Translate this page into:
Multicentric observational study of the possible side effects of the COVID-19 vaccine in relation to absorbable thread insertion
Address for correspondence: Dr. Irina Poleva, CGH, v. Nomentana 173, 00161, Rome, Italy. E-mail: info@irinapoleva.it
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Coronavirus disease 2019 (COVID-19) vaccination programs in Europe began in December 2020, and as the number of vaccinated people increased, more information emerged about the possible side effects of vaccines. Recently, some reports appeared around the association of adverse reactions following soft-tissue filler injections and the COVID-19 vaccines. This fact raised the concerns of esthetic practitioners regarding the possibility of the association of the COVID-19 vaccine and adverse effects in other esthetic treatments. Many of us wonder if botox injection, biostimulation, or other procedure could trigger the same or other adverse reactions after the COVID-19 vaccination. Many colleagues postpone esthetic treatments, canceling the appointments.
Objective:
The objective of our investigation was to understand if any adverse reactions have been observed in the patients who underwent threads implantation before and after the vaccination.
Materials and Methods:
Four medical centers have collected the data of the patients treated with absorbable threads before the vaccination and after vaccination for COVID-19. The dossiers of 190 patients with a mean age of 50.4 years were evaluated. Three questionnaires were administered 1 week, 1 month, and 3 months after thread implantation.
Results:
There were no adverse reactions in the groups of patients under monitoring. Only one patient presented signs of inflammation and infection, but they are more probably attributable to the contamination during thread insertion.
Conclusions:
No patients treated with absorbable threads developed adverse events in relation to the COVID-19 vaccine. The only case of inflammation is attributable probably to the contamination of the thread during the procedure.
Keywords
Absorbable threads
COVID-19 vaccination
side effects
INTRODUCTION
Coronavirus disease 2019 (COVID-19) affects all organs and systems. Due to the rapid spread and severe consequences of the new coronavirus infection for the human body, there is a need for urgent vaccination.[1] Vaccines against COVID-19 have been developed in different countries by different manufacturers. According to the World Health Organization report, as of September 2, 2022, 171 candidate vaccines were in clinical trials and 198 vaccines were in preclinical trials. Eleven vaccines were included in the list of medicines recommended for emergency use.
There are four main types of vaccines currently available to protect from COVID-19:
-
-
Vector DNA vaccines: Sputnik V (Russia), Sputnic Lite (Russia), Oxford-AstraZeneca (Sweden), and Johnson & Johnson (USA);
-
-
mRNA vaccines: Pfizer (BNT162b2, USA) and Moderna (mRNA-1273, USA);
-
-
Whole virion vaccines (inactivated and attenuated vaccines): Sinopharm (China) and CoviVac (Russia);
-
-
Protein subunit vaccines: Novavax (USA) and EpiVacCorona (Russia).
Vaccination programs in Europe began in December 2020, and as the number of vaccinated people increased, more information emerged about the possible side effects of vaccines, including various skin reactions.[23]
The most common skin manifestations reported from clinical trials of COVID-19 vaccines are redness, swelling, and hardening of tissues, as well as itching and soreness at the injection site. Generally, these reactions appear within a week after vaccination and disappear without any medical care after a few days.[23]
Studies also report delayed local reactions at the injection site. Erythema, tissue induration, and pain may occur 8 or more days after vaccination.[4,5,6,7] These reactions were noted during phase III trials of a vaccine developed by Moderna.[37] Besides, similar manifestations have been reported outside clinical trials in patients who were vaccinated with mRNA vaccines.[67]
In the results of clinical studies, a number of other dermatological manifestations of varying severity were also reported. Less than 0.2% of participants vaccinated with Moderna experienced a variety of rashes, including allergic, atopic, contact, and exfoliative dermatitis, injection site urticaria, papular urticaria, and vesicular rashes. In addition, dermatitis acneiformis, allergic dermatitis, alopecia, petechial rash and eczema have been reported in less than 0.1% of patients vaccinated with Sputnik V.
In 43 participants in three observational studies, morbilliform rashes and maculopapular exanthema were observed. Of these 43 people, 21 (49%) received the BNT162b2 vaccine and 22 (51%) received the mRNA-1273 vaccine.[8]
There is also evidence of the occurrence of pernium-like lesions against the background of vaccination against COVID-19. Erythematous spots and purple papules usually appear on the hands and feet, and in some cases, these reactions become more pronounced with exposure to cold.[8]
Because medications against COVID-19 can cause skin reactions, it is necessary to find out how compatible vaccination against a new coronavirus infection is with different cosmetic procedures. To date, cases of side effects have been reported in patients who received hyaluronic acid fillers before vaccination.[9] Few studies suggest that after receiving a dose of the vaccine, inflammation and swelling may occur at the injection site of the filler.[10,11,12]
Objectives
Thread lifting is a modern technique for correcting the contours of the face, neck, and body without surgical intervention. The procedure is minimally invasive and does not require long-term recovery, which is why it is becoming increasingly popular among both women and men. In this regard, we considered it necessary to identify possible adverse reactions with a thread lift that may be caused by vaccination against COVID-19.
MATERIALS AND METHODS
Our multicentric observational study aimed to uncover if any uncommon adverse reaction is expected in COVID-19 vaccine-administered patients undergoing absorbable threads implantation.
Four medical centers participated in the study: one in Italy (Rome), two in Hungary (Budapest), and one in Russia (Moscow). In the study, absorbable threads made of polylactic acid and polycaprolactone (PLLA/PCL) were used. A questionnaire of subjective evaluation was designed to evaluate if the patients noticed one of the following general or local symptoms in the area treated with threads: fatigue, flu-like symptoms, pain, itch, rash, swelling, and fever. The questionnaire was filled by patients 1 week, 1 month, and 3 months after thread implantation. Data from 190 responder individuals were collected. These individuals were divided into two groups: group I—patients treated with the absorbable threads from 1 week to 3 months before vaccination (23 patients, 12%) and group II—patients treated with the absorbable threads few days or weeks (6 max) after vaccination (167 patients, 88%) [Figure 1]. The bigger number of patients in group II is explained by the fact that the vaccine became more available in time for the larger population, so more people became vaccinated during this observational study.
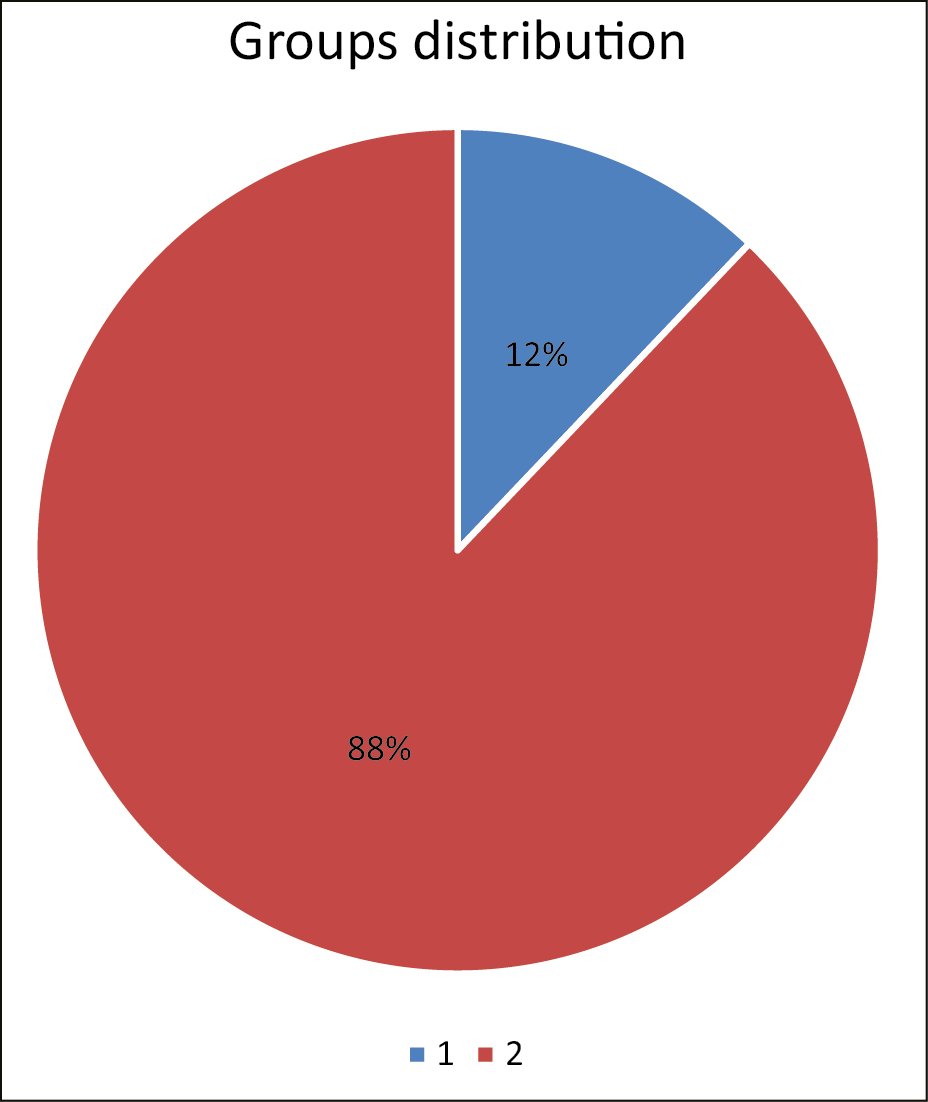
- Groups distribution
RESULTS
Demographic characteristics of the patients from both groups are demonstrated in Figure 2. There was no significant difference between the patients’ mean age in the two groups. All patients in group I were females, and only 3 of 167 patients in group II were males.
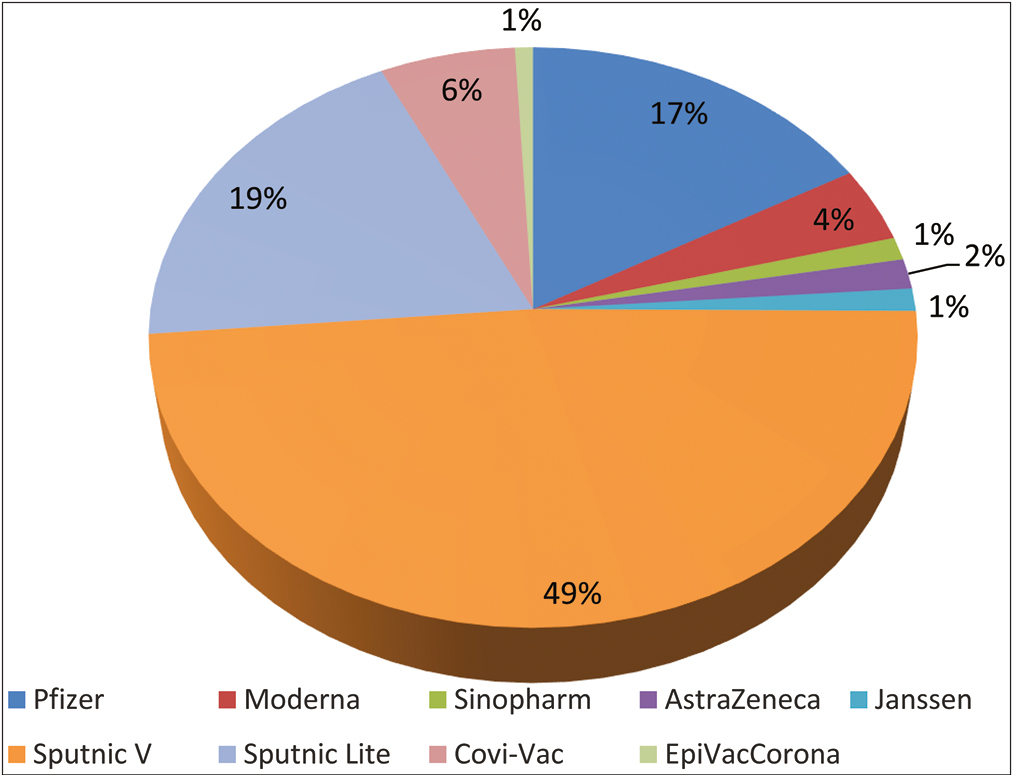
- Vaccine type distribution
Uptake of nine different COVID-19 vaccines including mRNA vaccines, vector vaccines, and protein subunit vaccines was detected among study subjects. Vaccine distribution is reflected in Table 1.
| Mean age | Age range | |
|---|---|---|
| Total | 50.4 | 25-70 |
| Goup I | 52.3 | 31-66 |
| Group II | 50.0 | 34-70 |
Readout of the side effects at three time points, 1 week, 1 month, and 3 months after thread lifting procedure, showed no important or prolonged adverse reaction at any time point in any of the two groups of patients under monitoring [Figure 3]. In group I, there were no side effects attributable to the thread insertion during all periods of follow-up (3 months). The number of light side effects observed (flu-like symptoms and prolonged swelling) were comparable to the side effects of vaccination, regardless of thread lifting [Figure 3]. In group II, some light side effects observed in the 1st week after vaccination could be due to the well-known side effects of the COVID-19 vaccine (flu-like symptoms, prolonged swelling, and fever). Noteworthy, only one patient presented signs of inflammation and infection, but these reactions were more probably attributable to the contamination during thread insertion: a hair was found in the implantation area. After the removal of the hair, the patient recovered quickly.
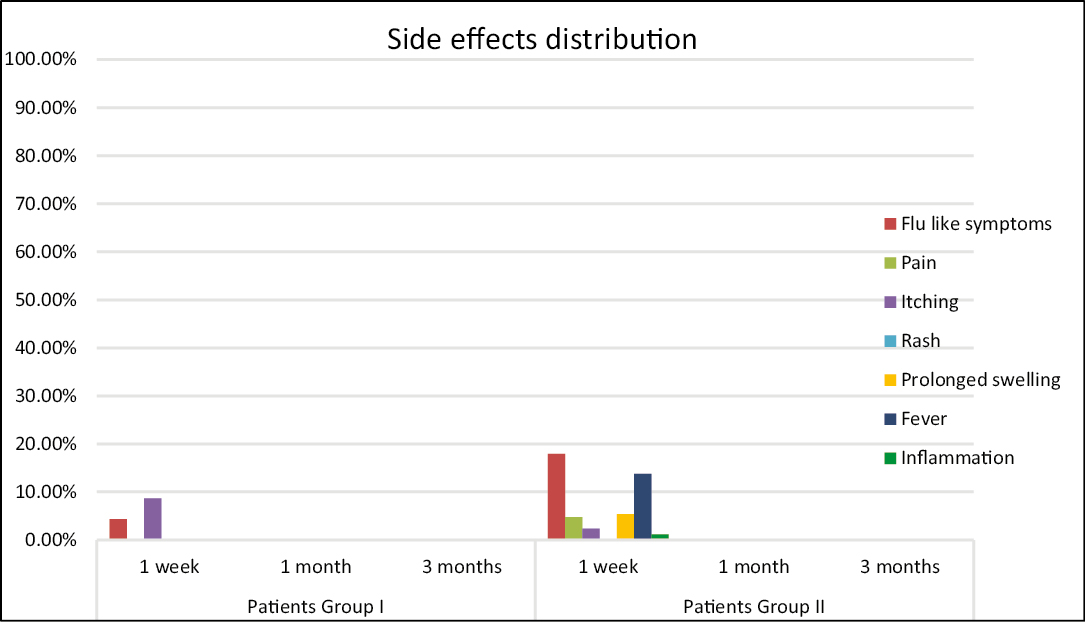
- Side effects distribution
DISCUSSION
Thread lifting is among the most popular minimally invasive esthetic procedures worldwide and is currently used for the rejuvenation of the face and body.[13,14,15] Among different types of absorbable lifting, PLLA/PCL threads have gained a high interest among esthetic practitioners due to their higher collagen-stimulating properties and long-term effects.[16,17,18] It is believed that the ideal lifting threads should provide biostimulating effects through neocollagenesis ensuring the improvement of skin roughness, radiance, elasticity, and turgor while minimizing adverse inflammatory reaction of the skin at the site of thread administration.[1920] Short recovery period together with long-lasting biostimulating effects are the main advantages of this treatment among other surgical or nonsurgical esthetic procedures.[19,20,21,22] Nevertheless, various adverse reactions associated with thread lifting are reported. These complications can be divided into two groups: early-onset and late-onset adverse effects. Early-onset complications occur up to several days posttreatment, among them are skin redness, uneven tone, irregular surface, burning, and itching sensation. Late-onset adverse effects that occur days to months postprocedure include infections, foreign body granuloma, dyspigmentation, scarring, erythema, edema, pain, and induration.[1920,23]
With the emergence of the coronavirus pandemic caused by the COVID-19 virus, numerous vaccines came available and the vaccinated population against COVID-19 is increasing. However, various adverse effects associated with different COVID-19 vaccines have been reported, and notably, cases of reactions from cosmetic procedures after COVID-19 vaccination have been observed in practice.[9,10,11,12] Such studies though are still limited, and there is no information regarding adverse reactions with a thread lift that may be caused by vaccination against COVID-19.
To our knowledge, this is the first study that evaluates the complications caused by COVID-19 vaccine in patients treated with PLLA/PCL absorbable threads. Subjective evaluation was performed in patients’ groups treated with lifting threads before or after COVID-19 vaccination to evaluate the following general or local symptoms: fatigue, flu-like symptoms, pain, itch, rash, swelling, and fever. Thread lifting procedure showed no important short or prolonged adverse reaction at any measured time points (1 week, 1 month, and 3 months after procedure) in any of the groups of patients under monitoring.
This study reports for the first time the safety of COVID-19 vaccines for patients undergoing thread lifting with PLLA/PCL threads. Although the incidence of COVID-19 infection is declining, there are still some categories of the population to whom vaccination is recommended (centre of disease control and prevention recommendations last updated on 23 May 2023). Moreover, this information may be useful for future vaccinations. It is a demonstration that the PLLA/PCL threads seem not to have immunogenic potential in relation to COVID-19 vaccination.
In spite of the fact that this information is critically important for esthetic practitioners and patients, the limitations of the study should be acknowledged and may become a focus for future research. First, the study considered only one type of the threads, namely PLLA/PCL threads. Currently, the most popular threads are polydioxanone threads. However, in the centers that contributed to the study, only PCL/PLLA threads are used, and these threads are recently conquering an increasingly large portion of the esthetic market. Second, the study lacks objective evaluation. Finally, the small sample size, especially in the first group of patients, diminishes the feasibility of drawing conclusive clinical outcomes. Overall, however, this study opens new avenues to further larger scale research to establish clinically relevant recommendations in esthetic medicine.
Financial support and sponsorship
Nil.
Conflicts of interest
IP is an Aptos trainer and received compensation for Aptos methods training. ES is a part of the company that distributes Aptos threads in Hungary. BH declares that she has no conflict of financial or nonfinancial interests. OZ is an Aptos trainer.
REFERENCES
- The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg. 2020;78:185-93.
- [Google Scholar]
- Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-16.
- [Google Scholar]
- COVID-19 vaccines and the skin: The landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39:653-73.
- [Google Scholar]
- Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. JAMA Dermatol. 2021;157:1000-2.
- [Google Scholar]
- Cutaneous reactions following booster dose administration of COVID-19 mRNA vaccine: A first look from the American Academy of Dermatology/International League of Dermatologic Societies registry. JAAD Int. 2022;8:49-51.
- [Google Scholar]
- Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273-7.
- [Google Scholar]
- Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- [Google Scholar]
- Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: A worldwide review. Microorganisms. 2022;10:624.
- [Google Scholar]
- Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination—A case report. J Cosmet Dermatol. 2021;20:2684-90.
- [Google Scholar]
- COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: A challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res. 2022;314:1-15.
- [Google Scholar]
- Late inflammatory reactions in patients with soft tissue fillers after SARS-CoV-2 infection and vaccination: A systematic review of the literature. J Cosmet Dermatol. 2022;21:1361-8.
- [Google Scholar]
- Hypersensitivity reaction to hyaluronic acid dermal filler after the Pfizer vaccination against SARS-CoV-2. Int J Infect Dis. 2021;113:233-5.
- [Google Scholar]
- Removal of facial soft tissue ptosis with special threads. Dermatol Surg. 2002;28:367-71.
- [Google Scholar]
- Facial thread lifting with suture suspension. Braz J Otorhinolaryngol. 2017;83:712-9.
- [Google Scholar]
- Outcomes in thread lift for face and neck: A study performed with Silhouette Soft and Promo Happy Lift double needle, innovative and classic techniques. J Cosmet Dermatol. 2019;18:84-93.
- [Google Scholar]
- Erratum to: Recent advances in face lift to achieve facial balance. J Maxillofac Oral Surg. 2017;16:134.
- [Google Scholar]
- Outcomes in thread lift for facial rejuvenation: A study performed with happy LiftTM revitalizing. Dermatol Ther. 2014;4:103-14.
- [Google Scholar]
- Biostimulatory effects of polydioxanone, poly-d, l lactic acid, and polycaprolactone fillers in mouse model. J Cosmet Dermatol. 2019;18:1002-8.
- [Google Scholar]
- Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. J Cosmet Laser Ther. 2015;17:99-101.
- [Google Scholar]
- Facial lift and patient satisfaction following treatment with absorbable suspension sutures: 12-month data from a prospective, masked, controlled clinical study. J Clin Aesthet Dermatol. 2019;12:18-26.
- [Google Scholar]
- Randomized, controlled, multicentered, double-blind investigation of injectable poly-L-lactic acid for improving skin quality. Dermatol Surg. 2019;45:718-24.
- [Google Scholar]
- Satisfaction of patients and surgeons with combined Aptos thread lifting treatment, fat grafting and laser treatment. Aesthetic Plast Surg 2022 doi.org/10.1007/s00266-022-03019-x
- [Google Scholar]






