Translate this page into:
Study to compare the efficacy of 50% glycolic acid with 65% trichloroacetic acid in the treatment of atrophic acne scar by CROSS technique
*Corresponding author: Olympia Rudra, Department of Dermatology, Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal, India. olympia.rudra@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Roy G, Gayen T, Sen S, Rudra O. Study to compare the efficacy of 50% glycolic acid with 65% trichloroacetic acid in the treatment of atrophic acne scar by CROSS technique. J Cutan Aesthet Surg. 2024;17:198-204. doi: 10.25259/jcas_18_21
Abstract
Objectives:
Acne scars often become challenging to treat with satisfactory results. The chemical reconstruction of skin scars (CROSS) technique has been used with high concentrations of trichloroacetic acid (TCA) which often produces unacceptable side effects. There is a dearth of data, with 50% glycolic acid (GA) for the same indication in the management of acne scars in the Indian population. This study aimed to assess the clinical response of acne scars after the application of 50% GA with that after the use of 65% TCA in a similar manner.
Material and Methods:
An institution-based prospective comparative study was performed with patients aged 16–45 years of either sex with acne scars and not been treated within the past 1 year. Subjects were assigned to receive one of the formulations (50% GA [Group A] and 65% TCA [Group B]). Grading of scars was done on day 1 and day 35 based on patients’ assessment on a four-point visual scale and physicians’ assessment by the Goodman–Baron qualitative global acne scar grading scale. The procedure was repeated every fortnightly for three such. Data were analyzed by Statistica version 6 (Tulsa, Oklahoma: StatSoft Inc. 2001).
Results:
The visual improvement scale of patients showed 41.38% (n = 12) fair improvement in group A, whereas 58.06% (n = 18) showed good improvement in group B. Grading by the Goodman–Baron scale showed 9.68% (n = 3) showed four grade changes in group B.
Conclusion:
Improvement is best observed with 65% TCA. Adverse effects were noted more with 65% TCA, especially acneiform eruption which was lacking in the previous studies. GA can be a safer alternative to TCA with acceptable results. Our study opens the scientific window for future research on different concentrations of GA as a CROSS agent.
Keywords
Acne scar
Chemical reconstruction of skin scars
Glycolic acid
Trichloroacetic acid
INTRODUCTION
Acne is a common chronic inflammatory disorder involving the pilosebaceous unit and presents most commonly as comedones, papules, pustules, nodules, and cysts.1 Scarring is a well-known complication of acne which is disturbing and may even precipitate psychological disturbances including depression.2,3 The factors that are important in the development of acne scars are the severity of acne and the delay in starting treatment. The degree of severity of scarring depends on the depth and extent of inflammation.4 There are different types of acne scars such as ice pick, rolling, or box scar.5 In the chemical reconstruction of skin scars (CROSSs) technique, a higher concentration of the chemical is applied which penetrates deeper tissue. Thus, it is useful for treating deep scars such as ice-pick scars. Goodman and Baron proposed a qualitative grading system that differentiates four grades according to scar severity.6 In this grading system, Grades I, II, III, and IV represent macular, mild, moderate, and severe atrophic and hypertrophic lesions, respectively [Table 1].7 The CROSS technique involves applying small amounts of the desired chemical at high concentrations at the surface of the atrophic scar.8 This leads to upper dermal collagen necrosis, followed by dermal collagen remodeling and neocollagenesis. The desired chemicals should be safe and not absorbed into the systemic circulation. Hence, high concentrations can be used safely.9 Acne scars often become challenging for dermatologists to treat with satisfactory results.
| Grade | Level of disease | Characteristics |
|---|---|---|
| 1 | Macular | Erythematous, hyper-or hypopigmented flat marks were visible to the patient or observer at any distance. |
| 2 | Mild | Mild atrophy or hypertrophy that may not be obvious at social distances of 50 cm or greater and may be covered adequately by makeup or the normal shadow of shaved beard hair in men or normal body hair if extrafacial. |
| 3 | Moderate | Moderate atrophic or hypertrophic scarring that is obvious at social distances of 50 cm or greater and is not covered easily by makeup or the normal shadow of shaved beard hair in men or body hair if extrafacial, but is still able to be flattened by manual stretching of the skin (if atrophic). |
| 4 | Severe | Severe atrophic or hypertrophic scarring that is obvious at social distances>50 cm and is not covered easily by makeup or the normal shadow of shaved beard hair in men or body hair if extrafacial and is not able to be flattened by manual stretching of the skin. |
After an extensive search on PubMed and Medline databases, we found few studies with trichloroacetic acid (TCA) as a CROSS agent, but no literature exists on the efficacy of 50% glycolic acid (GA) as a CROSS agent in the management of acne scar. There is a dearth of data with 50% GA (v/v in aqueous solution) or 65% TCA (v/v in aqueous solution) as a CROSS agent in the management of acne scar which prompted us to undertake the present study.
Our main objectives were to assess clinical response based on patients’ assessment on a four-point visual scale where 0=unsatisfactory result, 1=fairly satisfactory result, 2=good satisfactory result, and 3=excellent result; and physician’s clinical assessment by Goodman and Baron qualitative global acne scar grading scale. Apart from this, secondary outcomes such as post-inflammatory hyperpigmentation (PIH), adverse drug reaction, prolonged local irritation or erythema, and sub-optimal response were also measured.
MATERIAL AND METHODS
An institution-based prospective comparative study was performed with patients aged 16–45 years of either sex having Fitzpatrick skin type IV and V, with clinically evident atrophic acne scars and no other local dermatosis. Those not being treated within the past 1 year were included in the study. Patients with active inflammatory lesions, keloidal tendency or infection; immunocompromised patients; having facial cancer, severely damaged skin; having known hypersensitivity to any of the two chemicals; patients who received oral isotretinoin over the past 6 months, pregnant or lactating mothers, and uncooperative patients; or patients with unrealistic expectations were excluded from the study. Prior ethical clearance was taken. We estimated that a total of 60 subjects would be required to detect a 30% difference in clinical improvement between groups with 80% power and a 5% probability of type I error. Further assuming a 10% dropout and rounding off, the recruitment target was set at 30 subjects per group. Sampling was purposive and took place in the dermatology outpatient department 3 days/week. On each day, the first two subjects fulfilling the eligibility criteria were approached for informed consent and recruited if they were willing. No priming was done before initiating treatment. Subjects were apportioned (by software generated list which was kept in the custody of a senior investigator) to receive any one of the formulations (50% GA [v/v in aqueous solution] in Group A or 65% TCA [v/v in aqueous solution] in Group B). A detailed history was taken and a clinical examination of these patients was done. Each subject was asked to wash his/her face with a gentle cleanser and then pat dry with a clean gauze piece. Patients were made to lie on a recliner. The skin was degreased with acetone/absolute alcohol solution and allowed to dry. The grading of acne scars was done with the help of a hand lens and scoring was done with the help of the Goodman–Baron global acne scar qualitative grading score system. Pre-procedure, intraprocedure, and post-procedure serial photographs were taken with a digital camera (Nikon D 3200). The skin of the face was stretched and the pointed end of a wooden toothpick was just dipped into the drug solutions and then touched carefully to the base of the scar to prevent spillage of the drug to the surrounding skin. The endpoint for 50% GA was the appearance of uniform erythema throughout the application site and frosting for 65% TCA. After achieving the endpoint ice water was applied to the application sites.
Post-procedure, patients were asked to rate their burning sensation on a scale of 0–10 where 0 indicated no burning and 10 indicated maximum intolerable burning. A non-comedogenic moisturizer and sunscreen with SPF50 was applied. Every patient was informed about the possible side effects and was asked to follow up every week or early for any adverse effects. The procedure was repeated every fortnightly for three such applications. The patient was asked to rate their improvement on a four-point visual scale where 0=unsatisfactory result, 1=fairly satisfactory, 2=good, and 3=excellent result. The principal investigator also graded acne using the Goodman–Baron qualitative scale on day 1 of reporting and day 35 of follow-up. All relevant data were recorded in a pre-tested, pre-designed, and semi-structured schedule and later imported to a Microsoft Excel sheet and computed by Statistica version 6 (Tulsa, Oklahoma: StatSoft Inc. 2001).
RESULTS
A total of 66 patients were enrolled in our study (31 in Group A and 35 in Group B), among them six patients dropped out after the very first application. Statistical data were calculated based on results obtained from 60 patients. The software used was Statistica version 6 (Tulsa, Oklahoma: StatSoft Inc. 2001).
The youngest patient was 16 years in both groups and the oldest patient was 40 years and 41 years of age in Group A and Group B, respectively. The mean and median age were 23.84 ± 6.75 years and 22 years in Group A; and 27.6 ± 6.47 years and 28 years in Group B, respectively.
Out of a total of 66 subjects, group A had 16 male and 15 female patients, whereas group B contained 19 male and 16 females.
Subjects were examined for immediate or late adverse reactions following topical application of the drugs and were divided into early (within 72 h) reactions which included drug allergic reaction, photosensitivity, and patient’s subjective burning sensation. Out of a total of 66 patients, seven patients developed hypersensitivity reactions (6.45% [n = 2] in Group A and 14.29% [n = 5] in Group B) and 11 patients (9.68% [n = 3] in Group A and 22.86% [n = 8] in Group B) reported photosensitivity. About 74.19% (n = 23) of patients in Group A noted mild burning sensation whereas 42.86% (n = 15) of patients in Group B noted severe burning sensation [Table 2].
| Adverse reactions/side effects (Early) | Group A | Group B | P-value | ||
|---|---|---|---|---|---|
| Present | Absent | Present | Absent | ||
| Drug allergic reaction | 2 (6.45%) | 29 (93.55%) | 5 (14.29%) | 30 (85.71%) | 0.433 (Fisher’s exact 2-tailed test) |
| Photosensitivity | 3 (9.68%) | 28 (90.32%) | 8 (22.86%) | 27 (77.14%) | 0.196 (Fisher’s exact 2-tailed test) |
| Patient’s subjective | Mild (23 [74.19%]) | Mild (0 [0%]) | <0.001 (Chi-square test) | ||
| burning sensation | Moderate (8 [25.81%]) | Moderate (20 [57.14%]) | |||
| Sever (0 [0%]) | Sever(15[42.86%] | ||||
Late (>72 h) reactions comprising new acneiform eruption, scab formation, and PIH were noted. Acneiform eruption [Figure 1] was noted in 12.90% (n = 4) patients in Group A and 25.71% (n = 9) patients in Group B. Scab formation and PIH were found in 100% (n = 31) patients in Group A whereas 85.71% (n = 30) and 54.29% (n = 19) in Group B showed scab formation and PIH, respectively [Table 3]. Post-application erythema was noted in all patients of both groups which were treated with ice water, non-comedogenic moisturizer, and sunscreen with SPF50.
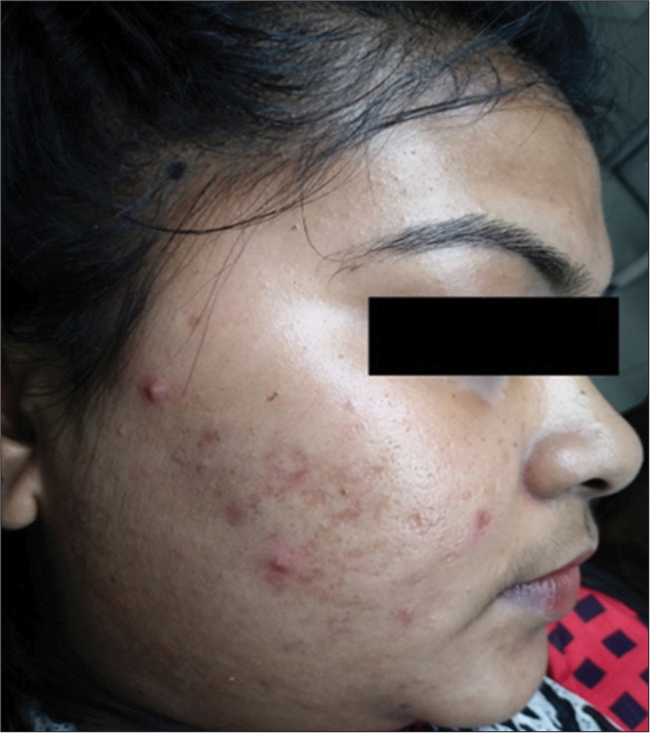
- Acneiform eruptions with 50% glycolic acid (GA) in Group A.
| Adverse reactions/side effects (Late) | Group A (%) | Group B (%) | P-value | ||
|---|---|---|---|---|---|
| Present | Absent | Present | Absent | ||
| New acneiform eruption | 4 (12.90) | 27 (87.10) | 9 (25.71) | 26 (74.29) | 0.228 (Fisher’s exact 2-tailed test) |
| Scab formation | 0 (0) | 31 (100) | 5 (14.29) | 30 (85.71) | 0.055 (Fisher’s exact 2-tailed test was used to calculate the data) |
| Post-inflammatory hyperpigmentation | 0 (0) | 31 (100) | 19 (54.29) | 16 (45.71) | Fisher’s exact 2-tailed test with P<0.001 was used to calculate the data |
Out of a total of 66 subjects, 10.00% (n = 6) opted out of the study without completing the scheduled sittings (6.45% [n = 2] were from Group A and 11.43% [n = 4] from Group B). Fisher’s exact 2-tailed test with P < 0.001 was used to calculate the data. The patient’s assessment on the visual improvement scale showed that 41.38% (n = 12) patients had fair improvement in Group A whereas 58.06% (n = 18) patients showed good improvement in Group B [Table 4]. The Chi-square test was used for the patient’s visual improvement scale and data were computed (n = 60).
| Patient group | Number of patients with their remarks | |||
|---|---|---|---|---|
| Unsatisfactory | Fair Good | Excellent | Row total | |
| A | 34.48% (n=10) | 41.38% (n=12) 20.69% (n=6) | 3.45% (n=1) | 29 |
| B | 6.45% (n=2) | 25.81% (n=8) 58.06% (n=18) | 9.68% (n=3) | 31 |
| Total | 12 | 20 24 | 4 | 60 |
The Goodman–Baron scale was used for grading acne by the investigator on day 1 and day 35 and data were analyzed. It showed 9.68% (n = 3) noted four grade changes in Group B whereas 65.52% (n = 19) of patients in Group A and 32.26% (n = 10) in Group B showed no change in grade. The Chi-square test was used for calculating statistical data where the P value was 0.010 where n = 60 [Table 5]. Change of grade in Group A [Figures 2a and b, 3a and b] and Group B [Figures 4a and b, 5a and b] of patients was considered a measure of improvement.3
| Patient group | Number of patients | |||||
|---|---|---|---|---|---|---|
| No grade change | Grade one change | Grade two change | Grade three change | Grade four change | Row total | |
| A | 65.52% (n=19) | 20.68% (n=6) | 6.89% (n=2) | 6.89% (n=2) | 0.00% (n=0) | 29 |
| B | 32.26% (n=10) | 48.38% (n=15) | 3.22% (n=1) | 6.45% (n=2) | 9.68% (n=3) | 31 |
| Total | 29 | 21 | 3 | 4 | 3 | 60 |
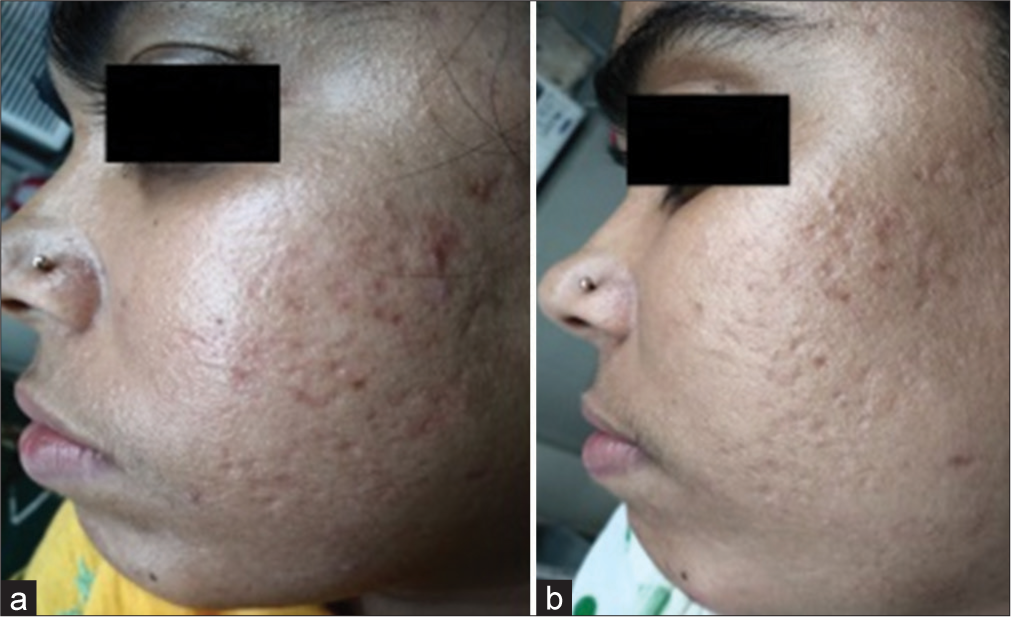
- Improvement in acne scars with 50% glycolic acid (GA) in Group A comparing (a) day 1 and (b) day 35.
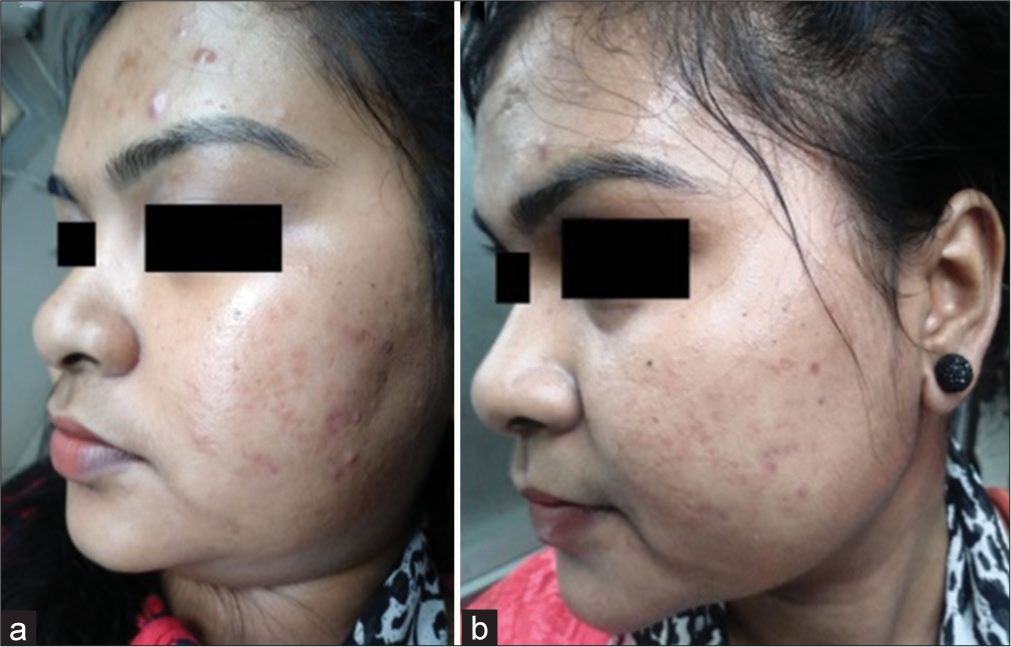
- Improvement in acne scars with 50% glycolic acid (GA) in Group A comparing (a) day 1 and (b) day 35 in another patient.
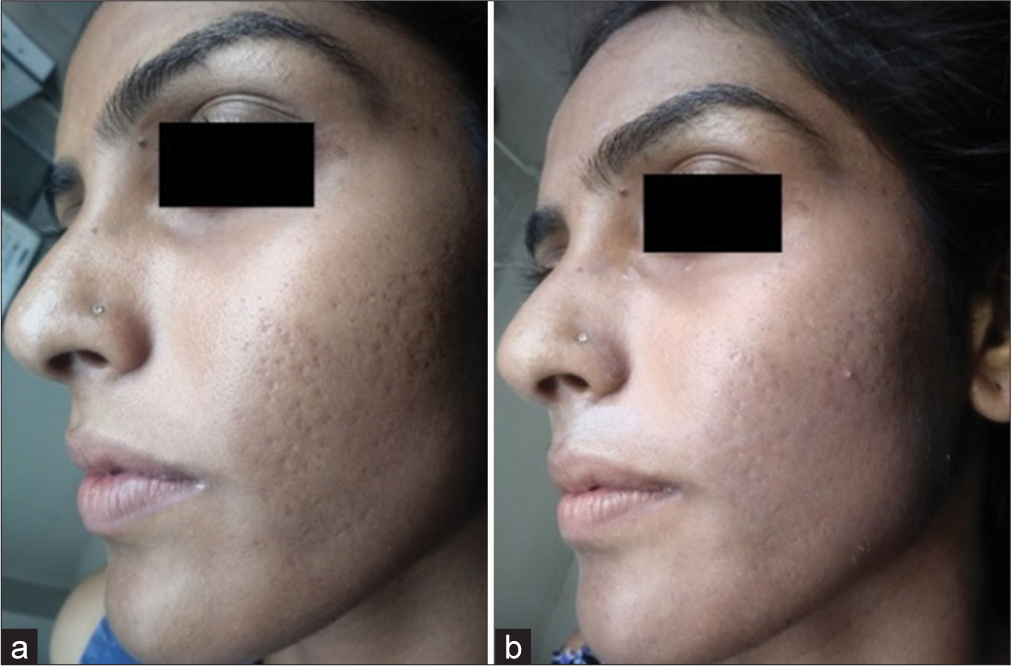
- Improvement in acne scars with 65% trichloroacetic acid (TCA) in Group B comparing (a) day 1 and (b) day 35.
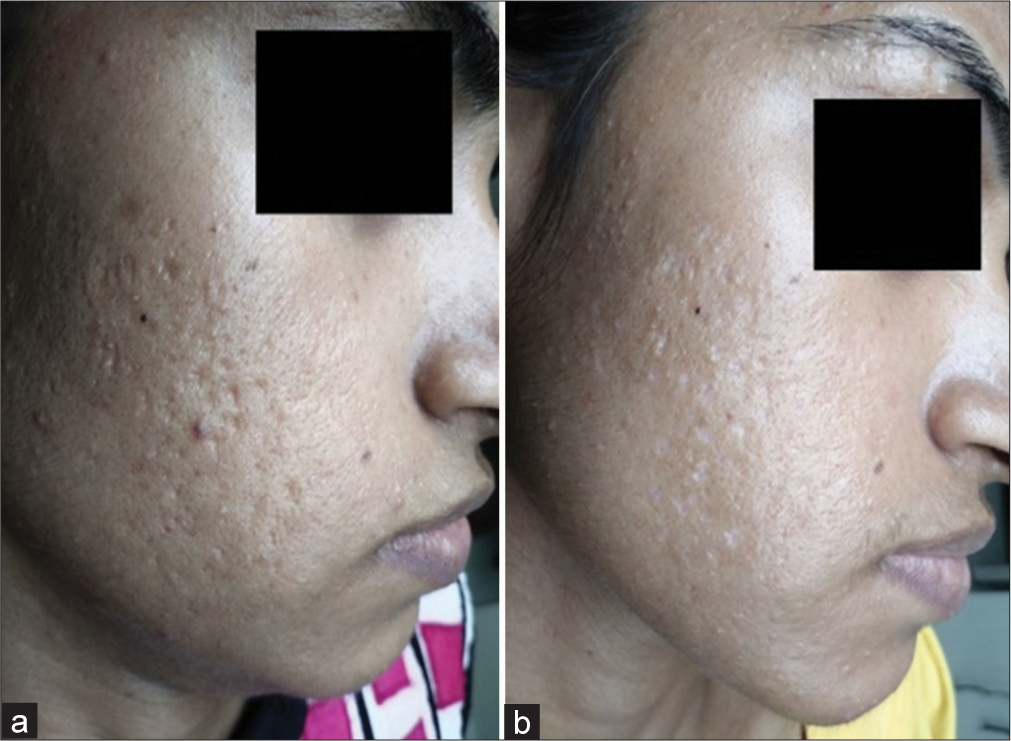
- Improvement in acne scars with 65% trichloroacetic acid (TCA) in Group B comparing (a) day 1 and (b) day 35 in another patient.
DISCUSSION
Post-acne scarring is a very common problem existing in society.10 Acne scars may have a negative psychological effect on social life and relationships.11 Acne and post-acne scars are among the most common causes of depression among the adolescent population around the world.12,13 GA is an alpha hydroxy acid, soluble in alcohol, derived from fruit and milk sugars. It acts by thinning the stratum corneum, promoting epidermolysis, and dispersing basal layer melanin. It increases dermal hyaluronic acid and collagen gene expression by increasing secretion of Interleukin-6.14,15 GA is avoided in pregnancy, active dermatitis, and glycolate hypersensitivity.
Side effects include temporary hyperpigmentation or irritation which are short-lived. The level of skin penetration depends on the concentration of GA used.16 In our study, 41.38% (n = 12) in Group A reported fair results. Change of grade by the Goodman–Baron scale showed 20.69% (n = 6) improvement by one grade and no change in grade in 65.52% (n = 19) in Group A. We could not compare the data as no literature exists on the efficacy of 50% GA as a CROSS agent.
TCA application to the skin causes protein denaturation/kerato-coagulation resulting in a readily observed white frost.17-19 The degree of tissue penetration and injury is dependent on several factors including the percentage of TCA used, anatomic site, and skin preparation. Local application of TCA causes inflammatory reactions leading to the formation of new collagen fibers.20,21 As TCA is a self-neutralizing agent it does not get absorbed in the circulation; hence, high concentrations can be safely used.13 In our study, 58.06% (n = 18) patients reported good results and 25.81% (n = 8) reported fair results whereas 9.68% (n = 3) subjects reported excellent results at the end of the procedure. Lee et al.,20 using a concentration of 65% TCA for atrophic acne scars on five patients, found that 20% of patients showed excellent visual improvement after three sessions and 50% showed excellent visual improvement after six sessions. Results in our study were comparable with the results of other studies available with TCA as a CROSS agent. Patients in Group B had better visual results than those in Group A.
In Group B, 48.38% (n = 15) showed one grade; 3.22% (n = 1) showed two grades; and 9.68% (n = 3) had a four-grade improvement in their acne grades. In a study by Puri22 with 90% TCA, after three courses, 20%, 15%, and 10% of patients developed excellent, good, and fair responses, respectively. In a study conducted by Garem et al. with 50 % TCA, 19 (63.3%) patients showed good and 11 (36.7%) patients showed fair improvement. None showed excellent or poor improvement based on the Goodman–Baron scale.23 Studies conducted exclusively with 65% TCA as CROSS are rare. Lee et al.20 reported good clinical response in 82% of patients treated with 65% TCA CROSS but he did not categorize the results on a scale of no improvement, fair, good, and excellent. Hence, we compared our results with the available studies with different concentrations of TCA used in CROSS studies. In a study with 12 patients by Bhardwaj and Khunger13 with 100% TCA as a CROSS agent, they found that 80% of patients showed excellent improvement, and 20% showed good results after four sessions. Our results were fairly comparable with these studies.
Drug allergic reaction was characterized by erythema, edema, burning, and inflammation around the site of drug application.24 Overall 7 (10.61%) out of the total subjects (n = 66) developed hypersensitivity reactions. The statistics were comparable with other studies. In a case report by Vishal et al. from Kerala, a patient undergoing 35% GA peel for acne vulgaris developed contact urticaria.25 Photosensitivity was another adverse reaction noted by the test subjects.26-28 A total of 11 subjects reported photosensitivity which was mostly due to not following advice to use broad-spectrum sunscreen during the study period. The burning sensation was reported immediately on application of both drugs, but sensations were subjective and varied in intensity from subjects across both Groups.29,30 In our study, 57.14% (n = 20) and 42.86% (n = 15) of patients in Group B experienced moderate and severe burning sensations, respectively. Garem et al. reported that 60% (n = 18) patients had mild and 40% (n = 12) patients had moderate discomfort with 50% TCA as a CROSS agent.23 In a study by Puri with 70% TCA CROSS for post-acne scar, erythema, and burning sensation were noted in 15% and 10% of patients, respectively.22 In contrast, subjects treated with 50% GA (Group B), 23 out of 31 (74.19%) reported mild burning sensation while the rest, that is, 8 (25.81%) reported moderate burning sensation.30
In Group B, 54.19% (n = 19) presented with PIH following treatment. Pigmentary problems can occur, despite a reasonable avoidance of solar radiation due to melanocytic sensitivity caused by the irritating effect of the peel.31 Acneiform eruption was noted in 12.90% (n = 4) patients in Group A and 25.71% (n = 9) patients in Group B. Literature is present to support the instance of new acne eruption following GA peels29,30 but data are lacking concerning TCA CROSS. Chemical peel (TCA, GA, salicylic acid, and Jessner’s peel) is the most commonly used peel for superficial and pigmented acne scars. Microdermaabrasion, microneedling, and subcision are used in rolling and box acne scars. The punch excision technique is indicated in the ice peak and box scar. Dermal fillers may be performed for atrophic scars. Cryotherapy, topical silicon dioxide gel, and intralesional corticosteroids are used for hypertrophic and keloid scars. Ablative lasers such as carbon dioxide, erbium-doped yttrium aluminium garnet (Er: YAG), and non-ablative lasers (Q switch Nd: YAG, ER glass, and long pulse dye lasers) are also used for rolling, box, and atrophic scar.32
CONCLUSION
In our study, improvement in acne scar grades by the CROSS technique is best observed with 65% TCA. On the other hand, adverse effects such as hypersensitivity, photosensitivity, burning sensation, scab formation, and PIH were noted to be more prevalent in patients treated with 65% TCA than those treated with 50% GA as a CROSS agent. Studies on TCA CROSS done previously lack any evidence of post-procedural acneiform eruption. In our study, a significant number of patients developed acneiform eruption during and after the procedure. This is a new finding in our study. TCA is an established agent in the management of atrophic acne scar, but in our study, we found out that GA can be a safer alternative to TCA with acceptable results in patients with low scores on the Goodman–Baron Global Acne grading scale. No study exists on using 50% GA as a CROSS agent in the treatment of atrophic acne scars. Our study provides an opportunity for future research on different concentrations of GA as a CROSS agent in the future.
Authors’ contributions
All the contributors worked together in conceptualizing, designing and defining the intellectual content of the article, literature search including those of clinical studies, manuscript preparation and editing, data acquisition, data analysis and statistical analysis were performed by all the four authors. Finally the manuscript review was accomplished by all the contributory authors.
Ethical approval
The research/study was approved by the IPGME&R Research Oversight Committee (Institutional Ethics Committee) with memo no. Inst/IEC/2016/599 on 10.12.2016.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Disorders of the sebaceous glands In: Burns T, Breathnach S, Cox N, Griffiths C, eds. Rook’s text book of dermatology Vol 43. (7th ed). United States: Blackwell Science; 2004. p. :1-75.
- [Google Scholar]
- Acne, rosacea, and related disorders In: Clinical dermatology: A colour guide to diagnosis and therapy (4th ed). Edinburgh: Mosby; 2004. p. :168-70.
- [Google Scholar]
- What’s new concerning the pathophysiology of acne? Ann Dermatol Venereol. 2003;130:101-6.
- [Google Scholar]
- Early events in innate immunity in the recognition of microbial pathogens. Expert Opin Biol Ther. 2007;7:907-18.
- [CrossRef] [PubMed] [Google Scholar]
- Hormonal therapy for acne. Semin Cutan Med Surg. 2008;27:188-96.
- [CrossRef] [PubMed] [Google Scholar]
- Postacne scarring: A qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-66.
- [CrossRef] [PubMed] [Google Scholar]
- The assessment of acne: An evaluation of grading and lesion counting in the measurement of acne. Clin Dermatol. 2004;22:394-7.
- [CrossRef] [PubMed] [Google Scholar]
- An acne grading method using photographic standards. Arch Dermatol. 1979;115:571-5.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of healthcare outcomes and costs related to medication use in patients with acne in the United States. Cutis. 2006;77:251-5.
- [Google Scholar]
- Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-16.
- [CrossRef] [PubMed] [Google Scholar]
- An assessment of the efficacy and safety of CROSS technique with 100% TCA in the management of ice pick acne scars. J Cutan Aesthet Surg. 2010;3:93-6.
- [CrossRef] [PubMed] [Google Scholar]
- Chemical peeling: How, when, why? J Eur Acad Dermatol Venereol. 1997;8:1-11.
- [CrossRef] [Google Scholar]
- Management of post-acne scarring: What are the options for treatment? Am J Clin Dermatol. 2000;1:3-17.
- [CrossRef] [PubMed] [Google Scholar]
- The art of the trichloroacetic acid chemical peel. Clin Plast Surg. 1998;25:53-62.
- [CrossRef] [Google Scholar]
- Medium-depth chemical peels in the treatment of acne scars in dark-skinned individuals. Dermatol Surg. 2002;28:383-7.
- [CrossRef] [PubMed] [Google Scholar]
- Focal treatment of acne scars with trichloroacetic acid: Chemical reconstruction of skin scars method. Dermatol Surg. 2002;28:1017-21.
- [CrossRef] [PubMed] [Google Scholar]
- CROSS technique: Chemical reconstruction of skin scars method. Dermatol Ther. 2008;21:29-32.
- [CrossRef] [PubMed] [Google Scholar]
- A study on the efficacy of TCA CROSS for the management of acne scars. J Pak Assoc Dermatol. 2013;23:184-9.
- [Google Scholar]
- Chemical reconstruction of skin scars (CROSS) technique using trichloroacetic acid 50% in different types of atrophic acne scars. Egypt J Dermatol Venerol. 2013;33:37-41.
- [CrossRef] [Google Scholar]
- Psychological problems in the acne patient. Dermatol Ther. 2006;19:237-40.
- [CrossRef] [PubMed] [Google Scholar]
- Contact urticaria to glycolic acid peel. J Cutan Aesthet Surg. 2012;5:58-9.
- [CrossRef] [PubMed] [Google Scholar]
- Contact urticaria: Present scenario. Indian J Dermatol. 2009;54:264-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of complications in chemical peeling. J Cutan Aesthet Surg. 2010;3:186-8.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of chemical face peeling. Plast Reconstr Surg. 1974;54:397-403.
- [CrossRef] [PubMed] [Google Scholar]
- Step by step chemical peels. (1st ed). New Delhi: Jaypee Medical Publishers; 2009. p. :279-97.
- [CrossRef] [Google Scholar]
- The therapeutic value of glycolic acid peels in dermatology. Indian J Dermatol Venereol Leprol. 2003;69:148-50.
- [Google Scholar]
- Comparison of a 1550 nm erbium: Glass fractional laser and a chemical reconstruction of skin scars (CROSS) method in the treatment of acne scars: A simultaneous split-face trial. Lasers Surg Med. 2009;41:545-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acne scarring-pathogenesis, evaluation, and treatment options. J Clin Aesthet Dermatol. 2017;10:12-23.
- [Google Scholar]







