Translate this page into:
The effect of combining humic and fulvic acids poultice on wound healing in male rats
Address for correspondence: Dr. Fatemeh Samiee-Rad, Clinical Research Development Unit, Kowsar Hospital, Qazvin University of Medical Sciences, Qazvin 3419759811, Iran. E-mail: fsamieerad@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Finding new compounds to accelerate wound healing is critical today. Humic substances or fulvic acid each have anti-inflammatory properties.
Aims and Objectives:
The purpose of this study is to determine the effects of poultice 0.5% containing humic and fulvic acids on wound healing in male rats.
Materials and Methods:
An animal model was arranged by making a full-thickness skin wound was created in each rat. Animals were randomly divided into control, sham, and treatment groups. To investigate the effect of humic and fulvic acids combining poultice, the wound area and histological analyses of the number of inflammatory cells, fibroblasts, and angiogenesis were evaluated for 21 days.
Results:
The animals in the treated group showed higher wound healing percentage, angiogenesis, and fibroblast distribution compared with the control (P < 0.001). Moreover, the topical administration of humic and fulvic acids 0.5% poultice decreased the mean number of inflammatory cells significantly than the other groups (P < 0.001).
Conclusion:
The topical administration of a poultice containing humic and fulvic acid accelerated wound healing by increasing angiogenesis and fibroblast and reducing inflammatory cell distribution in a rat model.
Keywords
Burns
fulvic acid
humic acid
inflammatory cells
wound healing
INTRODUCTION
The wound is a disruption of the epithelial layer leading to a disruption in the function and structure of normal tissue; homeostasis, inflammation, proliferation, and remodeling have formed the integrated and overlapping steps of wound healing.[1,2,3] These dynamic steps depend on the type of wound, local primary factors, and systemic diseases such as diabetes and hypertension.[4] This physiological process is influenced by various cells, cytokines, mediators, vessels, and extracellular matrix.[5] The secreted cytokines and mediators lead to thrombosis, angiogenesis, chemotaxis, lymphocyte migration and proliferation, prevention of dehydration, control of the inflammatory process, granulation tissue formation, and re-epithelization.[6] The correct levels of cytokines and inflammatory mediators can control infection and accelerate the wound-healing process.[7,8,9] Finally, regulation of cytokine release reduces the migration of neutrophils, eosinophils, and other inflammatory cells into the wound area, resulting in an increase in cellular proliferative processes in the wound bed.[910] The secretion and function of cytokines are altered by various factors.[10] Therefore, the identification of biological materials that can regulate the inflammatory process in wound healing is one of the interesting topics in wound healing research.[9,10,11,12,13,14,15,16] Humic substances (HS) are produced during soil decompositions.[17] It has been previously shown that HS enhance the wound healing process due to its antibacterial and anti-inflammatory properties.[18] They regulate and maintain abnormal and normal cell growth, respectively. Three compounds have been identified in their structure, including humic acid (HA), which is soluble in alkaline substances, humin which is insoluble in alkaline, water, and acids; and fulvic acid, which is soluble in alkaline, water, and acids.[19,20,21,22] HA is effective in improving wound healing and cancer treatment. The wound healing process needs additional O2. The requirement for O2 is induced by the phagocytosis process, which consumes a lot of O2. The cellular damage is mostly related to free oxygen, which is converted to OH. Therefore, lipid peroxidation is started and followed by necrosis. HA has been accepted as an electron acceptor–donor system. So HA is capable of producing and binding to oxygen reactive. This regulatory system of HS is important for wound healing.[23] On the other hand, fulvic acid has been shown to strengthen the body’s defenses against disease. Animal studies show that fulvic acid can increase immune defenses and antioxidant activity, and decrease inflammation.[2425] Research has shown that fulvic acid increases the expression of interleukin (IL)-10, one of the most important anti-inflammatory cytokines. Positive regulation of IL-10 accelerates wound healing.[26] Despite the effectiveness of HA for wound healing, the adverse effect of HA is not fully understood.[27] The increase in the incidence of wounds and the variety of treatment approaches has led researchers to search for a simple, inexpensive treatment with the fewest side effects. Despite previous experiences with the use of soils containing humic or fulvic acid,[20,21,22,23,23,24,25,26,27,28,29,30] the combined effect of humic and fulvic acid has not been studied simultaneously. The aim of this study was to investigate the efficacy of coadministration of HA and fulvic acid in wound healing in a rat animal model.
MATERIALS AND METHODS
Preparation of poultice
A 5% HA + fulvic acid poultice containing 8 g of carboxymethylcellulose (CMC) (Merck, Germany), 0.004 g of HA, and 0.004 g of fulvic acid (Sigma-Aldrich company, America) were mixed with 16 mL of normal saline within a glass beaker. The prepared poultice was stored at room temperature until treatment time.
Subjects
This experimental study was performed on 30 male Sprague Dawley rats (230–250 g) according to guidelines for working with animals. The ethical committee of Qazvin University of Medical Sciences approved this study (IR.QUMS.REC.1396.423). The rats were kept in clean solitary cages under standard conditions (environment temperature 25 ± 1°C, humidity 55%, proper ventilation with 12-h light–dark cycles) and had free access to water and food.[14]
Full-thickness wound and sampling
Animals were anesthetized with intraperitoneal Ketamine/xylazine (50/5 mg/kg; Merck, Germany) injection. The back skin was shaved with an electric clipper and disinfected with 70% ethanol. After it, the full-thickness skin lesions developed on the dorsal of the Th3–Th5 level by using a disposable punch with 2 cm in diameter.[14] Surgical procedures were performed under sterile conditions. Animals were randomly divided into three equal groups (n = 10), including control, sham, and treatment. Daily skin wounds were gently dipped in sterile gauze impregnated with normal saline for 21 days. CMC dressing was used daily in the sham group and in the treatment group; in addition to CMC dressing, 5% HA + fulvic acid poultices were used continuously for 21 days.
In all groups, animals were kept in a standard crouching position. The wound area was measured using drawing them on a transparent paper by a trained observer on 0, 7, 14, and 21 postoperative days and was calculated using AutoCAD software.[15] A photograph was also taken from the wound area of each sample. The percentage of wound healing was calculated with the following formula: wound healing percentage = (wound size at day zero - wound size at desired day)/wound size at day zero × 100.
Histological examination
Rats were anesthetized as described above, and their wound biopsies were taken from the edges of the wound on 0, 7, 14, and 21 post-treatment days. The samples were fixed in 10% buffered formalin, dehydrated in increasing 70%, 90%, and 100% ethanol (each for 30 min), immersed twice in xylene for 30 min, and impregnated in liquid paraffin for 30 min. An amount of 5 µm histological sections were prepared and stained using hematoxylin and eosin as well as Toluidine blue from each sample and were blindly investigated by an expert pathologist. Iwf-Iox-Holland ocular fragment was used for cell counting under a microscope.[31]
Histological scoring
The stained histological slides were first observed descriptively. The number of neutrophils, eosinophils, mast cells, and fibroblasts was counted using an objective lens (*400). To evaluate the angiogenesis, the wound areas were first observed at low magnification to find the area with a high density of new vessels. Three separate fields were then selected in these areas and evaluated at high magnification filed with an objective lens (*400). The coding for angiogenesis in the histological sections was according to Table 1. The wound-healing process was scored based on the number of inflammatory cells, fibroblasts, and the number of new vessels.[3233]
| Coding Index | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Vascular sections | Not seeing | 4–8 | 12–15 | 15–20 | >20 |
Statistical analysis
Data were analyzed using SPSS software version 20. All results were expressed as mean ± standard deviation (SD). One-sample Kolmogorov–Smirnov test was used to determine the normality of data distribution. The repeated measure and one-way ANOVA followed by a post hoc Tukey test were used to analyze the data. P < 0.05 was considered significant.
RESULTS
Wound healing percentage
The wound healing percentage in all three groups is shown in Figure 1. The wound healing rate was zero in all three groups on the 0 days of the study. The wound area was significantly decreased in the HA + fulvic acid treated group compared to the sham and control groups at all three interval times of post-treatment (P < 0.001) [Figure 2A–S]. On the other hand, the wound area was completely closed in the HA + fulvic acid treatment group on day 21 postsurgery [Figure 3A–I].
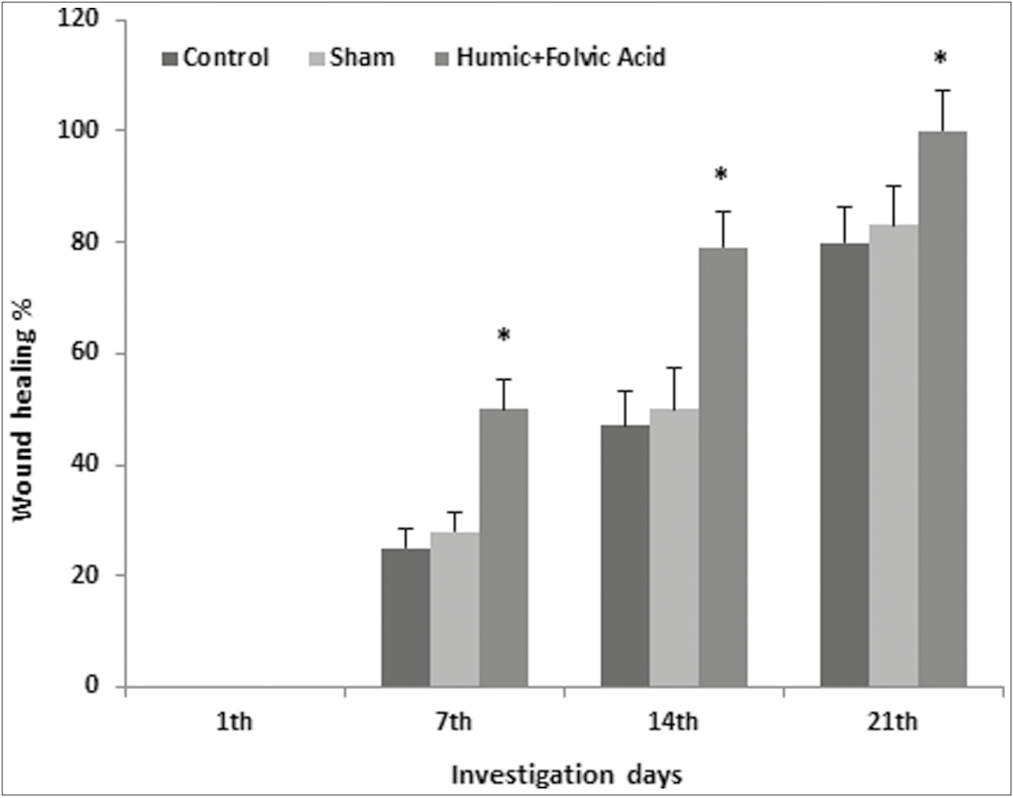
- The mean percentage of wound healing at different interval times of post-treatment
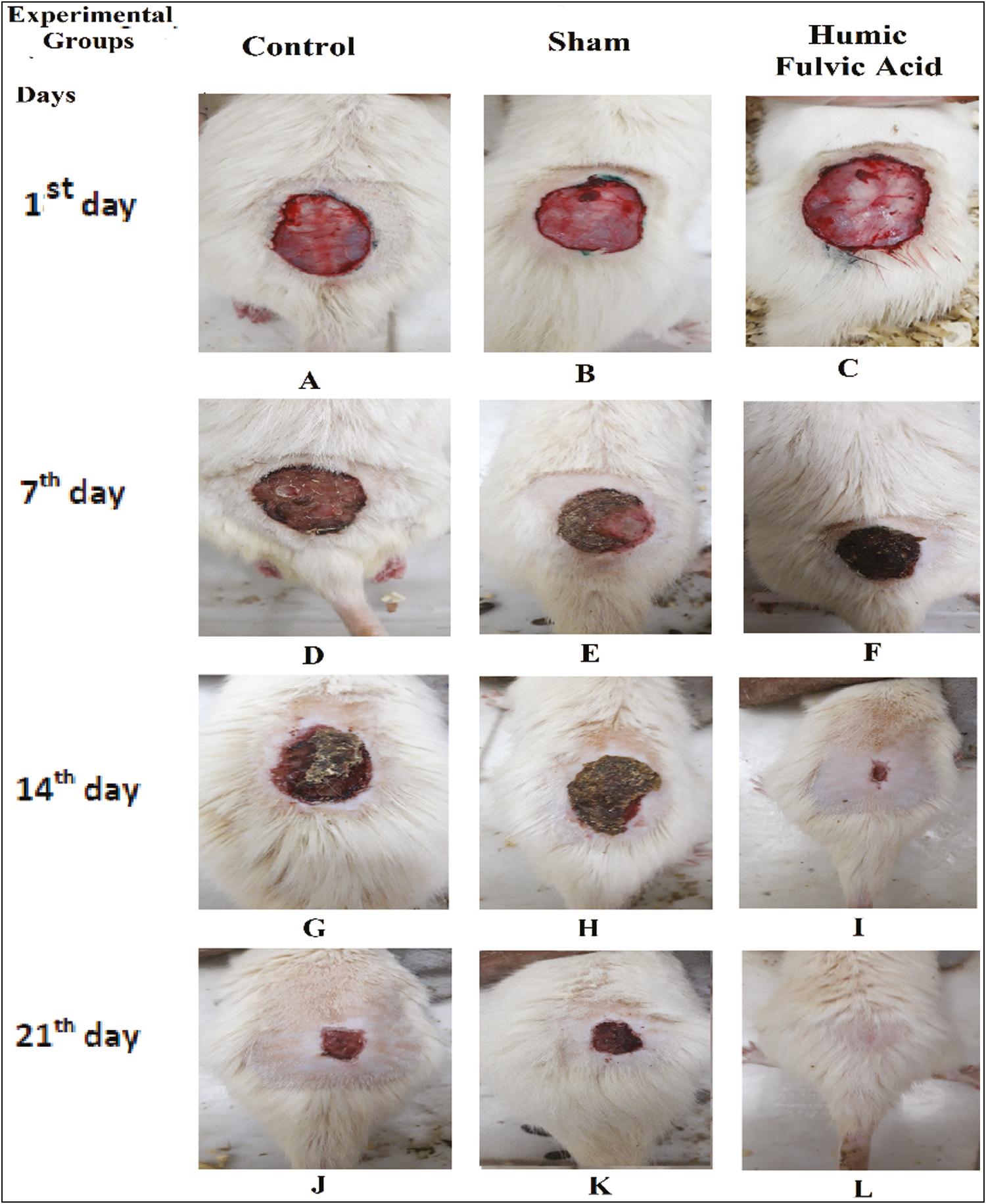
- Photographic representation of wound healing rate. Control (A), sham (B), and humic acid + fulvic acid (C) groups on the 0th day, Control (D), sham (E), and fulvic acid + humic acid (F) groups on the 7th day, Control (G), sham (H), and fulvic acid + humic acid group (I), at 14th days, Control (J), sham (K), and fulvic acid humic acid (L), groups at 21st days postwounding
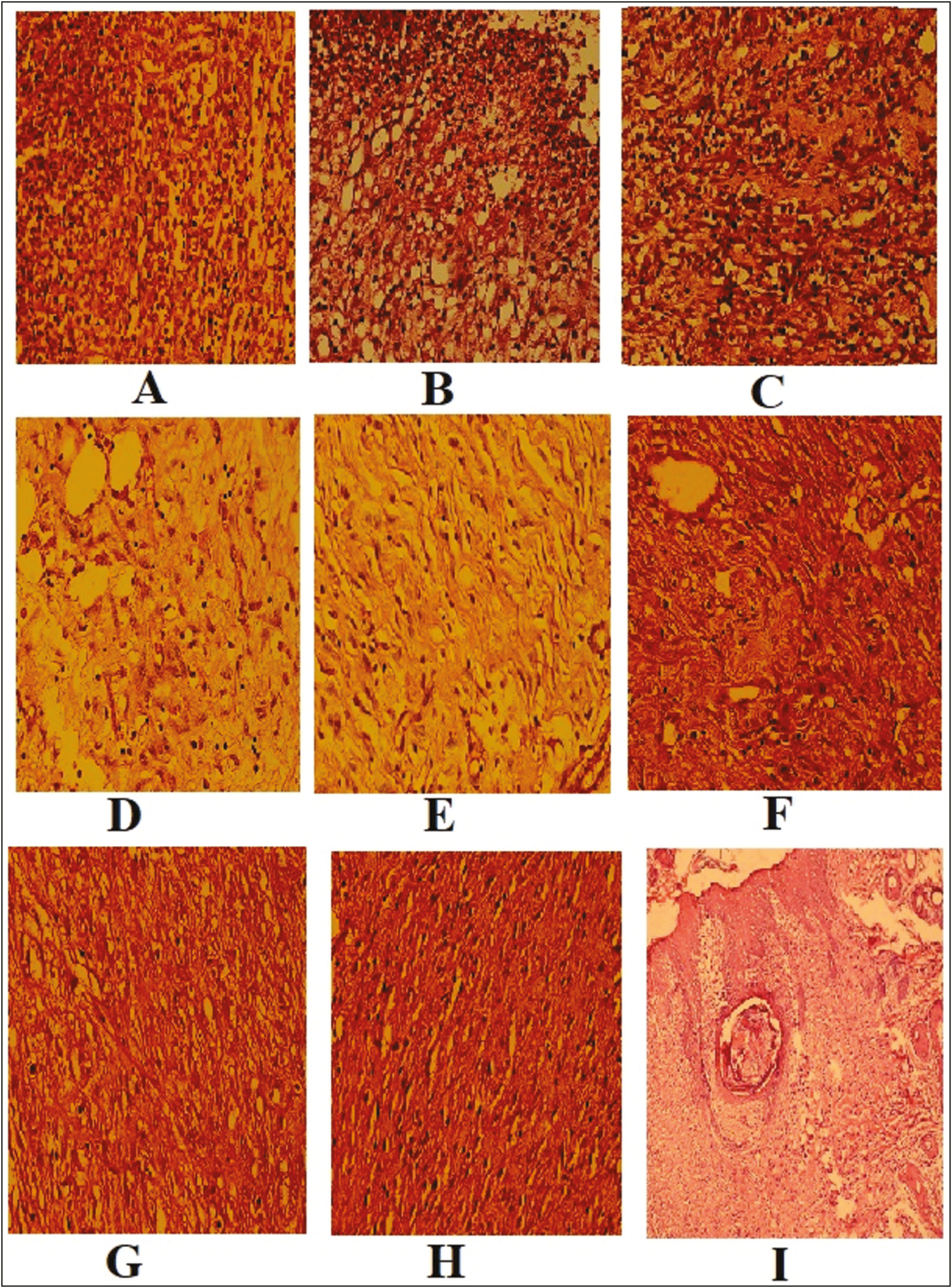
- Histomorphologic findings. The fibrinoleukocytes exudate, angiogenesis, and fibroblastic proliferation in a bed of ulcers in control (A), sham (B), fulvic +acid-humic acid (C) on the 7th day, control (D), sham (E), fulvic acid + humic acid (F), on the 14th day, control (G), sham (H), and the fulvic acid +humic acid (I) groups at 21 days postwounding. (Hematoxylin and eosin staining, ×400)
Neutrophils
The mean number of neutrophil measurements showed no significant difference between all three groups on 0 days of the study (P > 0.05). The number of neutrophils continuously decreased for all groups at the end of the study, respectively. A significant decrease of neutrophils was found in the HA + fulvic acid group compared to the sham and the control groups on days 7, 14, and 21 post-treatment (P < 0.001) [Table 2] [Figure 3A–C].
| Index | Group day | Control | Sham | Humic + fulvi acids |
|---|---|---|---|---|
| Neutrophils | 0 | 148.13 ± 8.40 | 148.88 ± 8.45 | 148.75 ± 9.67* |
| 7 | 89.75 ± 6.08 | 87.75 ± 4.20 | 49.63 ± 4.30* | |
| 14 | 70.38 ± 3.33 | 60.63 ± 4.62 | 29.25 ± 3.77* | |
| 21 | 49.75 ± 3.49 | 42.38 ± 3.06 | 9.13 ± 1.12* | |
| Eosinophils | 0 | 58.88 ± 3.52 | 59.63 ± 3.69 | 59.13 ± 4.22* |
| 7 | 50.13 ± 4.12 | 43.13 ± 1.88 | 23.00 ± 2.33* | |
| 14 | 37.50 ± 2.00 | 32.13 ± 2.35 | 12.88 ± 2.69* | |
| 21 | 28.75 ± 2.31 | 23.75 ± 2.81 | 5.13 ± 1.55* | |
| Mast cells | 0 | 45.00 ± 4.44 | 45.25 ± 3.37 | 45.00 ± 3.07* |
| 7 | 35.00 ± 3.78 | 30.00 ± 3.50 | 20.13 ± 3.60* | |
| 14 | 27.00 ± 4.30 | 23.00 ± 2.82 | 10.00 ± 2.50* | |
| 21 | 20.25 ± 3.53 | 15.00 ± 2.87 | 5.00 ± 1.85* | |
| Fibroblasts | 0 | (0.0) 0.0 | (0.0) 0.0 | (0.0) 0.0 |
| 7 | 14.5 ± 3.20 | 23.25 ± 3.69 | 60.13 ± 3.27* | |
| 14 | 30.63 ± 3.42 | 38.00 ± 3.11 | 90.00 ± 5.42* | |
| 21 | 74.88 ± 4.01 | 81.00 ± 4.07 | 129.75 ± 4.49* | |
| Formed new blood vessels (angiogenesis) | 0 | (0.0) 0.0 | (0.0) 0.0 | (0.0) 0.0 |
| 7 | 7.00 ± 2.56 | 12.00 ± 3.68 | 35.13 ± 3.68* | |
| 14 | 14.00 ± 3.42 | 19.50 ± 4.24 | 60.13 ± 4.70* | |
| 21 | 6.63 ± 2.20 | 10.00 ± 3.29 | 25.00 ± 4.10* |
*P < 0.01 compare with control and sham groups
Eosinophils
The mean number of eosinophils on day 0 of the study was not significantly different in all three groups (P > 0.05). However, a significant decrease in the mean number of eosinophils in the treatment group was observed on days 7, 14, and 21 after wounding compared to sham and control groups (P < 0.001) [Table 2] [Figure 3A–C].
Mast cells
There was no significant difference between all groups in terms of the mean number of mast cells on day 0 of the study (P > 0.05). Yet the mean number of mast cells was significantly lower in the HA + fulvic acid group compared to the sham and control groups on 7, 14, and 21 post-treatment days (P < 0.001) [Table 2] [Figure 3A–C]. There was no significant difference in the mean number of neutrophils, eosinophils, and mast cells on days 7, 14, and 21 post-treatment between the sham and control groups (P > 0.05) [Table 2] [Figure 3A–C].
Fibroblasts
No fibroblast was detected in any three groups on day 0 of the study. A significant difference in fibroblasts was detected between the treatment group with the sham and control groups on days 7, 14, and 21 post-treatment (P < 0.001). However, there was no significant difference between the sham and control on all days (P > 0.05) [Table 2] [Figure 3D–I].
Number of vessels
The mean numbers of vessels were significantly more in the HA + fulvic acid-treated group in comparison with control, and sham groups, on all three interval times of 7, 14, and 21 days post-treatment (P < 0.001) [Table 2] [Figure 3D–I].
DISCUSSION
In the present study, a dressing containing HA and fulvic acid was used to heal wounds. The results showed that treatment with a 0.5% poultice containing HA and fulvic acid significantly accelerated wound healing on all days 7, 14, and 21 after wounding. Also, the wound bed in the treated group with humic and fulvic acids was completely closed on the 21st-day post-wounding. The HA and fulvic acid combination also reduces inflammatory cells, including neutrophils, eosinophils, and mast cells in the wound area. In addition, the mean number of fibroblasts, as well as newly formed vessels (angiogenesis), was significantly higher in the treatment group than in the control and sham groups at all intervals after wounding, which is consistent with the results of other studies. It has been reported that HA activates the TGF-β/Smads Signaling, which is one of the mechanisms of the wound healing process. HA accelerates wound healing resulting from the promotion of angiogenesis and re-epithelization, fibroblast proliferation, and increase of granules containing collagen.[28] HS can prevent the migration and adhesion of inflammatory cells in wound areas. Also, substances containing fulvic acid regulate IL-10.[23] IL-10 is one of the major anti-inflammatory cytokines. Our findings are consistent with those reported by Ji and Zhao.[2628] Because following the use of a combined poultice, there was a reduction in inflammatory cells. On the other hand, reports indicate that topical administration of fulvic acid can activate the expression of the genes-related chemokine (C-C motif) ligand 7 (CCL-7), chemokine (C-C motif) ligand 12 (CCL-12), IL-6, and metalloproteinase (MMPs). MMPs have been shown to take part in the deposition of collagen, which is essential for wound healing.[34] CC chemokines such as CCL7 and CCL12 are found in the inflammatory phase of wound healing and directly promote angiogenesis.[34] Fulvic acid also reduces the inflammatory reaction by inhibition of TNF-alpha.[35] Similar to the Kinoshita findings, our result confirmed that topical drugs containing fulvic acid accelerate wound healing by enhancing fibroblast infiltration.[29] Animal model studies have confirmed that controlling inflammatory reactions can accelerate wound healing,[2836] and, as mentioned, sodium humate reduces inflammatory reactions caused by inflammatory agents.[28] Furthermore, the anti-inflammatory and antibacterial effect of fulvic acid has been previously described, which due to their anti-inflammatory properties, accelerates wound healing.[3738] In this regard, Zhao showed that the main mechanism of fulvic acid wound healing is happening in the regeneration stage.[26] This is consistent with our results in the regeneration phase, that’s mean is after the 21st-day application of the combined poultice, complete wound healing occurred. Another reason for the effectiveness of the poultice is the antioxidant activity of HS, which is important for wound healing.[4041] Potassium humate not only prevents the infiltration of phagocytes and inflammatory cells but also reduces the activity of superoxide dismutase.[39] The level of reactive oxygen species (ROS) plays a critical role in the normal and abnormal wound healing response. Therefore, an exact balance between proper levels of ROS is essential. It reported that antioxidant reagents regulate this balance and are used for new therapies. Antioxidant substances that maintain nontoxic ROS levels in the wound tissues could improve healing.[42] HS acts as an electron acceptor–donor and is capable of binding with oxygen reactive and reducing oxidative components.[23] Natural dressings have gained significant interest in recent years due to their biocompatibility. It was shown they are beneficial for tissue repair by accelerating the healing process, preserving a moist environment for wound healing, facilitating cell adhesion, migration, growth, and differentiation, and preventing secondary infections.[43] It appears that the ability of the humic and fulvic acid combination to accelerate wound healing in the treatment group requires further study to confirm or refute these hypotheses. The results showed that a poultice containing 0.5% humic and fulvic acids significantly reduced the wound area and the number of inflammatory cells and significantly increased newly formed vessels and fibroblasts compared to the control group on the days studied. It, therefore, appears that at least part of the effect of this combination dressing is due to a reduction in inflammation and an increase in angiogenesis and fibroblast proliferation. Of course, there may be other mechanisms involved that need to be evaluated. Further studies are needed to determine the exact molecular mechanisms involved in the multilayer wound healing process and to assess the potential use of this dressing for medical purposes.
The strength and weakness of the study
Previous studies have evaluated the effect of humic and fulvic acid alone on wound healing, however; in the present study, the 0.5% poultice containing both humic and fulvic acids was applied. The levels of effective cytokine in the wound-healing process have not been measured, which is the weakness of this study.
Limitations
The main limitation of the present study is poor information about the immunogenicity of poultices containing humic and fulvic acid in humans. Also, mention that no bacteriological information was collected (as fulvic acid is known to be effective against MRSA and Pseudomonas).
Financial support and sponsorship
Funding was provided by the Research Vice-Chancellor of Qazvin University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Funding was provided by the Research Vice-Chancellor of Qazvin University of Medical Sciences.
Acknowledgments
We are grateful to the Clinical Research Center of Kausar Hospital for conducting the research.
REFERENCES
- Effect of acacia honey-impregnated placenta membrane on pain and burn wound repair. Comp Clin Path. 2018;27:1457-63.
- [Google Scholar]
- Tissue concentrations of zinc, iron, copper, and magnesium during the phases of full thickness wound healing in a rodent model. Biol Trace Elem Res. 2019;191:167-76.
- [Google Scholar]
- A morphoelastic model for dermal wound closure. Biomech Model Mechanobiol. 2016;15:663-81.
- [Google Scholar]
- Low-level laser and bovine amniotic fluid-derived cream accelerating skin neck wound healing and reducing inflammation and wound scar in rat animal model. J Cutan Aesthet Surg. 2022;15:267-74.
- [Google Scholar]
- Comparison of topical sucralfate and silver sulfadiazine cream in second degree burns in rats. Adv Clin Exp Med. 2013;22:481-7.
- [Google Scholar]
- Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators Inflamm. 2017;2017:5217967.
- [Google Scholar]
- Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv Wound Care. 2018;7:209-31.
- [Google Scholar]
- Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol Life Sci. 2016;73:3861-85.
- [Google Scholar]
- Principles of wound healing. 2011. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]. Adelaide (AU): University of Adelaide Press; :23. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534261/
- [Google Scholar]
- Topical α-gal nanoparticles accelerate diabetic wound healing. Exp Dermatol. 2020;29:404-13.
- [Google Scholar]
- Accelerating dermal wound healing and mitigating excessive scar formation using LBL modified nanofibrous mats. Mater Design. 2020;185:108265.
- [Google Scholar]
- Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front Cell Dev Biol. 2020;8:638.
- [Google Scholar]
- Evaluation of healing effects of poultice containing 0.5% fulvic acid on male white-male rats with skin ulcer. J Cutan Aesthet Surg. 2022;15:40-7.
- [Google Scholar]
- The effect of honey-impregnated human placenta membrane on burn wound healing in rat. Comp Clin Path. 2015;24:263-8.
- [Google Scholar]
- Royal jelly accelerates healing of acetate induced gastric ulcers in male rats. Gastroenterol Hepatol Bed Bench. 2020;13:14-22.
- [Google Scholar]
- Comparison of the composition of humic and fulvic acids prepared by the IHSS method and NAGOYA method. Soil Sci Plant Nutr. 1994;40:601-8.
- [Google Scholar]
- The antiinflammatory properties of humic substances: A mini review. Phytother Res. 2015;29:791-5.
- [Google Scholar]
- Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev Environ Sci Bio. 2019;18:597-616.
- [Google Scholar]
- Production of border cells and colonization of maize root tips by Herbaspirillum seropedicae are modulated by humic acid. Plant Soil. 2017;417:403-13.
- [Google Scholar]
- Peat as a raw material for plant nutrients and humic substances. Sustainability. 2021; 13:6354.
- [Google Scholar]
- Potassium humate inhibits complement activation and the production of inflammatory cytokines in vitro. Inflammation. 2009;32:270-6.
- [Google Scholar]
- Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J Diabetes Res. 2018;2018:5391014.
- [Google Scholar]
- Carbohydrate-derived fulvic acid is a highly promising topical agent to enhance healing of wounds infected with drug-resistant pathogens. J Trauma Acute Care Surg. 2015;79:S121-9.
- [Google Scholar]
- Sodium humate accelerates cutaneous wound healing by activating TGF-β/Smads signaling pathway in rats. Acta Pharm Sin B. 2016;6:132-40.
- [Google Scholar]
- Effect of fulvic acid on ultraviolet induced skin aging: The effect of fulvic acid on fibroblasts and matrix metalloproteinase. Nishinihon J Dermatol. 2012;74:427-31.
- [Google Scholar]
- Humic acid enhances wound healing in the rat palate. Evid Based Complement Alternat Med. 2018;2018:1783513.
- [Google Scholar]
- Effects of the oral administration of silver nanoparticles on wound healing in male rats. Wound Pract Res. 2020;28:8.
- [Google Scholar]
- Restorative effect of Iranian probiotic bacteria lactobacillus casei on healing gastric stomach ulcers caused by acetic acid in male Wistar Rats. J Anim Biol. 2011;4:35-45.
- [Google Scholar]
- Topical estrogen accelerates wound healing in diabetic rats. Iran J Endocrinol Metab. 2011;12:544-51.
- [Google Scholar]
- Bimodal effect of humic acids on the LPS-induced TNF-α release from differentiated U937 cells. Phytomedicine. 2009;16:470-6.
- [Google Scholar]
- Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int J Biol Macromol. 2020;164:963-75.
- [Google Scholar]
- 2019. An investigation into the topical and systemic safety and efficacy of a new carbohydrate derived fulvic acid (CHD-FA) product. University of Pretoria; Available from: http://hdl.handle.net/2263/29191
- Carbohydrate-derived Fulvic acid (CHD-FA) inhibits Carrageenan-induced inflammation and enhances wound healing: Efficacy and toxicity study in rats. Drug Dev Res. 2012;73:18-23.
- [Google Scholar]
- An in vitro investigation of the anti-inflammatory properties of potassium humate. Inflammation. 2004;28:169-74.
- [Google Scholar]
- Humic acid suppresses the LPS-induced expression of cell-surface adhesion proteins through the inhibition of NF-κB activation. Toxicol Appl Pharmacol. 2000;166:59-67.
- [Google Scholar]
- Antioxidant dressing therapy versus standard wound care in chronic wounds (the REOX study): Study protocol for a randomized controlled trial. Trials. 2020;21:505.
- [Google Scholar]
- The role of allogenic keratin-derived dressing in wound healing in a mouse model. Wound Repair Regen. 2017;25:62-74.
- [Google Scholar]






