Translate this page into:
Delayed inflammatory reaction to hyaluronic acid filler following COVID-19 vaccination: A case report and review of the literature
*Corresponding author: Noureddine Litaiem, Department of Dermatology, Charles Nicolle Hospital, University of Tunis el Manar, Tunis, Tunisia. noureddine.litaiem@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Litaiem N, Fazzeni M, Hawilo A, Zeglaoui F. Delayed inflammatory reaction to hyaluronic acid filler following COVID-19 vaccination: A case report and review of the literature. J Cutan Aesthet Surg. doi: 10.25259/JCAS_31_2024
Abstract
Delayed inflammatory reactions (DIRs) to coronavirus disease 2019 (COVID-19) vaccines were rarely reported in patients receiving hyaluronic acid (HA) soft-tissue fillers. We present a case of a DIR to HA filler that occurred within days after receiving the second dose of the Pfizer-BioNTech COVID-19 messenger ribonucleic acid (RNA) vaccine and provide a comprehensive summary of its clinical presentation and treatment.
Keywords
Hyaluronic acid
Dermal fillers
Injection site reaction
Adverse effects
COVID-19
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Vaccination
Vaccines
Messenger RNA
INTRODUCTION
Hyaluronic acid (HA) is one of the most important ingredients in cosmetic products due to its remarkable anti-aging properties. This can be achieved through soft-tissue augmentation, improved skin hydration, collagen and elastin stimulation, and face volume restoration.1 The use of HA soft-tissue fillers for non-invasive esthetic procedures is rapidly increasing. In 2022 alone, over 4 million HA soft-tissue filler procedures were performed worldwide, making it the second most performed non-surgical procedure.2
With the emergence of coronavirus disease 2019 (COVID-19) vaccines, practitioners have raised concerns about the safety of HA-based fillers during the pandemic. However, the frequency of complications that could be attributed to COVID-19 disease or vaccination was low (0.06%).3
Delayed inflammatory reactions (DIRs) to fillers are defined as reactions occurring at least 2–4 weeks after injections and could manifest as discoloration (most commonly erythema), painful nodules, localized induration, and edema.4
DIRs to COVID-19 vaccines were rarely reported in patients receiving HA soft-tissue fillers.5-15 Its pathophysiological mechanism is not fully understood, and its treatment is not well codified. We present a case of a DIR to HA filler that occurred within days after receiving the second dose of the Pfizer-BioNTech COVID-19 messenger ribonucleic acid (RNA) vaccine and provide a comprehensive summary of its clinical presentation and treatment.
CASE REPORT
A 43-year-old female, physically fit and healthy, without any personal or family history of atopy, urticaria, or known drug allergies, presented with intermittent swelling of the lower eyelids for 2 weeks. She denied any prior inflammatory filler reactions. In June 2020, the patient received 3 ml of HA soft-tissue filler, with 1 ml of Juvederm Volbella and 2 mL of Juvederm Ultra, equally injected into her bilateral tear troughs to correct under eye hollowness. The injection was performed in the subdermal plane using a microcannula under proper sterile conditions. In March 2021, 3 days after receiving the second dose of the mRNA Pfizer-BioNTech COVID-19 vaccine, the patient reported moderate swelling and tenderness in her bilateral tear troughs. There was no recent history of trauma or infection, and she had not taken any medications recently. The examination revealed edema of the lower eyelids, mainly on the right side, but without erythema and mild tenderness on touch [Figure 1]. No palpable nodule or fluctuant mass was found. Magnetic resonance imaging showed multiple bilateral tiny foci of high transverse relaxation time (T2) signal rounded material and bilateral cosmetic material injection (filler) seen within maxilla and nasolabial folds with mild contour asymmetry, being larger on the right in the axial fluid-attenuated inversion recovery images [Figure 2]. Based on the appearance of localized edema within days after receiving the mRNA COVID-19 vaccine, the diagnosis of DIR secondary to the vaccine was made, as no other triggers, such as infection, trauma, dental, or medical procedures, were found. The patient received initial treatment with oral prednisone, 40 mg daily, which reduced swelling but resulted in recurrence after treatment tapering. The patient was subsequently started on a daily dose of 5 mg lisinopril, resulting in substantial improvement within 6 h [Figure 1] and complete resolution without recurrence at follow-up.
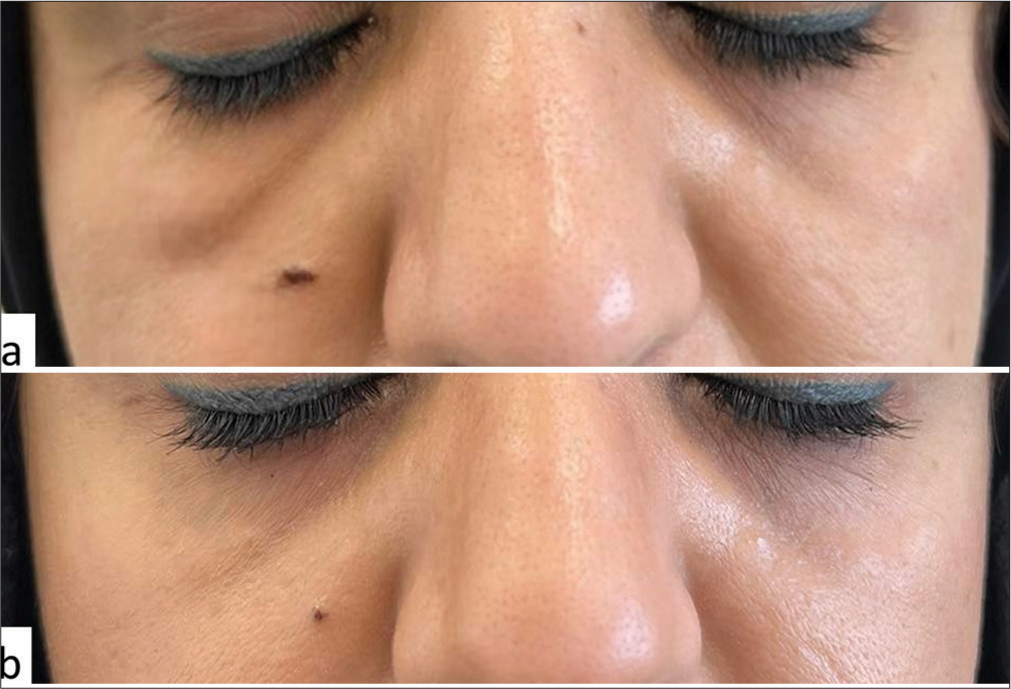
- (a) Edema of the lower eyelids following coronavirus disease 2019 (COVID-19) vaccination. (b) Rapid resolution after lisinopril initiation.
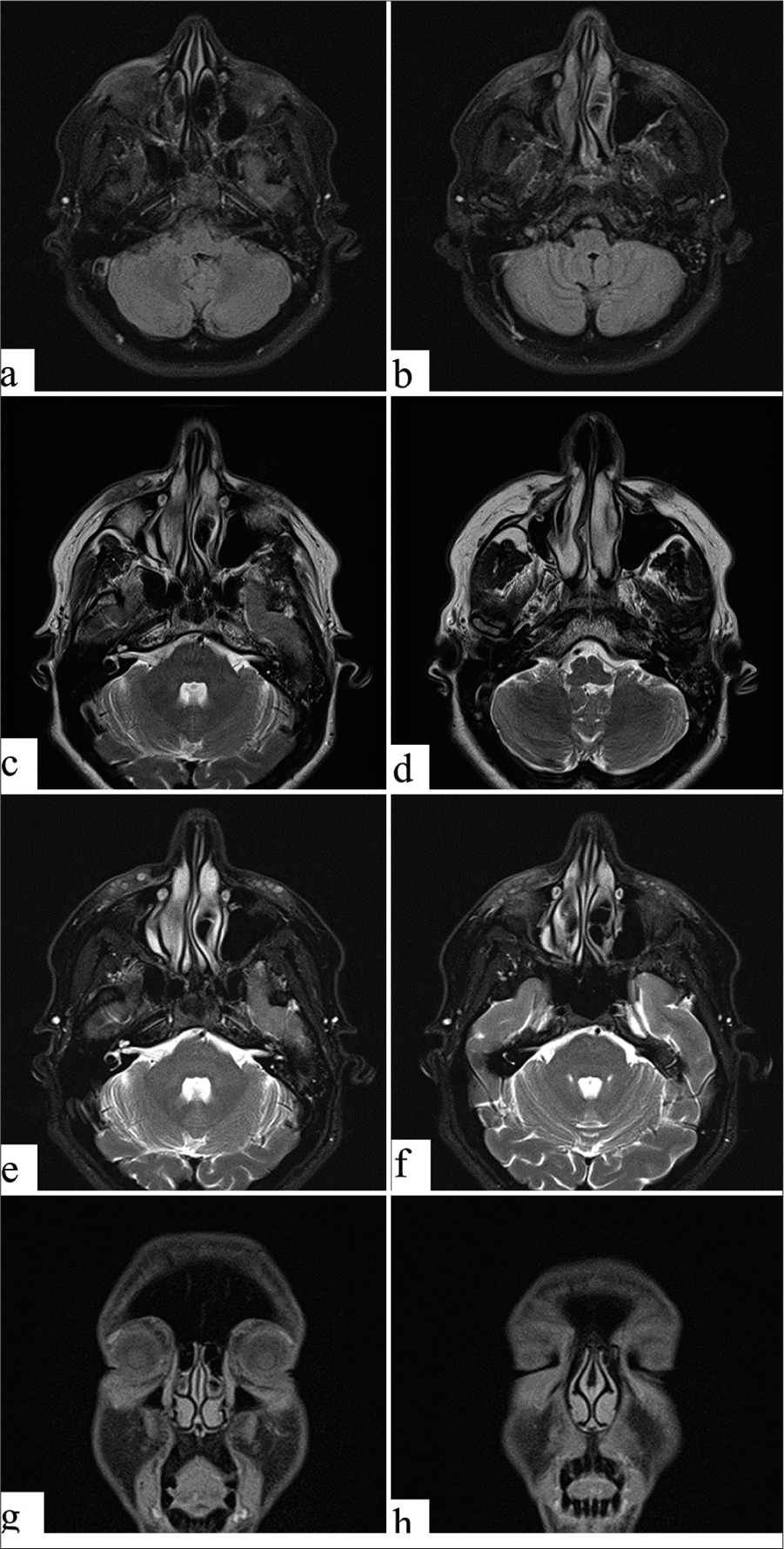
- Magnetic resonance imaging. (a and b) Axial fluid-attenuated inversion recovery images showing bilateral cosmetic material injection (fillers) injection seen within the maxilla and nasolabial folds with mild contour asymmetry, being larger on the right. (c and d) Axial transverse relaxation time (T2) images show bilateral tiny multiple foci of high T2 signal rounded material, multiple and more on the left side, being larger on the right. (e and f) Axial T2 Dixon images showing bilateral tiny multiple foci of high T2 signal rounded material, as described above. (g and h) Coronal longitudinal relaxation time (T1) Dixon images showing bilateral tiny multiple foci of intermediate to low T1 signal rounded material, as described above. No evidence of localized collection, distal migration, granulomatous changes, fibrotic changes, or scars.
DISCUSSION
All HA dermal fillers carry a risk of both acute and delayed reactions. Acute reactions can manifest as injection site reactions such as erythema, bruising, itching, injection site infections, lumps, and migrations due to technique errors and type I hypersensitivity reactions, which typically occur within minutes or hours of injection due to an immunoglobulin E-mediated immune response such as angioedema or anaphylactic reactions.16 Delayed reactions may occur weeks, months, or years after the initial injection and are characterized by induration, erythema, and edema in the injection site.17 The incidence rate of DIRs to HA soft-tissue fillers varies considerably between products, ranging from 0.02% to 4.25%.16,17
The full extent of the mechanisms involved in DIRs remains to be fully elucidated. Several triggers for DIRs have been suggested, including systemic infection, vaccination, local trauma, injection technique, product quality, filler volume, and previous or current dental procedures.17 Biofilm formation has also been implicated, but its role remains unclear.16,17 Both filler characteristics and host immune status appear to play a role in activating certain mechanisms leading to DIRs. Immune-mediated type IV hypersensitivity to fillers in patients with DIRs has been investigated in a case report by Azzouz et al. intradermal testing with 0.1 mL of suspected HA filler was performed and showed an inflammatory nodule within 2 weeks of application.6 However, according to the group consensus by Artzi et al., it is unclear whether DIRs should be considered as true hypersensitivity reactions.4
To date, DIR to COVID-19 vaccination was reported in 21 patients [Table 1]. All patients were female, and the mean age was 42.19 (range: 23–76 years). The mean interval between filler injection and reaction was 15.93 months (range: 0.5–60 months). Eleven developed symptoms after the first dose of vaccination, 10 after the second dose, and two after the third dose. The mean time interval between the vaccine injection and the DIR to filler was 8.96 days (range: 1–60 days). Fourteen patients developed symptoms after injection of the mRNA Pfizer-BioNTech vaccine, of which 6 after the first dose, 4 after the second dose, two after the third dose, and two exhibited symptoms both after the first and second dose. Five patients developed symptoms after injection of the mRNA Moderna vaccine, of which two after the first dose and three after the second dose. Two patients developed symptoms after injection of the AstraZeneca vaccine, of which one was after the first dose and one after the second dose. Given its rarity, it is unclear whether DIR is associated with a specific type of HA filler. In our patient, the reaction appeared 3 days after receiving the second dose of the mRNA Pfizer-BioNTech COVID-19 vaccine at the site of HA filler injection (8 months earlier).
| Sex, age History | Site of filler | Duration of filler | Type of vaccine | Delay of reaction | Clinical features | Treatment | Treatment response | |
|---|---|---|---|---|---|---|---|---|
| Munavalli et al.13 | F, 43 Prior history of idiopathic urticaria |
Temples, cheeks | 2 years | 2nd dose Pfizer BNT162b2 | 2 days | Moderate swelling in the periorbital area, and the medial and lateral cheeks. | 5 mg of Lisinopril (5 days) | Back to baseline after 2 days |
| F, 31 prior history of a filler reaction (edema following a respiratory infection) |
Earlobes Bilateral malar cheeks, nasolabial folds Nasolabial folds |
27 months 19 months 7 months |
2nd dose Moderna | 24 h | Swelling of the upper mucosal lip, the left earlobe, and bilateral zygomas | 5 mg of Lisinopril (2 days) 10 mg of Lisinopril (3 days) |
Minimal effect Back to baseline |
|
| F, 36 | Tear troughs, lips | 13 months | 1st dose Moderna | 18 h | Edema in the infraorbital and perioral regions, in the upper lip Swelling and inflammation expanded to the mid-cheeks |
Cetirizine (20 mg) 5 mg of Lisinopril (3 days) |
No improvement Improvement after 6 h, back to baseline at 24 h |
|
| F, 76 prior history of a filler reaction |
Cheeks | 1st dose Pfizer | 10 days | Panfacial and periorbital swelling. | 5 mg of Lisinopril (7 days) | Improvement within 4 days, back to baseline at 7 days | ||
| Munavalli et al.13 |
F, 39 No history |
Tear trough | 6 months | 1st dose Pfizer | 2 days | Tender, erythematous swelling at her left tear trough area. | Watchful waiting | Spontaneous resolution by day 5 |
| F, 61 No history |
Pan facial (chin, jawline, periorbital) | 10 months | 1st dose Pfizer | Few days | Intermittent facial swelling, mainly at the cheeks and under eye | Hyaluronidase | Complete resolution 48h after injection | |
| F, 45 No history |
Chin, lips | 10 months | 2nd dose Pfizer | 24 h | Swelling at the injection sites | Lisinopril 5 mg daily (7 days) | Improvement within 24h Complete resolution after 72h | |
| Savva et al.12 | F, 38 No history |
Lips | 1 month | 1st dose Pfizer 2nd dose Pfizer |
2 days 2 months |
Small erythematous nodules on lips mildly painful Painful erythematous edema on both lips |
Methylprednisolone (8 days) | Spontaneous resolution after 7 days Complete resolution 5 days |
| Calvisi9 | F, 60 No history |
Lips | 1 year | 1st dose Pfizer (after 2 prior doses of AstraZeneca) |
5 days | Swelling in the upper lip | Watchful waiting | Spontaneous resolution after 3 days |
| F, 45 No history |
Lips | 2 years | 3rd dose Pfizer | Angioedema in the upper lip | Prednisolone 25 mg for 3 days |
Complete resolution after 3 days | ||
| F, 40 No history |
Nasolabial folds | 5 months | 1st dose Moderna (after 2 doses of Pfizer) | Erythema and edema at the site of injection | Watchful waiting | Spontaneous resolution after 7 days | ||
| Beamish et al.7 | F, 23 No history |
Malar eminences, lips, jaw, and chin |
1 year | 2nd dose Pfizer | 6 weeks | Painful asymmetric swelling over her maxilla, lips, and lower jaw | Single dose of IV diphenhydramine 50 mg Single dose of IV dexamethasone 10 mg |
|
| Azzouz et al.6 | F, 43 HCTD, Hashimoto’s thyroiditis, and craniopharyngioma |
Multiple facial skin folds | 3 months | 2nd dose of Moderna (3 weeks before injection) | 24 h | Facial edema nodules and indurations on her cheeks and chin, at the sites of previous HA injections |
Oral prednisone Local steroid injections Hyaluronidase injections |
Moderate symptomatic improvement and nodule regression. |
| Neamatallah8 | F, 36 No history |
lips | 22 months | 1st dose Pfizer 2nd dose Pfizer |
3 days 12 h |
Dryness, induration, and erythematous swelling in the lower lip | Watchful waiting hyaluronidase and triamcinolone injection |
Symptoms reduced within two weeks improvement in the following days |
| Ortigosa et al.11 | F, 35 No history |
Malar, and chin regions lips, nasojugal furrow |
5 years 16 months |
1st dose AstraZeneca | 24 h | Induration and edema in her lips and chin | Oral prednisone (total of 21 days) hydroxyzine (50 mg/day) |
Improvement was achieved, persistence of mild lip edema. |
| F, 47 No history |
Around the eye | 18 months | 2nd dose Pfizer | 4 weeks | Edema in the lower eyelids Recurrence |
Prednisolone 20 mg/day for 4 days Lisinopril 5 mg |
Complete resolution after 7 days Resolution in 24 h |
|
| F, 34 No history |
Lips | 10 months | 3rd dose of Pfizer | 24 h | Pain and mild edema in the lips | Loratadine 10 mg/day for 2 days | Complete resolution in 72 h | |
| F, 56 No history |
Mandibula (jaw angle), chin and lips | 1st dose Pfizer | 48h | Induration and edema in the mandible and chin | Oral prednisone 20 mg twice a day for 3 days | Complete resolution was achieved | ||
| F, 43 No history |
Nasolabial folds and lips Lips |
1 year 15 days |
2nd dose AstraZeneca | 7 days | Edema, erythema, increase in the temperature of her lips | Ibuprofen 40 mg/day and prednisone 40 mg/day (tapered for 3 weeks) | Symptoms reduced after 7 days | |
| Obagi et al.15 | F, 29 No history |
Lips and under-eye region | 4 months | 1st dose Pfizer After 2 doses of Moderna |
Swelling of the lips and the bilateral under-eye compartments | Watchful waiting Hyaluronidase injection (under-eye) |
Lip swelling had resolved, but the inflammation around her eyes persisted Complete resolution in 1 day |
|
| Osmond and Kenny14 | F, 26 No history |
Chin and jaw | 3 years | 2nd dose Moderna | 24h | Chin swelling | Watchful waiting | symptoms gradually resolved in 48 h |
HCTD: Hereditary connective tissue disorder, HA: Hyaluronic acid
The exact mechanism of DIR to COVID-19 vaccination is still not fully understood. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry into host cells is mediated by its spike glycoprotein and binds the angiotensin-converting enzyme receptor 2 (ACE2) as the cellular receptor. Membrane-bound and soluble ACE2 catalyzes the conversion of the proinflammatory angiotensin II (AngII) to the anti-inflammatory metabolites angiotensin 1–7.13 The receptor-binding domain of the COVID spike protein irreversibly binds to membrane-bound ACE2, initiating membrane fusion and entry into the cell, effectively down-regulating ACE2 and its ability to locally control AngII.16 Accumulation of AngII upregulates the expression of monocyte chemoattractant protein type 1, tumor necrosis factor-alpha, interleukin 6, and interleukin 8, which are potent chemoattractants and activators of neutrophils and has been shown to upregulate cluster of differentiation 44 (CD 44) glycoprotein. CD44 glycoprotein has an affinity for binding free extracellular HA, particularly low molecular weight (LMW) HA, inciting a cluster of differentiation 8 (CD8) + The T helper cells (Th1) immune response.13
The role of AngII in DIR to COVID-19 vaccination could explain the efficacy of ACE inhibitors like lisinopril for the treatment of this DIR. ACE inhibitors reduce the AngII levels, thereby mitigating the effects of rising spike protein levels and, additionally, reducing AngII -induced aldosterone secretion by the adrenal cortex, which leads to an increase in sodium excretion and, subsequently, an increase in water outflow.
Seven patients were treated with lisinopril and showed rapid improvement and complete resolution of symptoms in 1–7 days [Table 1]. Lisinopril usually reaches peak efficacy within 6 h after oral intake, as seen in our patient, who experienced significant improvement 6 h after the first dose. 5 mg of lisinopril is a good starting dose for the treatment of DIRs, escalating to 10 mg if a partial improvement is seen. The suggested duration of therapy is 3–5 days or at least 1–2 days after the swelling subsides. Lisinopril is usually well tolerated, but clinicians should be aware of its adverse effects, such as hypotension, cough, and head and neck angioedema.5,13
CONCLUSION
The COVID-19 pandemic has seen a remarkable decline and the wave of mass vaccination has passed. It is clear that the benefits of vaccination far outweigh the risk of cutaneous DIR. Nevertheless, with the ever-increasing demand for dermal filler treatment, clinicians should be mindful of the risks of this reaction and its potential etiologies. Further studies are needed, especially with the emerging use of ACE-I for the prevention and treatment of DIRs to HA fillers.
Authors’ contributions
Conceptualization: Noureddine Litaiem, Mariem Fazzani. Methodology: Noureddine Litaiem, Mariem Fazzani, Abdulmooti Hawilo. Writing - original draft preparation: Mariem Fazzani. Writing - review and editing: Noureddine Litaiem, Mariem Fazzani. Abdulmooti Hawilo, Faten Zeglaoui.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120(Pt B):1682-95.
- [CrossRef] [PubMed] [Google Scholar]
- Global survey 2022. In: Full report and press releases. Available from: https://www.isaps.org/discover/about-isaps/global-statistics/reports-and-press-releases/global-survey-2022-full-report-and-press-releases [Last accessed on 2023 Dec 14]
- [Google Scholar]
- Good tolerance of hyaluronic acid filler injections during the COVID-19 pandemic. J Cosmet Dermatol. 2023;22:342-6.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed inflammatory reactions to hyaluronic acid fillers: A literature review and proposed treatment algorithm. Clin Cosmet Investig Dermatol. 2020;13:371-8.
- [CrossRef] [PubMed] [Google Scholar]
- ACE inhibitors-An effective treatment for hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination. J Cosmet Dermatol. 2022;21:1369-70.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed hypersensitivity reaction to cosmetic filler following two COVID-19 vaccinations and infection. Allergy Asthma Clin Immunol. 2023;19:31.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed inflammatory reaction to dermal fillers after COVID-19 vaccination: A case report. CJEM. 2022;24:444-6.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed inflammatory reaction to hyaluronic acid lip filler after the Pfizer-BioNTech COVID-19 vaccine: A case report. Heliyon. 2023;9:e18274.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronic acid delayed inflammatory reaction after third dose of SARS-CoV-2 vaccine. J Cosmet Dermatol. 2022;21:2315-7.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination-A case report. J Cosmet Dermatol. 2021;20:2684-90.
- [CrossRef] [PubMed] [Google Scholar]
- Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: A series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-70.
- [CrossRef] [PubMed] [Google Scholar]
- Hypersensitivity reaction to hyaluronic acid dermal filler after the Pfizer vaccination against SARS-CoV-2. Int J Infect Dis. 2021;113:233-5.
- [CrossRef] [PubMed] [Google Scholar]
- Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-8.
- [CrossRef] [PubMed] [Google Scholar]
- Reaction to dermal filler following COVID-19 vaccination. J Cosmet Dermatol. 2021;20:3751-2.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of delayed inflammatory response to hyaluronic acid soft tissue filler in a Pfizer-Boosted Moderna-vaccinated individual with hyaluronidase. Surg Cosmet Dermatol. 2022;14:1-4.
- [CrossRef] [Google Scholar]
- Relationship between delayed reactions to dermal fillers and biofilms: facts and considerations. Dermatol Surg. 2014;40:1175-9.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: Cause and management. Dermatol Surg. 2015;41:929-39.
- [CrossRef] [PubMed] [Google Scholar]






