Translate this page into:
A comparative study of the efficacy of potent topical corticosteroids per se versus combination of potent topical corticosteroids with fractional CO2 laser in the management of vitiligo vulgaris
*Corresponding author: Rashmi Mahajan, Department of Dermatology, Venerology and Leprosy, Smt. Bhikhiben K Shah Medical Institute and Research Center, Vadodara, Gujarat, India. rsoodmahajan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ninama K, Mahajan R, Baxi D, Patil RS, Marfatia Y. A comparative study of the efficacy of potent topical corticosteroids per se versus combination of potent topical corticosteroids with fractional CO2 laser in the management of vitiligo vulgaris. J Cutan Aesthet Surg. 2025;18:42-7. doi: 10.25259/JCAS_84_2024
Abstract
Objectives:
The study aimed to compare the efficacy of two treatment modalities in vitiligo vulgaris that is – potent topical corticosteroid (betamethasone-dipropionate lotion 0.05%) with FrCO2 laser versus Potent topical corticosteroid (PTS) alone, and to observe side effects if any.
Material and Methods:
Sixty cases with stable vitiligo (vitiligo vulgaris including focal vitiligo) >12 years of age with <27% body surface area were included in the study. The Institutional Ethics Committee approval was taken. After taking consent and clinical photographs, cases were randomly allocated into two groups of 30 patients each: Group A received FrCO2 laser (power-6W, random mode) once a month + twice daily application of PTS (betamethasone dipropionate lotion 0.05%) over patches and Group B applied PTS only twice daily (betamethasone dipropionate lotion 0.05%). The response was assessed at the end of 3 months.
Results:
The mean vitiligo area scoring index (VASI) score at baseline for Group A was 1.17 ± 0.59, and after 3 months was 0.60 ± 0.56. For Group B, the mean VASI at baseline was 0.87 ± 0.35 and after 3 months was 0.63 ± 0.50. The p value of group B was 0.003 and for group A was 0.001. Even though the reduction in mean VASI was statistically significant in both groups, Group A showed a better response. Group A showed good to excellent response in 16.67% while Group B only cases in 3.3%. This suggests that the addition of FrCO2 to steroids may provide superior repigmentation.
Conclusion:
The addition of FrCO2 laser to topical corticosteroids offers a safe and promising approach and can be considered in patients who are unresponsive to other treatment modalities.
Keywords
Fractional CO2
Topical corticosteroid
Vitiligo
INTRODUCTION
Vitiligo is an acquired primary disorder of pigmentation of unknown etiology characterized clinically by depigmented macules and patches.1 It is frequently associated with leukotrichia over the involved skin as a result of the destruction and loss of functioning melanocytes from the affected area.2 Vitiligo has an adverse impact on the quality of life. Multiple treatment modalities have been established for treating vitiligo. However, due to the multifactorial and polygenic nature of the disease, combination therapy is more useful in these patients.3
Multiple clinical trials are advocating the benefits of fractional CO2 (FrCO2) laser in the treatment of vitiligo. In this article, we provide our experience on the use of FrCO2 laser in conjunction with potent topical corticosteroids, comparing the results with potent topical corticosteroids alone in the treatment of vitiligo. FrCO2 laser stimulates the migration of melanocytes and the differentiation of melanocyte stem cells.3 It acts by the mechanism of fractional photo thermolysis. The formation of tiny microchannels facilitates the penetration of topically applied drugs.4
MATERIAL AND METHODS
The Institutional Ethics Committee approved this study. All cases attending the outpatient department within the study period of 1½ years with a clinical diagnosis of vitiligo vulgaris (including focal vitiligo) with body surface area (BSA) <27% and age>12 years were included in the study. Patients with unstable vitiligo and suffering from other dermatological conditions were excluded from the study.
Informed consent (>18 years) and assent (12–18 years) forms were taken of the cases and were entered in a structured pro forma.
Consecutive cases were designated into two groups. Group A was treated with FrCO2 laser (power 6W, duration 2.5 ms, interval 1.0 ms, distance 0.8 mm, and random mode with 12 × 12 dimension) and topical betamethasone dipropionate lotion 0.05% and Group B was treated with topical betamethasone dipropionate lotion 0.05% only. Both groups applied lotion twice daily on the target areas.
Clinical photographs of the patients were taken at baseline and monthly until 3 months. Side effects such as erythema, itching, burning sensation, ecchymosis, and skin atrophy were noted.
VASI score and BSA (in-hand units) were calculated at baseline and on every follow-up. The mean VASI score was calculated at baseline and then after 3 months to calculate the reduction in the same and assess improvement. Improvement of vitiligo was also scored using a quartile grading scale:
Grade 0 – No improvement
Grade 1: 1–25% repigmentation – minimal
Grade 2: 26–50% repigmentation – moderate
Grade 3: 51–75% repigmentation – good
Grade 4: >75% repigmentation – excellent.
All data were presented in mean and standard deviation. Further, analysis was done using parametric tests such as student t-test, analysis of variance (ANOVA), and Chi-square test. All qualitative data were presented in percentages. A non-parametric test was used to find the significance level between the two groups.
Treatment for Group A patients
After the application of topical anesthetic cream (lidocaine 70 mg + tetracaine 70 mg) over the selected patches for 45 min, patients were subjected to FrCO2 laser over the target lesions and the perilesional apparently healthy skin of about 1 cm. The laser settings were power of 6W,3 duration 2.5 ms, interval 1.0 ms, distance 0.8 mm, random mode, and square shape with 12 × 12 dimensions, after which the patient was advised to apply betamethasone dipropionate lotion 0.05% twice daily over patches and once daily for lesions over the face. The procedure was repeated at 1-month intervals for a total of three sessions. Patients were followed up monthly for a period of 3 months.
Treatment for Group B patients
Patients were advised to apply betamethasone dipropionate lotion 0.05% twice daily over the affected lesion over the body and once daily for lesions over the face. Patients were followed up monthly for a period of 3 months.
RESULTS
A total of 60 patients were included and treated throughout the study, with 30 in each group. The most common site to be affected was the lower extremities (33.33%), followed by the face and neck (23.33%) [Graph 1]. The average age of patients in the study was 27.32 (standard deviation [SD] = 13.76) years; 60% of the patients were female. 24 male patients and 36 female patients were enrolled and the mean age of the patients in the study was 27.32 (standard deviation [SD]=13.76) [Table 1]. Leukotrichia was seen in 15% of patients.

- Sites affected.
| Gender | n | Mean age | SD |
|---|---|---|---|
| Male | 24 | 26.88 | 13.317 |
| Female | 36 | 27.61 | 14.225 |
| Total | 60 | 27.32 | 13.76 |
SD: Standard deviation
Both treatment groups showed a reduction in mean VASI – in FrCO2 + topical corticosteroid (TCS) group, mean VASI reduced from 1.17 (SD = 0.59) to 0.60 (SD = 0.56), which was statistically significant (P = 0.001) and in TCS group mean VASI reduced from 0.87 (SD = 0.35) to 0.63 (SD = 0.50) which was also statistically significant (P = 0.003). However, no statistical difference between the two groups was observed (P = 0.173) [Tables 2 and 3].
| Treatment | VASI | n | Mean | SD | P-value |
|---|---|---|---|---|---|
| FrCO2+TCS | Baseline | 30 | 1.17 | 0.59 | 0.001 |
| 3 month | 30 | 0.60 | 0.56 | ||
| TCS | Baseline | 30 | 0.87 | 0.35 | 0.003 |
| 3 month | 30 | 0.63 | 0.50 |
SD: Standard deviation, VASI: Vitiligo area scoring index, FrCO2: Fractional CO2, TCS: Topical corticosteroid
| VASI | Treatment | n | Mean | SD | P-value |
|---|---|---|---|---|---|
| Baseline | FrCO2+TCS | 30 | 1.17 | 0.59 | 0.021 |
| TCS | 30 | 0.87 | 0.35 | ||
| 3 month | FrCO2+TCS | 30 | 0.60 | 0.56 | 0.808 |
| TCS | 30 | 0.63 | 0.50 |
SD: Standard deviation, VASI: Vitiligo area scoring index, FrCO2: Fractional CO2 , TCS: Topical corticosteroid
Percentage improvement in repigmentation was calculated using a quartile grading system in both treatment groups. It was observed that good to excellent repigmentation (51–75, >75%) was observed in 5 patients (16.67%) in FrCO2 + TCS group, as compared to 1 (3.33%) in TCS group, moderate response was observed in 9 (30%) in FrCO2 + TCS group as compared to 5 (16.67%) in TCS group. Minimal response was seen in 13 (43.33%) in the FrCO2 + TCS group as compared to 18 (60%) in TCS group. No improvement was seen in three and six patients, respectively [Graph 2, Figures 1-4, Table 4]. It was observed that patients with leukotrichia showed a poor response in both treatment modalities.
| Repigmentation % | FrCO2+TCS (%) | TCS (%) |
|---|---|---|
| 1–25 | 13 (43.33) | 18 (60.00) |
| 26–50 | 9 (30.00) | 5 (16.67) |
| 51–75 | 5 (16.67) | 0 (0.00) |
| >75 | 0 (0) | 1 (3.33) |
| No improvement | 3 (10.00) | 6 (20.00) |
| Total | 30 (100) | 30 (100) |
FrCO2: Fractional CO2, TCS: Topical corticosteroid
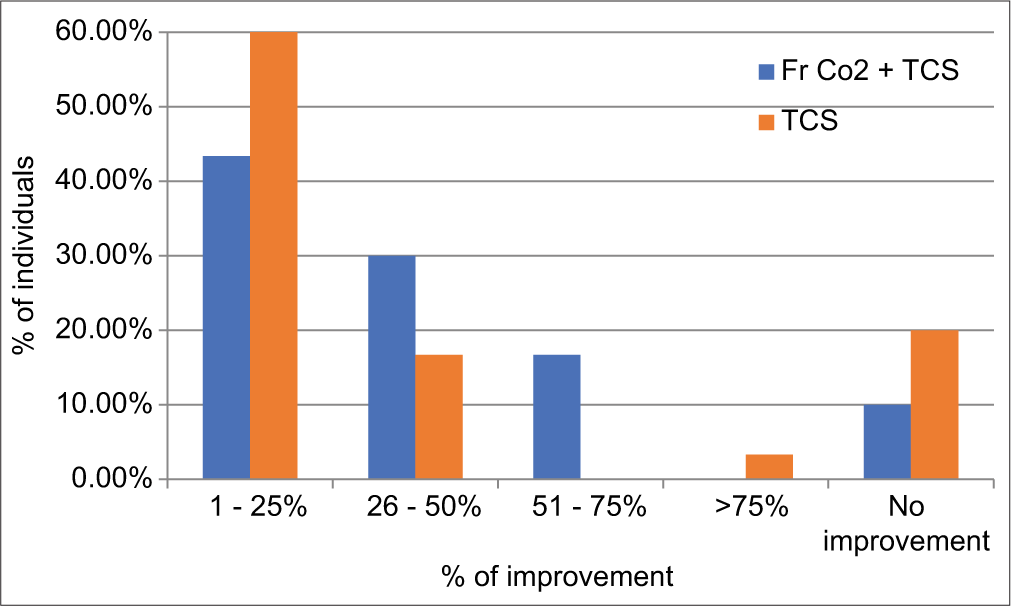
- Graph showing percentage of individuals showing minimal, moderate, good, and excellent response. FrCO2: Fractional CO2. TCS: Topical corticosteroid.
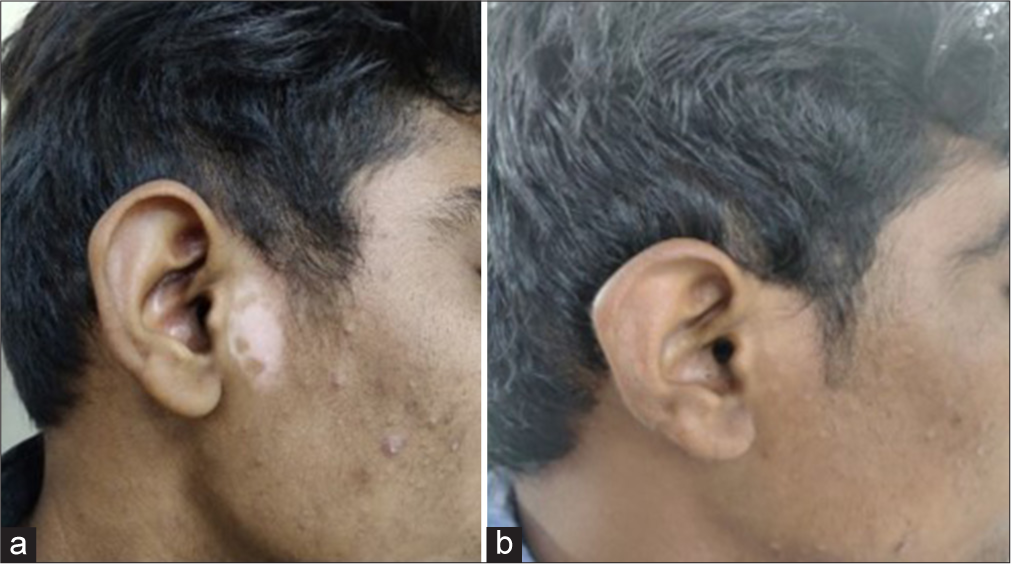
- Patient treated by fractional Co2 laser with potent topical corticosteroid - (a) baseline visit (b) at or after 3 months.
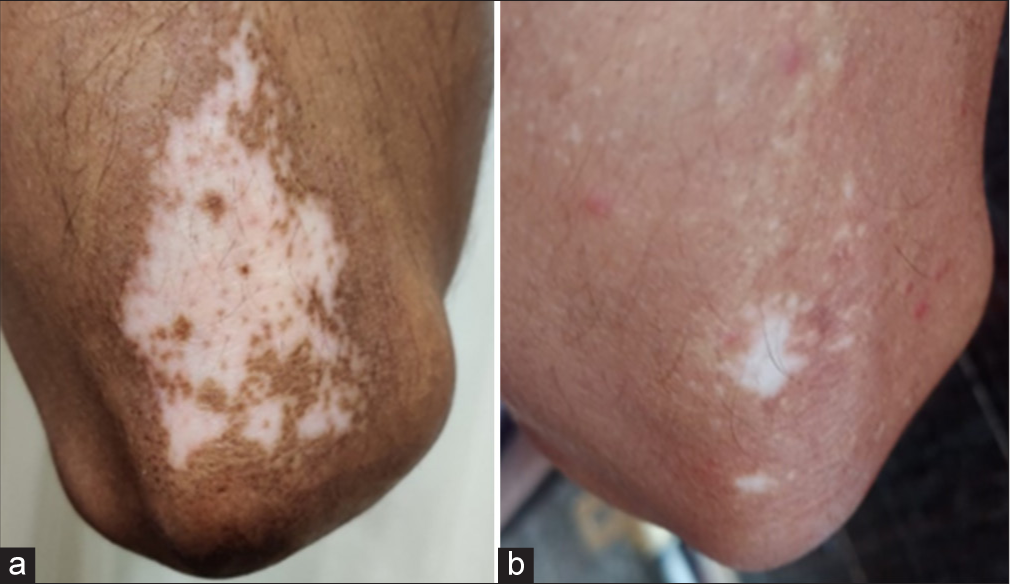
- Patient treated by fractional CO2 laser with potent topical corticosteroid - (a) baseline visit (b) at or after 3 months.
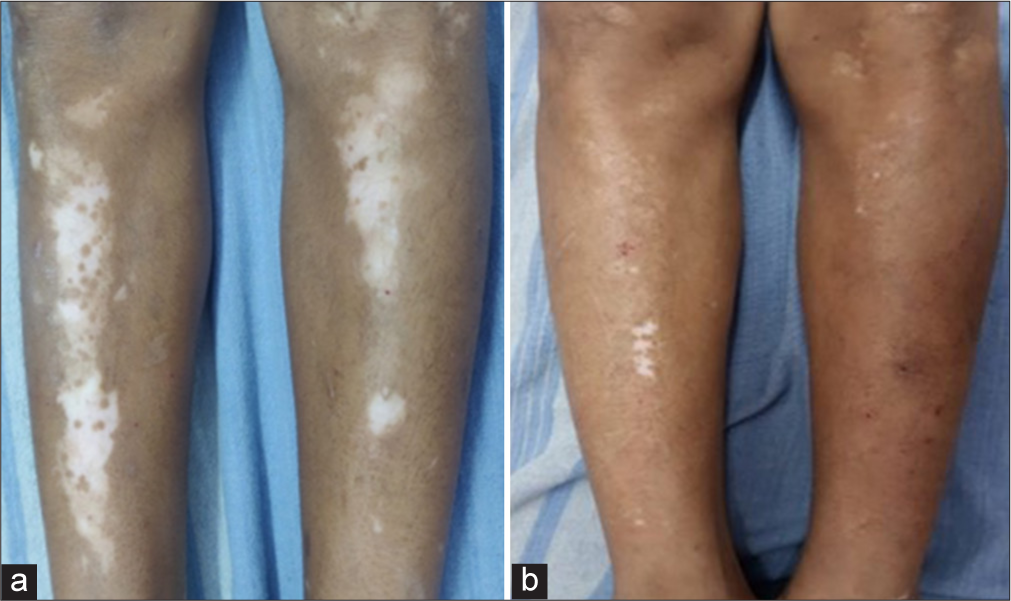
- Patient treated by potent topical corticosteroid alone - (a) baseline visit (b) at or after 3 months.
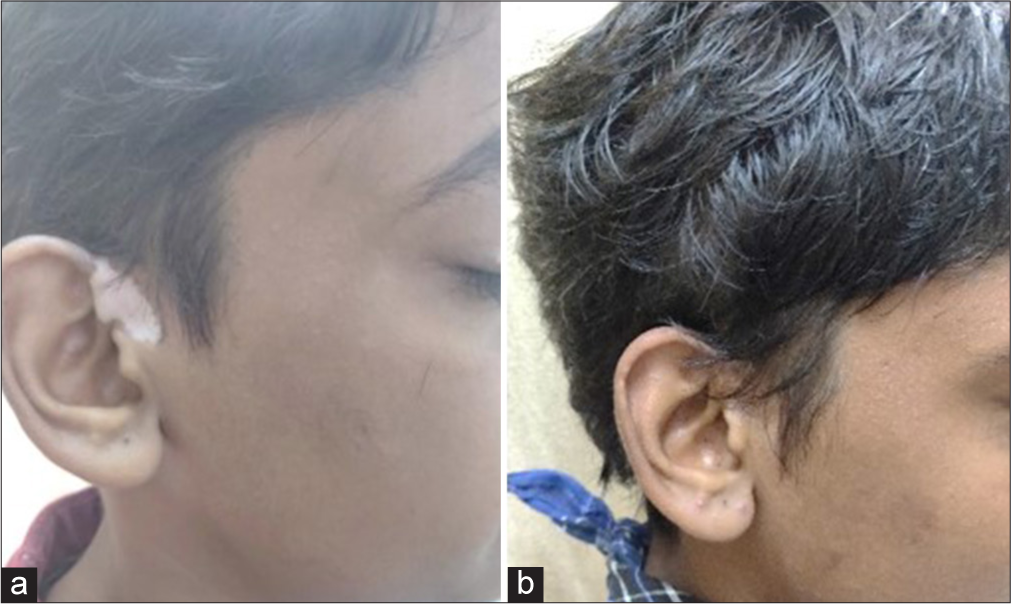
- Patient treated by potent topical corticosteroid alone - (a) baseline visit (b) at or after 3 months.
Side effects developed in 7 patients (23.33%) in the FrCO2 + TCS group as compared to 3 patients (10%) in the TCS group. The most commonly observed side effects were erythema (10% in FrCO2 + TCS and 3.33% in TCS group), acneiform eruptions (6.67% in FrCO2 + TCS and 3.33% in TCS group), telangiectasia (6.67% in FrCO2 + TCS), and striae and atrophy (3.33% in TCS group) [Table 5, Figures 5-7].
| Side Effect | Treatment | Total | P-value | |||
|---|---|---|---|---|---|---|
| FrCO2+TCS (%) | % | TCS | % | |||
| Acneiform eruption | 2 | 6.67 | 1 | 3.33 | 3 | 0.325 |
| Erythema | 3 | 10.00 | 1 | 3.33 | 4 | |
| Striae and atrophy | 0 | 0.00 | 1 | 3.33 | 1 | |
| Telangiectasia | 2 | 6.67 | 0 | 0.00 | 2 | |
| None | 23 | 76.67 | 27 | 90.00 | 50 | |
| Total | 30 | 100.00 | 30 | 100.00 | 60 | |
FrCO2: Fractional CO2 , TCS: Topical corticosteroid
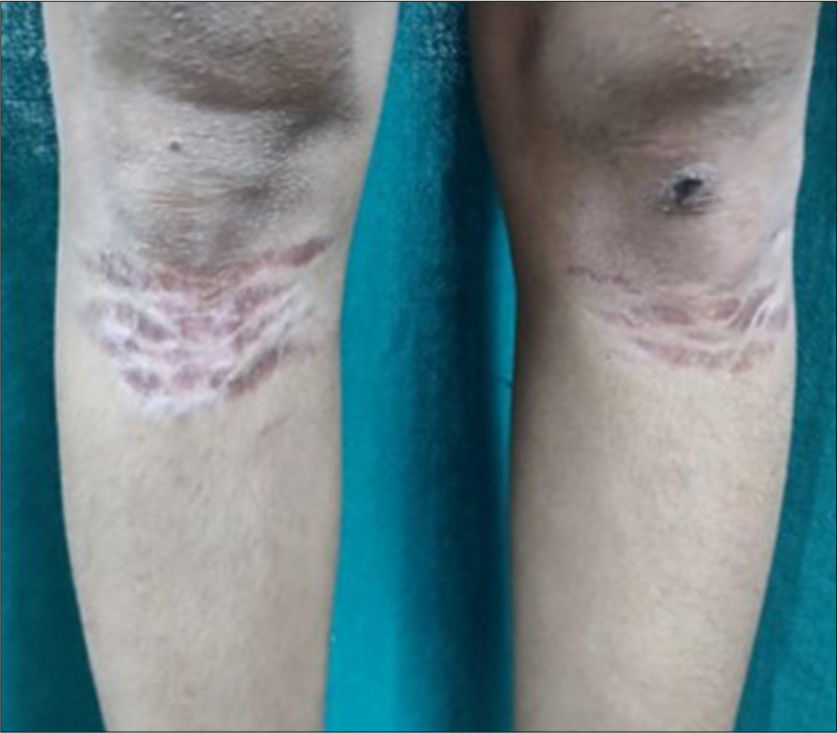
- Case 26/Female/14 years with striae and atrophy.
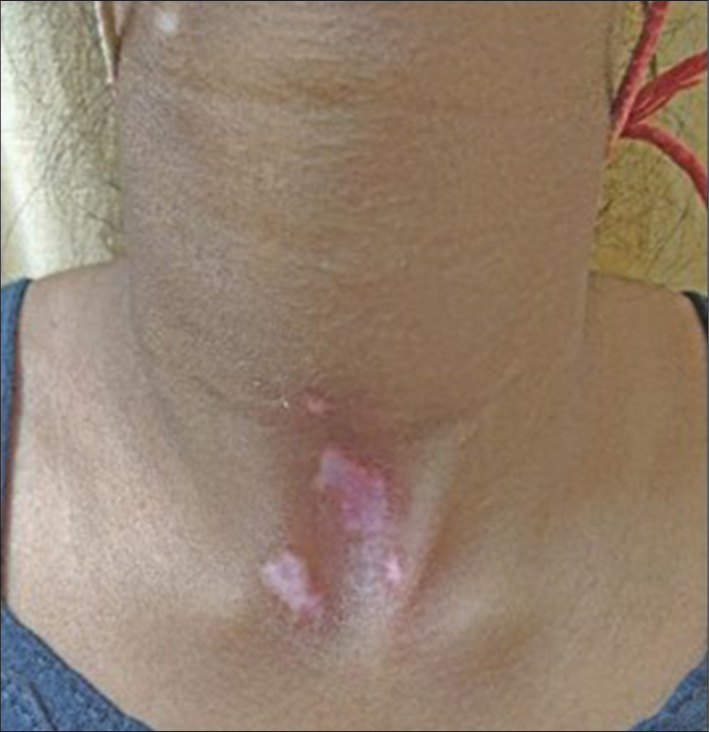
- Case 2/Female/14 years with erythema.
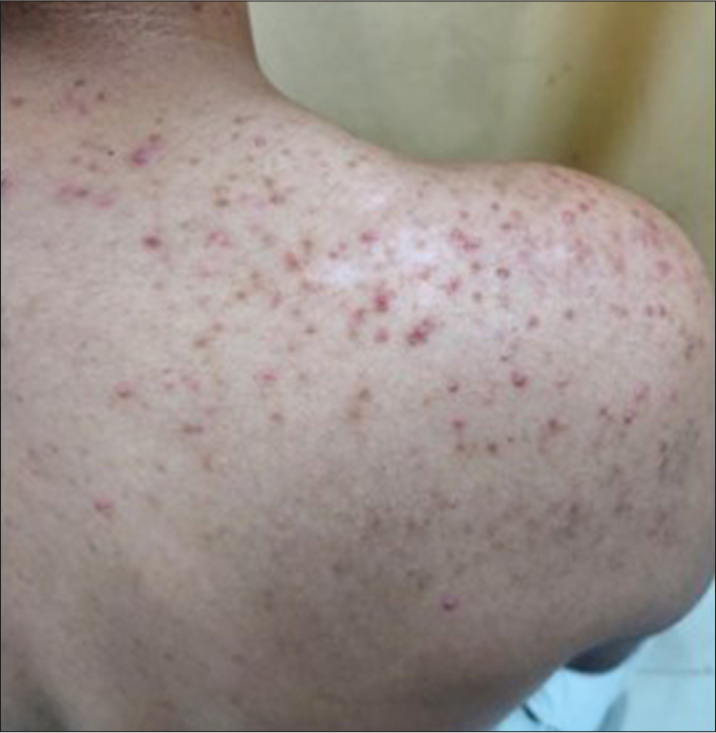
- Case 3/Male/25 years with acneiform eruption.
DISCUSSION
Our study shows that out of a total of 30 cases, none of the patients showed an excellent response; 5 patients (16.67%) showed good response, 9 (30%) showed moderate response, and 13 (43.33%) showed minimal response. No improvement was observed in 3 (10%) cases. Similar studies were carried out by Wen et al.,5 and Vachiroman et al.6 results of which were comparable to our study and suggested that the addition of FrCO2 laser with a conventional treatment modality improved the treatment outcome in patients to some extent. However, the conventional treatment modalities in their studies were different, with topical tacrolimus being used in the study by Wen et al.5 and narrowband ultraviolet B (NBUVB) and topical corticosteroid (clobetasol propionate) being used in the report by Vachiroman et al.6
Another observation in our study was that the reduction in mean VASI after 3 months was significant in both groups; however, no group achieved a significantly lower VASI score than the other. Overall, the reduction in mean VASI score for Group A was slightly better but not statistically significant, suggesting that the addition of FrCO2 may help in providing better outcomes. In a similar study conducted by Bakr et al.,7 where cases were allocated into three groups instead of two groups as in our study, it was observed that there was no statistical significance between Group A (FrCO2 laser + Topical tacrolimus 0.03% ointment) and Group B (FrCO2 laser + topical calcipotriol ointment); however, Group C (FrCO2 + Narrow Band UVB [NBUVB]) achieved significantly lower VASI score suggesting that combination of FrCO2 + NBUVB provided a better response as compared to other combination modalities.
Side effects such as acneiform eruption, erythema, and striae and atrophy were seen in a total of 3 patients (10%). These side effects were due to the use of topical corticosteroids at a higher frequency than what was explained to them. Given the paucity of similar studies with a high number of patients in previous case studies, there is insufficient data to curate a meaningful comparison of how our complication rate compares to the other authors.
Human skin behaves as a protective barrier, with physicochemical limitations to the amounts of topical drugs that can penetrate the cutaneous barrier.5 The therapeutic response of a topical drug depends on its ability to traverse the superficial layers of the skin and gain entry into the targeted cells. The major barrier to percutaneous absorption is passage across the stratum corneum. Various modalities have been introduced which can lead to the formation of channels or pores in skin, which enable few topical preparations to traverse through them and penetrate into the skin. The FrCO2 laser forms microscopic vertical canaliculi known as “microscopic treatment zones”, causing a fractional disruption of the epidermal barrier, allowing the penetration of certain topically applied drugs. As seen in the above tables, few studies showed improvement with the addition of FrCO2 laser to conventional topical treatments showed better outcomes, and few studies showed no superior repigmentation with the addition of FrCO2 laser. Going by these explanations, a superior repigmentation might have been expected; however, a few factors impede the absorption of topically applied steroids, such as post-treatment erythema, erosion, and oozing, which may last for 7–10 days. Microscopically, a perilesional zone of tissue necrosis around the microchannels may also hinder drug absorption.
Percentage improvement in repigmentation was calculated in both treatment groups. It was observed that good to excellent repigmentation (51–75, >75%) was observed in 5 patients (16.67%) in FrCO2 + TCS group, as compared to 1 (3.33%) in TCS group, moderate response was observed in 9 (30%) in FrCO2 + TCS group as compared to 5 (16.67%) in TCS group. Minimal response was seen in 13 (43.33%) in the FrCO2 + TCS group as compared to 18 (60%) in the TCS group. No improvement was seen in three and six patients, respectively.
Out of 60 patients, it was observed that 20 (33.33%) patients had lesions over the lower extremities making it the most common site affected in our study.
Limitation of study
The main limitation of the study is the small sample size of cases with vitiligo enrolled under potent topical corticosteroid with fractional CO2 laser group and potent topical corticosteroid group alone.
CONCLUSION
The addition of FrCO2 laser to topical corticosteroids offers a safe and promising approach in the treatment of patients with stable vitiligo. Studies with larger sample sizes and site-specific evaluation of each treatment modality can throw more light on the comparative efficacy of each modality.
Authors’ contributions
All authors participated in drafting the work or revising it critically for important intellectual content. Dr. Kishan Ninama: Conceptualization, formal analysis and investigation, manuscript revision, drafting of manuscript, intellectual revisions, mentorship, final approval. Dr. Rashmi Mahajan: Conceptualization, formal analysis and investigation, manuscript revision, drafting of manuscript, final approval. Dr. Disha Baxi: Drafting of manuscript, final approval. Dr Raveena Sanjay Patil: Formal analysis and investigation, drafting of manuscript, manuscript revision, intellectual revisions. Dr. Yogesh Marfatia: Conceptualization, formal analysis and investigation, drafting of manuscript, final approval.
Ethical approval
The research/study approved by the Sumandeep Vidyapeeth Institutional Ethics Committee, number SVIEC/ON/MEDI/BNPG19/D20023, dated 14th February 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Phototherapy in vitiligo: A comparative evaluation of various therapeutic modalities. J Egypt Women Dermatol Soc. 2012;9:123-35.
- [CrossRef] [Google Scholar]
- A randomized trial to evaluate the efficacy and safety of 1% pimecrolimus cream vs. 0.05% clobetasol propionate cream for the treatment of childhood vitiligo. J Dermatitis. 2018;3:2.
- [Google Scholar]
- Combined treatment with fractional carbon dioxide laser, autologous platelet-rich plasma, and narrow band ultraviolet B for vitiligo in different body sites: A prospective, randomized comparative trial. J Cosmet Dermatol. 2018;17:365-72.
- [CrossRef] [PubMed] [Google Scholar]
- Preliminary study on the treatment of vitiligo with carbon dioxide fractional laser together with tacrolimus. Lasers Surg Med. 2018;50:829-36.
- [CrossRef] [PubMed] [Google Scholar]
- A preliminary study of fractional CO2 laser added to topical tacrolimus combined with 308 nm excimer lamp for refractory vitiligo. Dermatol Ther. 2019;32:e12747.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of a preceding fractional carbon dioxide laser on the outcome of combined local narrowband ultraviolet B and topical steroids in patients with vitiligo in difficult-to-treat areas. Lasers Surg Med. 2016;48:197-202.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study on the use of Fractional CO2 laser with Tacrolimus or Calcipotriol or NB-UVB in treatment of stable non segmental vitiligo. Dermatol Ther. 2020;34:e14604.
- [CrossRef] [Google Scholar]






