thread. This signal is expresed in nanometers or the wavelength peaks.
In Graph 1, the spectrum in nanometers of the PDO suture alone (without Arg) is observed, whose absorbance region is recorded between the 220 and 225 nm approximately.
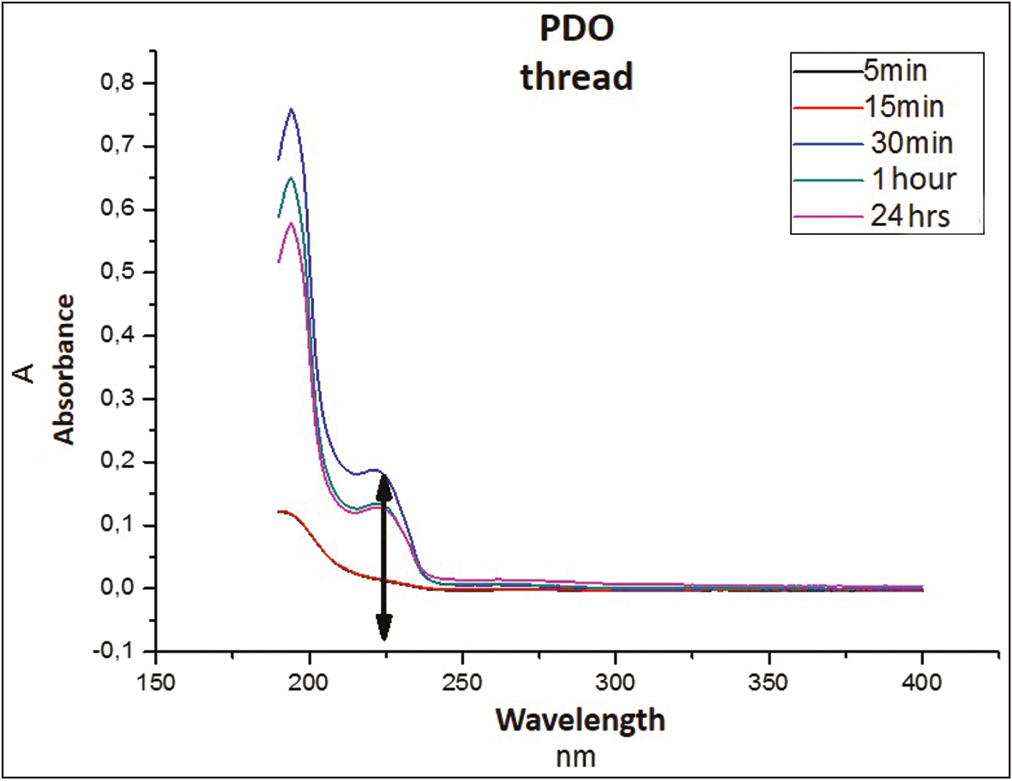
- Absorbance spectrum of PDO suture
Graph 2 shows that the suture emits a maximum absorbance peak of 0.17686 at 30 min, stabilizing again after 1 h, and remaining similar until 24 h.
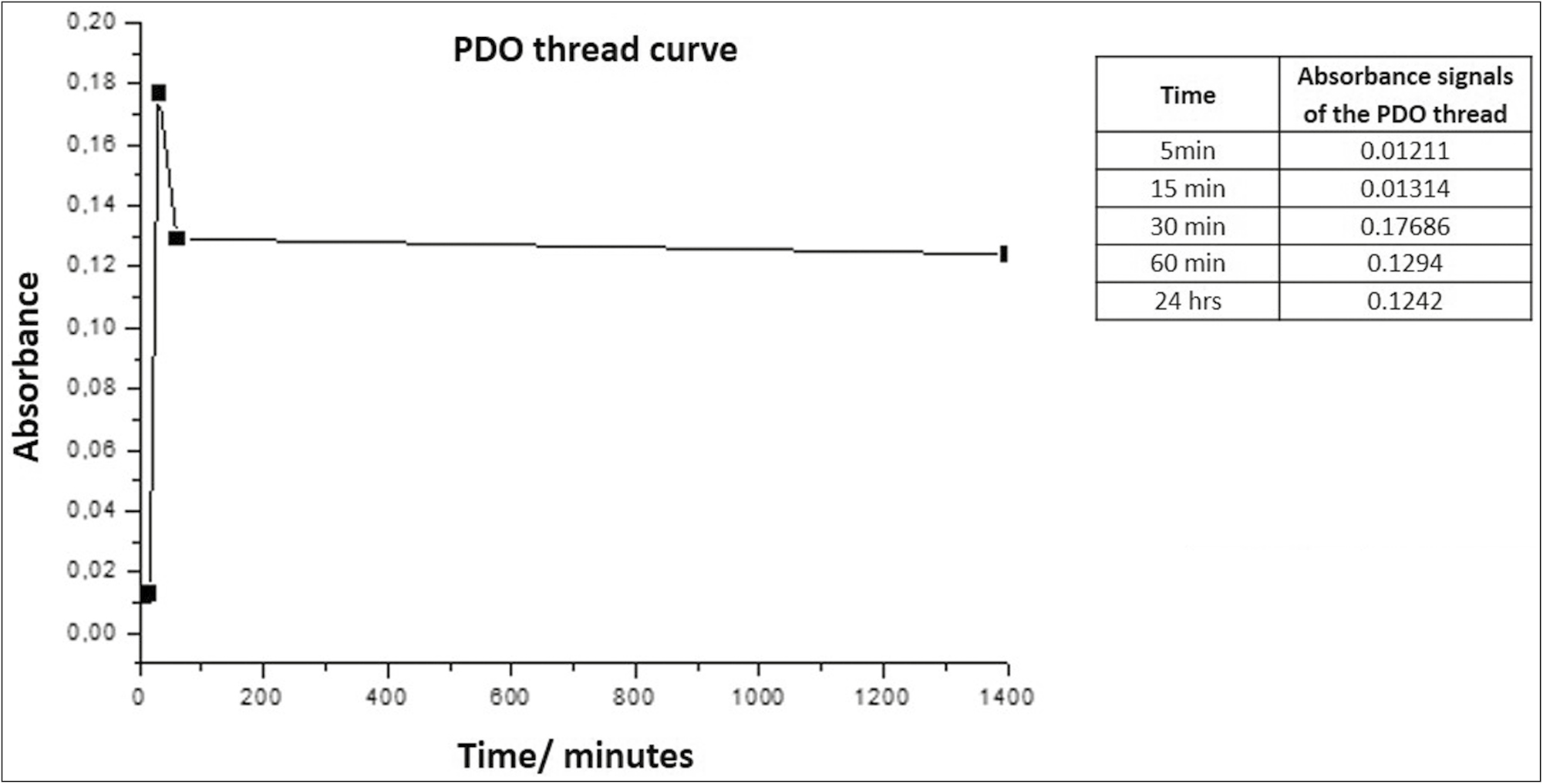
- Absorbance vs. time curve for PDO
This sweep of spectra of the thread suggests that the peak observed corresponds to the amorphous substance of the polymer that begins to be released into the medium by an incipient hydrolysis process that fails to destabilize and dissolve the structure.
Acetyl hexapeptide release from polydioxanone suture
Once the Arg is incorporated into the PDO, the release in distilled water is measured by UV-vis spectroscopy, Graph 3 evidences the UV-Visible spectrum of release as a function of time.
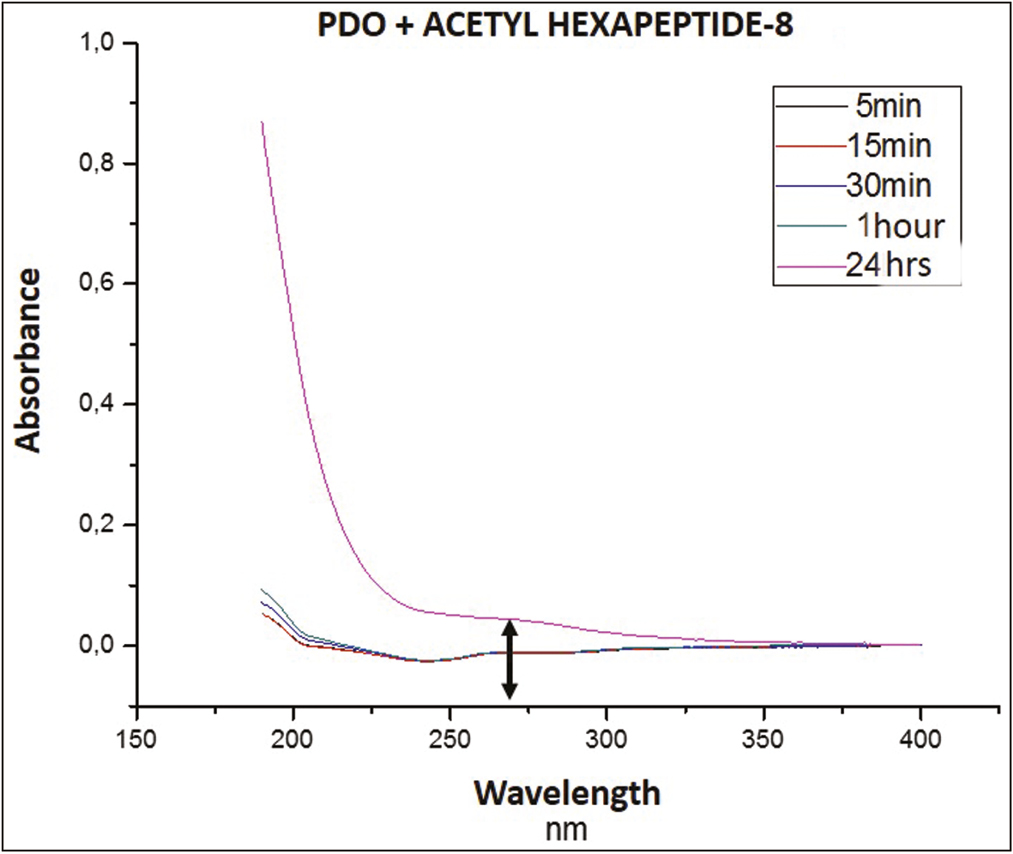
- UV-Vis spectrum of PDO at the time intervals, in which the release of Arg was measured
It can be seen that the absorbance area of the system is at a wavelength of approximately 270 nm. Regarding the signals captured over time, it can see that, at 15 min (red line), a signal was recorded in the middle that remained very close until 60 min (green). At 24 h (pink line), the highest absorbance peak was generated, among the registered signals.
This could correspond to an abrupt exit of the rest drug into the medium, due to the total degradation of the thread, that to break released by complete the Arg in the middle, this being the strong signal captured by the equipment.
Estimation of the release kinetics of acetyl hexapeptide from polydioxanone
The estimate of the release kinetics was calculated by plotting the absorbance as a function of time. Table 2 shows the results of the highest wavelength or highest peak absorbance of the PDO suture fragment with Arg studied at 5 min, 30 min, 60 min, and 24 h, data taken for the graphic representation of the release curves corresponding to each scaffold.
| Time | PDO + Arg |
|---|---|
| 5 min | −0.01175 |
| 15 min | −0.01228 |
| 30 min | −0.01082 |
| 60 min | −0.01015 |
| 24 h | 0.04341 |
As it was not certain whether the spectrophotometer detected the acetyl hexapeptide-8 (Arg) signal from the scaffold (peak observed in Graph 3), it was decided to determine the order of release kinetics, for which the absorbance was plotted as a function of time [Graph 4 and Graph 5].
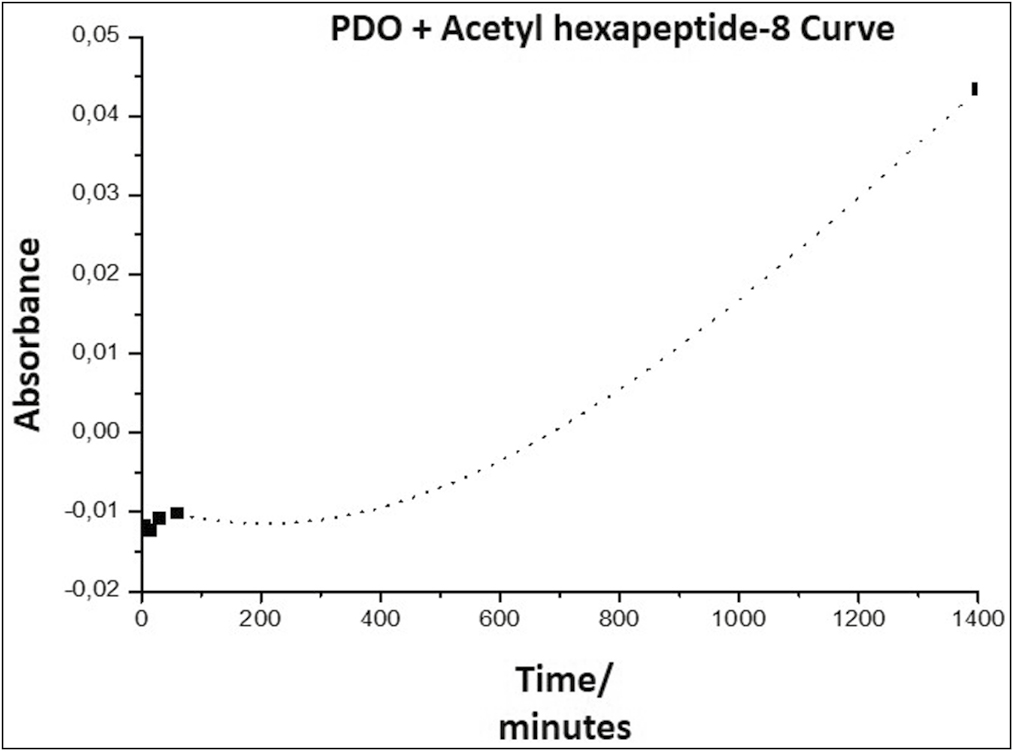
- Arg release curve from the PDO thread, representing the absorbance as a function of time
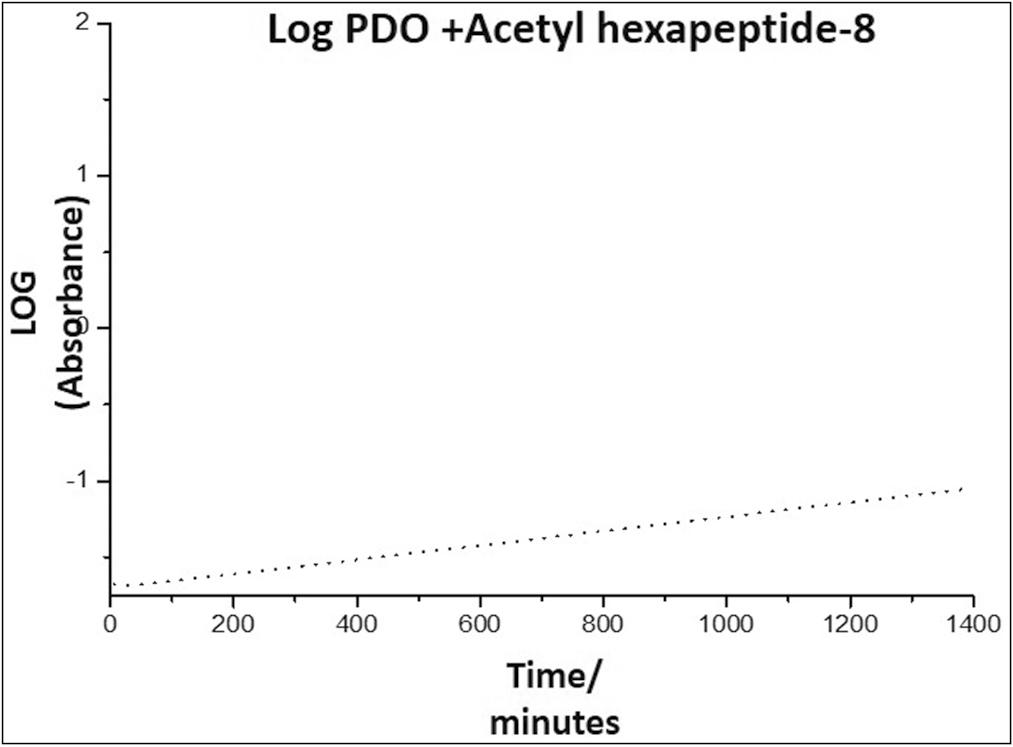
- Kinetics of the release of Arg from the PDO thread, representing the absorbance as a function of time
Graph 4 shows a slope in the form of a curve, indicating that the release of Arg from the PDO occurs with an order higher than zero order. This indicates that it is a first-order reaction, in which the speed depends on the concentration of a reagent raised to the first power; therefore, the speed will depend on the concentration of species released into the environment; a graph of the logarithm of absorbance as a function of time must be made to confirm the release kinetics [Graph 5].
Graph 5 reveals that at short times, the release kinetics obeys a straight line, which indicates that this system follows the first-order release, that is, it depends on the concentration of a substance in the system, kinetics that can be expressed according to Equation 1
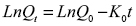
Where Qt is the amount of substance released at time t, Q0 is the initial amount of drug in solution, which is generally zero, and K0 is the rate constant in first-order kinetics.
The appearing shape of the slope indicates that the PDO thread is a good controlled release system for this drug and would allow a sustained and slow release of the same.
PDO ± Arg released concentration versus time
To determine the amount or concentration released, the absorbance of the drug was converted to milligrams/milliliters, using the conversion factor formula according to the Lambert-Beer law, because the absorbance is directly proportional to the concentration, recording the concentration for each of the absorbance recording periods studied (5 min, 15 min, 30 min, 60 min, and 24 h).
Around the release rate represented in Graph 6, it was observed that the peptide concentration in the medium was released in similar concentrations from 5 to 60 min, when the curve falls to its minimum concentration until 24 h, probably due to exhaustion of the drug and saturation of the medium betwen the 60 min y the 24h.
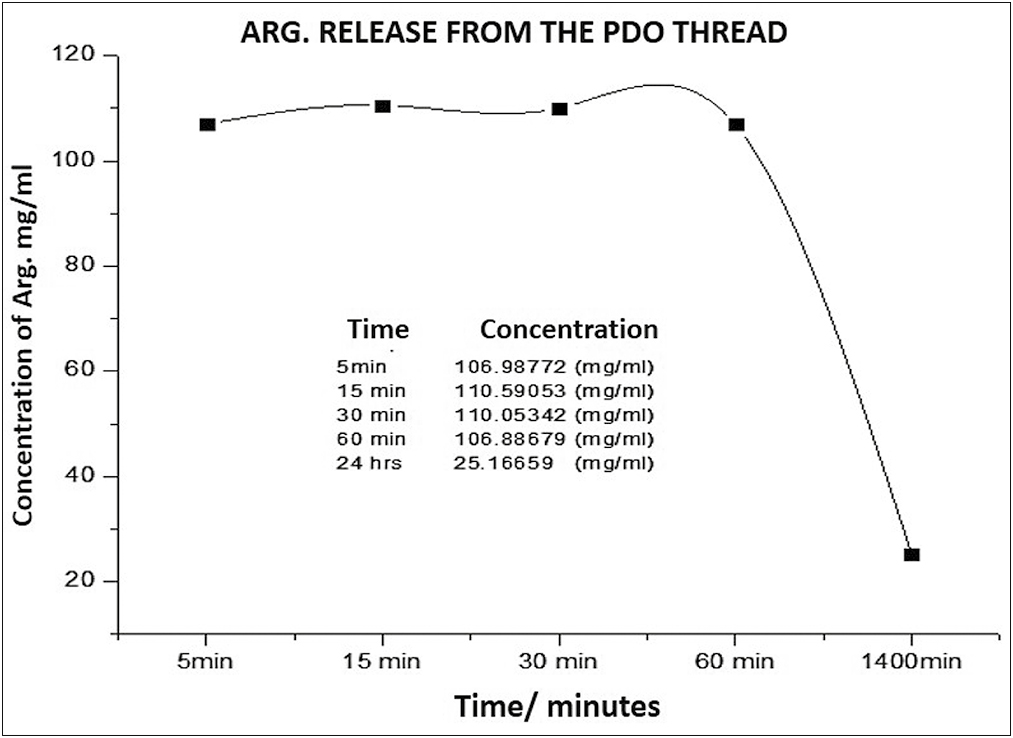
- Arg concentration released over time from the PDO thread
Clinical case
Once demonstrate the ability to the PDO suture to function as a system for the controlled release of Arg, this suture charged with Arg was apply in deep wrinkles of the upper third. For this we designed a protocol for its application.
Our protocol is a modification of the technique proposed by Kang et al.[8] [Figure 2], who only propose inserting PDO thread segments into deep wrinkles.
Instead, our protocol is to exploit the ability of the PDO suture as a delivery system to and not just “fill in a deep static wrinkle.” Our technique using “SMART PDO” consists of inserting the threads in the same direction of the deep wrinkle and in the perpendicular direction to the wrinkle.
Once inserted the PDO thread, connected into the luer lock of the cannula a syringe of 1 mL charged with 0.5 mL of the Arg carefully without removing the guide pin of the PDO thread [Figure 3]. Then, the PDO suture been soaked slowly with the Arg by retroinjection, and so, by capillarity the PDO suture begin to release the substance inside the tissue.
A reversal of deep wrinkles was observed with this protocol. According to the prolonged release curve (preclinical test), it is presumed that from 24 h after the thread is embedded with the Arg, the tissues begin to be affected by the biomimetic peptide generating relaxation.
We assumed that the PDO suture with Arg acts as how neuromodulator of the area, inasmuch as described in [Figure 4], at 24 h, the skin appears more hydrated and firm, which is a very satisfactory postoperative period for patients. As described in Figure 4, at 24 h, the skin appears more hydrated and firm, which is a very satisfactory postoperative period for patients. We suggested to wait for the biodegradation time of the PDO thread to repeat this technique if necessary, that is, to repeat at maximum to the 180 days.
DISCUSSION
Acetyl hexapeptide-8 (Arg) is a hexapeptide, that is, a chain of six synthetically linked amino acids that has been positioned as a novel biomimetic active for the effect of botulinum toxin.[9] Its antiwrinkle efficacy is the result of a double action: on the one hand, it causes muscle relaxation by a mechanism similar to that of botulinum toxin; on the other hand, it causes the relaxation of fibroblasts and a lifting effect.
This inhibitory peptide of presynaptic neuronal exocytosis is used in antiwrinkle formulations because the peptide in the active ingredient inhibits the contraction of the mimic muscles, especially the frontal, the cutaneous of the neck, those of the nasal area, and orbicularis of the eyelids.[10] It is made up of chains of six acetylated amino acids at the N-terminal residue; it is a weight molecule of 875 Da.[1112]
The N-terminal end of acetyl hexapeptide-8 associates with the synaptosomal receptor for the synaptic protein SNAP25 in the SNARE complex, essential for neuronal exocytosis. This complex is destabilized, preventing the fusion of the catecholamine and acetylcholine vesicles with the membrane, blocking Ca2 + release; it is dependent on neurotransmitters (acetylcholine) at the neuromuscular junction, which mimics the activity of botulinum neurotoxin (BoNT) type A but without destroying the SNARE complex.[1213]
The effectiveness of acetyl hexapeptide-8 in preventing the formation of skin rhytids or its skin-antiaging effect has been confirmed in several in vitro and in vivo tests in healthy volunteers.[12] Despite its excellent mimesis with BoNT A, transdermal penetration of acetyl hexapeptide-8 is limited due to its large molecular weight.[14]
Apart from modifying the formulation, various techniques have been proposed for permeation through the stratum corneum, for example, chemical enhancers, iontophoresis,[15] microneedles,[16171819] son phoresis,[20] thermal ablation,[21] radiofrequency ablation,[22] jet injectors,[23] and electroporation.[2425] While these methods of skin permeation or skin penetration enhancers work for entry, a major obstacle to transdermal delivery remains their high molecular weight.
With a high molecular weight of 889 Dalton, Arg remains primarily on the surface of the skin after topical application. A total of 0.01% of the peptide reaches the epidermis without traces of the peptide in the dermis. Poor skin permeation can lead to significant peptide waste, resulting in an ineffective final dosage form.[26]
It is because of this limited transdermal permeation that we propose to load the PDO suture as an intradermal delivery system for the acetyl hexapeptide, used successfully in the neuromodulation of the frontal and periocular areas under a new protocol of bioactive sutures for facial harmonization.
The PDO threads analyzed in this investigation emit the highest signal of Arg release at 24 h. Not studied for a longer period at to 24h, because it was considered that at this period the Arg it has been fully released form the PDO thread. The plateau shape is maintained for 1 h, indicating that in these 60 min, the release of almost all the content of the peptide from the PDO occurs, but is carried out in a sustained and controlled manner. This indicates that it is a favorable release profile for the supply of the active ingredient and its progressive and selective action on the SNARE complex, concentrating in 1 h what would probably be achieved topically by intradermotherapy after 4 weeks.[15]
However, evidence found in the literature indicates that in PDO threads as release matrices, there is a spike in a release after approximately 30–40 days, caused by mechanisms of controlled degradation in longer times,[27] which could result in a favorable becoming.
In addition, the absorbance region observed versus time graphs for the PDO + Arg system between 200 and 270 nm is similar to the range determined by other authors for Arg alone, which has its maximum peak at 215 nm when acetyl analyzed hexapeptide by UV-Vis[28] spectroscopy. This similarity between our results and those of these authors suggests that, indeed, what captured by the spectrophotometer in the aqueous medium corresponds to Arg being released into the medium and not traces of PDO suture.
This seems to be the result of the combination of a diffusion and a degradation effect, so that PDO threads made of nanofibers are resistant to fluid absorption once inside the body and, therefore, produce a much smaller diffusion. therefore, produce a much smaller diffusion of the solute outside the polymer and therefore more controlled.[27]
The hydrolytic degradation of PDO suture is partially affected by the degree of crystallinity of the compound but be accelerated by the action of the impregnated substance. In general, the swelling of the PDO suture and its subsequent degradation begins in the amorphous regions and spreads toward the crystalline regions,[729] as observed in the optical photomicrographs of the present study. However, the electronic microphotographs confirm that despite the swelling experienced by the PDO thread due to its capillarity, the topography of the PDO thread–Arg remains without physical degradation in the short periods evaluated.
Likewise, the peptide release graphs from the PDO suture reveal that the thread constitutes an ideal system for the sustained release of acetyl hexapeptide-8, this being a kinetic similar to that observed in the most versatile polymer devices currently used for other purposes pharmacists. However, clinical trials must be carried out to determine the efficacy of PDO thread as an acetyl hexapeptide-8 delivery system, which is expected to generate a lifting superior to its intradermal injection. Its clinical results should also be compared with its gold-standard counterpart, botulinum toxin type A.
So, being a biomimetic peptide of the toxin, it causes the relaxation of fibroblasts and a lifting effect, allowing some neurotransmission; as a result, muscle contraction is attenuated, which prevents the formation of wrinkles.[30] Into the bargain, our created system (PDO-Arg) would also serve a dual purpose: serve as a filler for deep grooves and release the biomimetic peptide for neuromodulation of the area, without completely paralyzing muscle contraction.
Although no signs of cytotoxicity have been observed in human dermal fibroblasts and human epidermal keratinocytes, when Arg is administered in concentrations between 10 μg/mL and 1 mg/mL, if there is an anticell proliferation effect when Arg is administrate at doses of 100 μM (90 μg/mL). Therefore, the use of acetyl hexapeptide-8 (Arg) in very high doses or for a very long period should be considered potentially dangerous.[31] Consequently, it suggested attending to this dosage when applying directly intradermally using PDO threads impregnated with acetyl hexapeptide.
This study presents the results of a single clinical case and although the preclinical results of the physicochemical analysis were also very conclusive, it is necessary to apply this technique in subsequent clinical studies on a split face, comparing the efficacy of this biomimetic peptide with its gold-standard reference, that is, botulinum toxin.
CONCLUSIONS
The instrumental analysis of the PDO suture under four types of analytical techniques, evidenced for the first time in the scientific literature, the capacity of the PDO suture as an intelligent system for the controlled release of acetyl hexapeptide-8. The fluid absorption test by electronic weighing revealed that the PDO thread has capillarity, as it doubles its weight every 24 h until 72 h of evaluation when soaked with acetyl hexapeptide-8. Controlled release tests show that PDO thread is a good osmotically controlled release system for acetyl hexapeptide-8, allowing a slow and sustained release of it from 5 min to 24 h. In a period of 60 min, the biomimetic peptide is released almost completely but in a sustained and controlled way (plateau shape), so this release profile is favorable for the subdermal supply of the action since its action on the complex SNARE concentrates in 1 h what would be achieved progressively topically through intradermotherapy after 4 weeks. Both the optical photomicrographs and the electronic photomicrographs confirm that despite the swelling experienced by the PDO thread due to its capillarity, the topography of the thread is embedded with acetyl hexapeptide remains without physical degradation in the evaluated lapses. Capillarity can be an ally in facial harmonization if PDO sutures are used as a controlled release system, turning PDO sutures into bioactive scaffolds, capable of releasing Arg while maintaining its resistance modulus without prematurely degrading. It suggested carrying out clinical trials to determine the anti-wrinkle efficacy of the PDO thread loaded with acetyl hexapeptide-8, to demonstrate that this system and novel proposal will generate a lifting superior to its intradermal injection. Its clinical results should also be compared with its gold-standard counterpart, botulinum toxin type A.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank the Laboratory of Electrochemistry at the Faculty of Science at the University of the Andes, Venezuela, for facilitating access to the laboratory facilities and for carrying out the in vitro degradation test and monitoring their implementation.
The authors would also like to thank “Dr Ernesto Palacios Pru,” Scanning Electron Microscopy Center at the Medicine Faculty of the University of the Andes, Venezuela, for the help in the processing of samples, guidance in the microscopical observation, and recording pictures.
REFERENCES
- Nanofibrous and nanoparticle materials as drug-delivery systems. In: Andronescu E, Grumezescu AM, eds. Nanostructures for Drug Delivery. Amsterdam: Elsevier Inc; 2017. p. :239-70.
- [Google Scholar]
- Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J Control Release. 2018;292:91-110.
- [Google Scholar]
- In Vitro degradation of polydioxanone lifting threads in hyaluronic acid. J Cutan Aesthet Surg. 2019;12:145-48.
- [Google Scholar]
- Electrospun polymeric core-sheath yarns as drug eluting surgical sutures. ACS Appl Mater Interfaces. 2016;8:6925-34.
- [Google Scholar]
- Transforming nanofibers into woven nanotextiles for vascular application. ACS Appl Mater Interfaces. 2018;10:19449-58.
- [Google Scholar]
- Wedge-shaped polydioxanone threads in a folded configuration (“Solid fillers”): A treatment option for deep static wrinkles on the upper face. J Cosmet Dermatol. 2019;18:65-70.
- [Google Scholar]
- Role of topical peptides in preventing or treating aged skin. Int J Cosmet Sci. 2009;31:327-45.
- [Google Scholar]
- Preparation and stability of cosmetic formulations with an anti-aging peptide. J Cosmet Sci. 2007;58:157-71.
- [Google Scholar]
- A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-10.
- [Google Scholar]
- Small peptides patterned after the N-terminus domain of SNAP25 inhibit SNARE complex assembly and regulated exocytosis. J Neurochem. 2004;88:124-35.
- [Google Scholar]
- In vitro skin penetration of acetyl hexapeptide-8 from a cosmetic formulation. Cutan Ocul Toxicol. 2015;34:46-52.
- [Google Scholar]
- Permeación cutánea iontoforética de péptidos: Una investigación sobre la influencia de las propiedades moleculares, las condiciones iontoforéticas y los parámetros de formulación. Drug Deliv Transl Res. 2014;4:222-32.
- [Google Scholar]
- Disolver parches de microagujas de polímero para una administración transdérmica rápida y eficiente de insulina a ratas diabéticas. Acta Biomater. 2013;9:8952-61.
- [Google Scholar]
- [Development of a novel transdermal delivery system of peptide and protein drugs using microneedle arrays] Yakugaku Zasshi. 2014;134:63-7.
- [Google Scholar]
- The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J Control Release. 2012;161:933-41.
- [Google Scholar]
- Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS One. 2014;9:e101956.
- [Google Scholar]
- Rectangular cymbal arrays for improved ultrasonic transdermal insulin delivery. J Acoust Soc Am. 2007;122:2022-30.
- [Google Scholar]
- Microporation applications for enhancing drug delivery. Expert Opin Drug Deliv. 2009;6:343-54.
- [Google Scholar]
- Transdermal delivery of human growth hormone through RF-microchannels. Pharm Res. 2005;22:550-5.
- [Google Scholar]
- Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov. 2006;5:543-8.
- [Google Scholar]
- Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24:1282-90.
- [Google Scholar]
- Enhanced skin permeation of anti-wrinkle peptides via molecular modification. Sci Rep. 2018;8:1596. Erratum in: Sci Rep. 2018 Apr 20;8(1):6500
- [Google Scholar]
- Long-term drug delivery using implantable electrospun woven polymeric Nanotextiles. Nanomedicine. 2019;15:274-284.
- [Google Scholar]
- Investigation of the binding properties of the cosmetic peptide Argireline and its derivatives towards copper (II) ions. J Solution Chem. 2018;47:80-91.
- [Google Scholar]
- Estudio de la degradación hidrolítica de la polidioxanona para la predicción del tiempo de vida útil Rev. Tec Ing Univ Zulia. 1998;21:170-8.
- [Google Scholar]
- The study of cellular cytotoxicity of argireline—An anti-aging peptide. Acta Biochim Pol. 2014;61:29-32.
- [Google Scholar]






