Translate this page into:
A clinico-epidemiological study of different dermoscopic patterns in hyperpigmented facial lesions in a tertiary care centre
Address for correspondence: Dr. Vikas Solanki, Department of Dermatology, B. Y. L. Nair Charitable Hospital & Topiwala National Medical College, Mumbai 400008, Maharashtra, India. E-mail: solankivikas807@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction:
Facial pigmentation is a common presentation of patients attending dermatology out patient department (OPD) and is of great concern to patients. Facial pigmentation may be multifactorial and is only rarely diagnosed accurately by a detailed history and clinical examination. Pigmentary disorders cause psychological distress and negatively impact the quality of life of an individual.
Aims and Objectives:
(1) To study different dermoscopic patterns in facial melanosis. (2) To estimate the frequency of different dermoscopic patterns.
Materials and Methods:
Patients with facial hyperpigmentation attending the dermatology OPD were recruited after taking their written consent. A detailed history was taken to collect demographic data. Clinical examination and dermoscopy were done in all patients. Biopsy was done as and when required. Descriptive statistics has been used to describe the quantitative data. Qualitative data were presented as frequency and percentage for clinical and dermoscopic patterns.
Results:
The study included 100 patients with 15 different facial melanoses. The most common age group affected was 21–40 years in 53 (53%) cases. The female-to-male ratio was 1.63:1. Melasma was reported as the most common cause of facial melanosis constituting 49 (49%) of the total cases. Out of the total melasma cases, epidermal melasma constituted 22 (45%) cases, dermal melasma constituted four (4%) cases and mixed melasma constituted 23 (47%) cases. Other cases included were lichen planus pigmentosus (14; 14%), facial acanthosis nigricans (14; 14%), periorbital hyperpigmentation (7; 7%), post-inflammatory hyperpigmentation (4; 4%), exogenous ochronosis (2; 2%), lentigines (2; 2%), frictional melanosis (2;2%), and one case each of Becker’s nevus, nevus of Ota, olanzapine-induced hyperpigmentation, Riehl’s melanosis, macular amyloidosis, and tanning.
Conclusions:
Melasma was reported as the most common cause of facial melanosis. The most common dermoscopic feature was accentuated pseudopigment network. The study is beneficial in understanding the different clinical and dermoscopic patterns of facial melanosis, thus helping the physician to effectively manage the conditions and reduce the need of biopsy.
Limitations:
(1) A small sample size. (2) Histopathological correlation was not done in all cases.
Keywords
Dermoscopy
facial melanosis
melasma
INTRODUCTION
Dermoscopy is a non-invasive technique for observation and diagnosis of pigmented skin lesions. The improved visualization of surface and subsurface structures allows the recognition of morphologic features within the lesions that would not be detected otherwise.
Facial pigmentation is a common presentation of patients attending dermatology out patient department and is of great concern to patients. Facial pigmentation may be multifactorial and is only rarely diagnosed accurately by a detailed history and clinical examination. Multiple factors including genetic and racial factors play an important role. The common clinical presentations of facial pigmentation are melasma, Reihl’s melanosis, lichen planus pigmentatosus, nevus of Ota, peri-orbital hyperpigmentation, post-inflammatory hyperpigmentation, etc. Acquired dermal macular hyperpigmentation (ADMH) is an umbrella term that is clinically characterized by small and large pigmented macules/patches and histopathologically shows an evidence of current or resolved interface dermatitis with pigment incontinence, without clinically significant prior inflammatory phase. ADMH includes pigmented contact dermatitis, lichen planus pigmentosus, ashy dermatosis, and idiopathic macular eruptive pigmentation.[1] Among the facial pigmentary disorders, melasma is the most common and extensively studied and researched.
Pigmentary disorders cause psychological distress and negatively impact the quality of life of an individual.[2] Most facial pigmentation disorders are commoner in darker skin. Both light and photosensitizing chemicals play an important role. There are only few studies which correlate different clinical and dermoscopic patterns of facial melanosis.[34]
Aims and objectives
To study different dermoscopic patterns in facial melanosis.
To estimate the frequency of different dermoscopic patterns.
MATERIALS AND METHODS
This observational cross-sectional study was done in 100 patients over 18 months in the Department of Dermatology after approval by the institutional ethics committee. All patients presenting to dermatology with facial hyperpigmented lesions, belonging to all age groups and both sexes, were included in the study after taking written informed consent. Patients with other associated pathological/diseased states or deformities of the face were excluded from the study. A detailed history was taken regarding age, sex, complaints, duration, aggravating factors, past and family history were recorded. Cutaneous examination was done and clinical diagnosis was made. Biopsy was done in cases where definitive diagnosis could not be done, clinically and/or dermoscopically. Dermoscopy was done from the representative lesion using polarizing mode of universal serial bus 2.0 videodermatoscope at 50× and 200×. The criteria used while doing dermoscopy were the pigment pattern, pigment depth, vascular morphology and arrangement, and any other specific pattern diagnostic of a particular disease. Descriptive statistics have been used to describe the quantitative data. Qualitative data were presented as frequency and percentage for clinical and dermatoscopic patterns.
RESULTS
The study, conducted in dermatology department at a tertiary center included 100 patients presenting with facial hyperpigmented lesions belonging to all age groups and of both sexes. Thirty-eight (38%) patients belonged to age group 31–40 years followed by 23 (23%) in 21–30 years, 23 (23%) in 41–50 years, 10 (10%) in >50 years, and 6 (6%) in <20 years. Mean value of age (years) of study subjects was 36.72 ± 10.1 with median (25th–75th percentile) of 37 (30–44). Sixty-two (62%) were female patients and 38 (38.00%) were male patients. Majority [89 (89%)] of patients were asymptomatic followed by symptoms of itching [10 (10%)]; burning was present in a single patient. Among 23 patients with significant past medical or surgical history, hypothyroidism was found in 3 (13.04%) patients followed by chronic myeloid leukemia [2 (8.70%)], diabetes mellitus [2 (8.70%)], hypertension [2 (8.70%)], and rubbing [2 (8.70%)]. History of burn, chronic liver disease, dye application, gastric ulcer, HIV, pemphigus vulgaris, schizophrenia, seizure disorder and systemic lupus erythematosus were present in only one each out of 23 patients (4.35%).
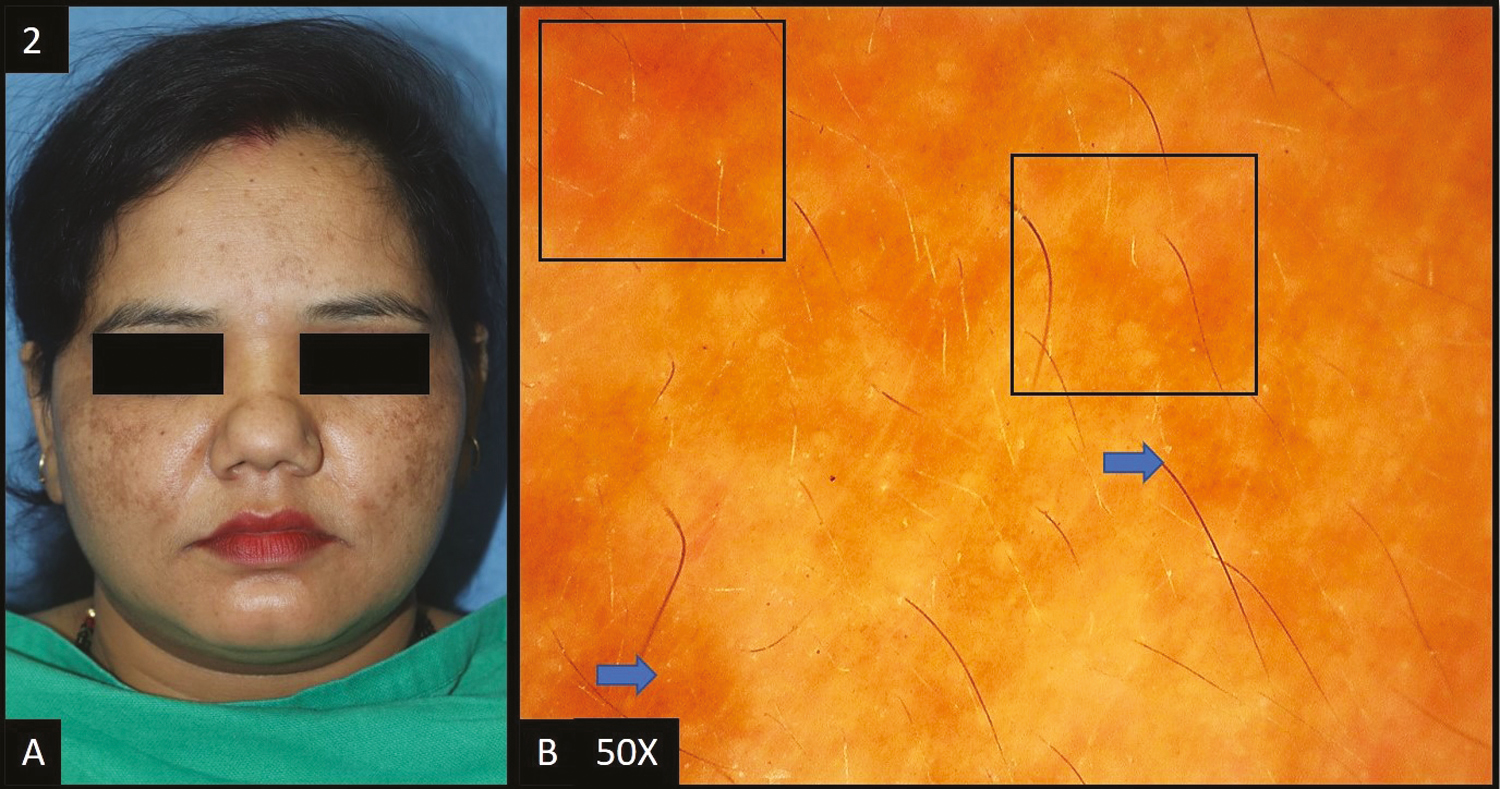
Melasma was reported as the most common cause of facial melanosis constituting 49% of the cases. History of prolonged photo exposure was present in nine (18%) cases of melasma. In two (4%) cases, the implicated agent causing melasma was imatinib. In our study, 12 cases (24%) gave a history of the application of triple combination and five cases (10%) gave a history of topical steroid use. Out of the total melasma cases, epidermal melasma [Figure 1A] constituted 22 (45%) cases, dermal melasma [Figure 2A] constituted four (8%) of cases and mixed melasma [Figure 3A] constituted 23 (47%) cases. In 21 (95.45%) out of 23 patients of epidermal melasma, dermoscopic features [Figure 1B] were accentuated pseudopigment network, brown dots/globules each followed by dark brown dots/globules in 19 (86.36%) patients, and telangiectasia in 11 (50%) patients. In all four patients, dermoscopic feature [Figure 2B] of dermal melasma was accentuated pseudo pigment network, light brown dots/globules each followed by brown dots/globules in 2 (50.00%) patients and telangiectasia in only one (25%) out of four patients. In all patients of mixed melasma, dermoscopic features [Figure 3B] were accentuated pseudopigment network followed by brown dots/globules in 22 (95.65%) cases, light brown dots/globules in 19 (82.61%) patients, telangiectasia in 15 (65.22%) patients, dark brown dots/globules in 4 (17.39%) cases and slate grey dots/globules in only 1 out of 23 patients (4.35%). Overall, accentuated pseudopigment network was the most common dermoscopic pattern seen in melasma. The second most common finding was brown dots and globules seen in 45 cases (92%) of melasma. Telangiectasia was present in 26 cases (53%) of melasma.
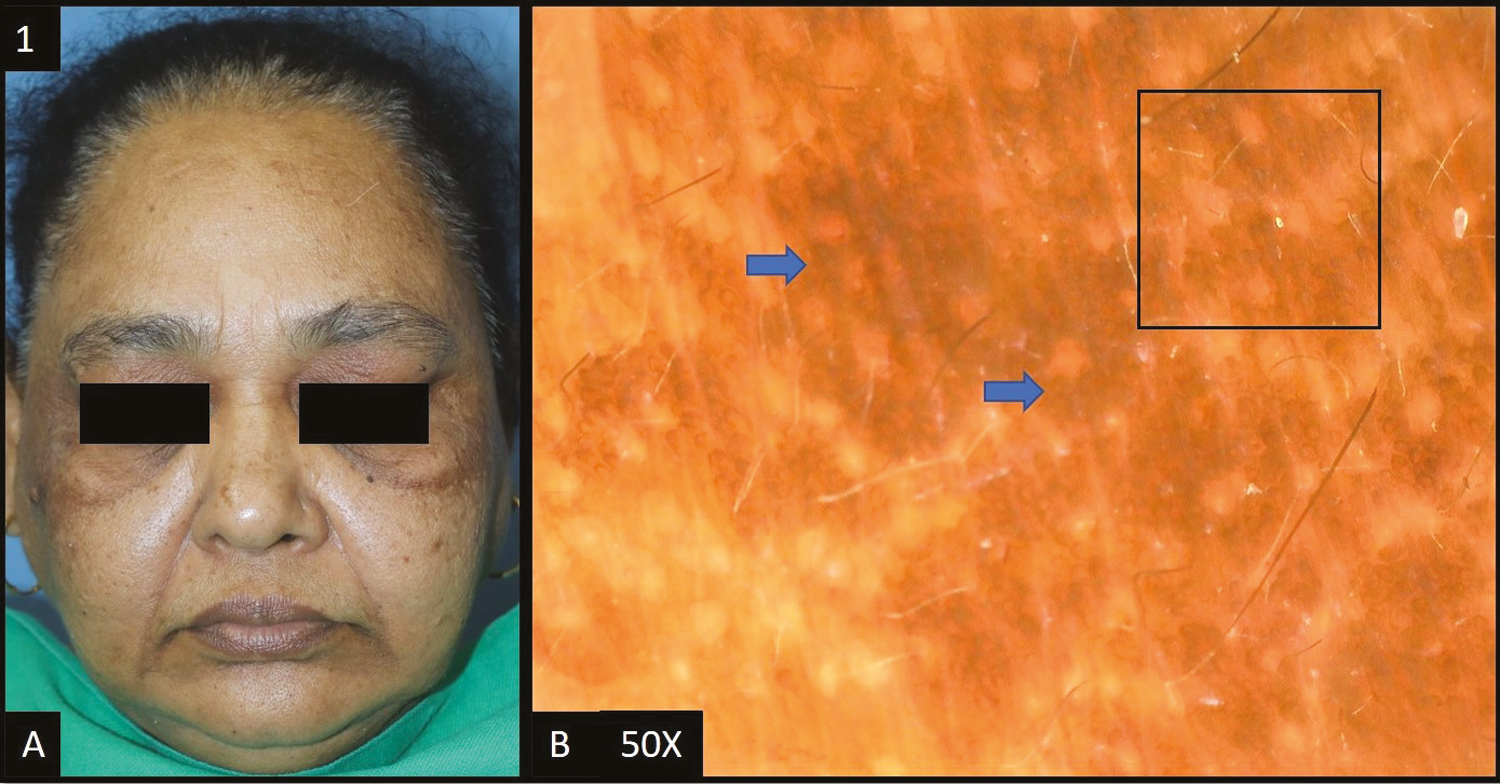
- (A) Epidermal melasma—multiple brown to dark brown hyperpigmented patches over bilateral malar and temple area of the face. (B) Dermoscopy (50×) shows accentuated pseudopigment network with perifollicular and perieccrine sparing (black square). Brown to dark brown dots and globules deposited in the pseudoreticular network (blue arrows)
- (A) Dermal melasma—multiple light brown hyperpigmented patches over bilateral malar area of the face. (B) Dermoscopy (50×) shows accentuated pseudopigment network with light brown colored dots and globules (blue arrows) and background erythema (dark blue squares)
- (A) Mixed melasma—multiple brown to dark brown, hyperpigmented patches over bilateral cheeks, nose and forehead. (B) Dermoscopy (50×) shows accentuated pseudopigment network (black square) with light brown to brown colored dots and globules (blue arrows) deposited in the network
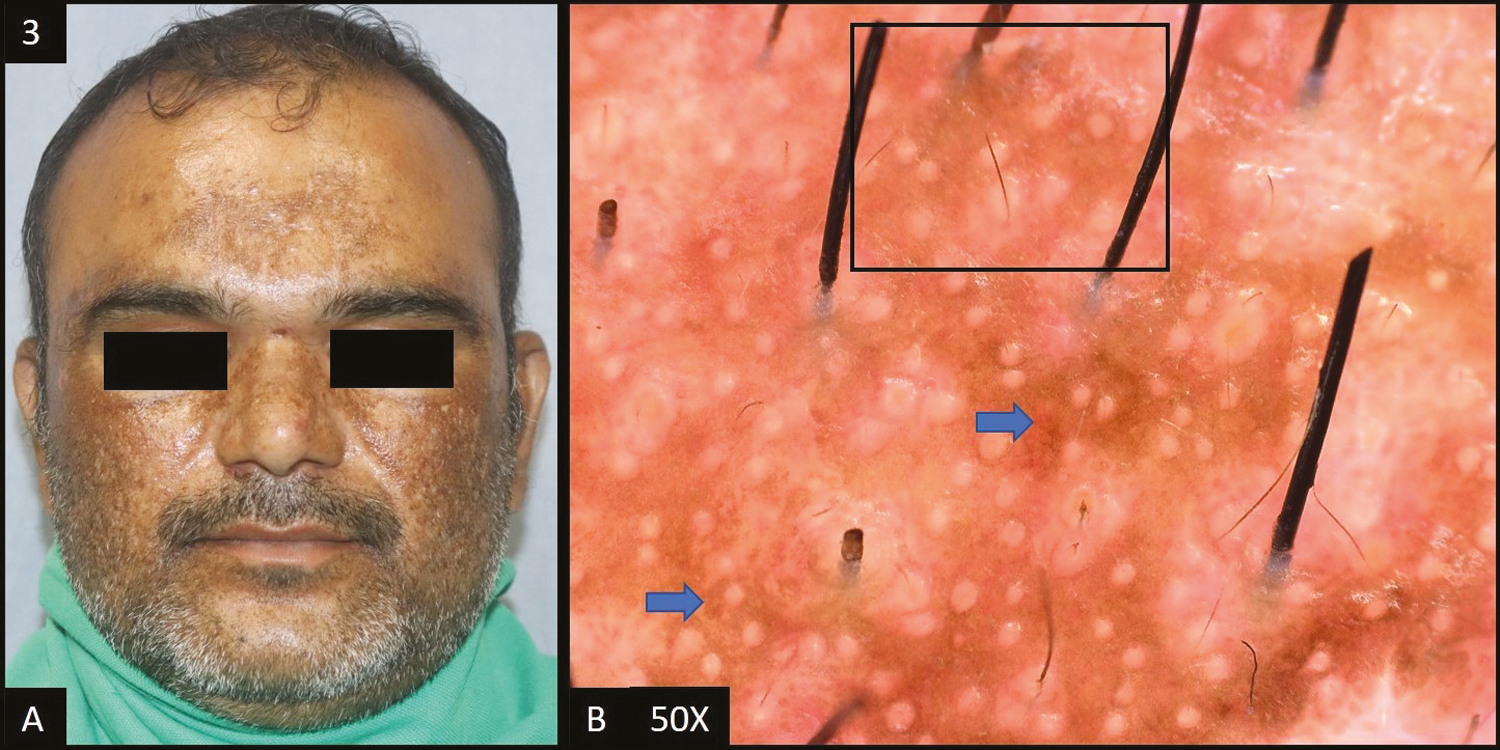
Facial acanthosis nigricans (FAN) was reported in 14 (14%) cases [Figure 4A].
- (A) Facial acanthosis nigricans—diffuse dark brown hyperpigmentation over forehead extending to temple area of the face. (B) Dermoscopy (50×) shows presence of crista sulci (yellow arrow) and crista gyri (blue arrow) with brown to dark brown colored dots and globules deposited in the network and dilated follicular openings (red arrows)
In 13 (93%) of patients, dermoscopic features [Figure 4B] of facial acanthosis nigricans were dark brown dots/globules followed by accentuated pseudo pigment network, brown dots/globules and sulcus cutis/crista cutis in nine (64%) cases each, telangiectasia in two (14%) cases, and slate grey dots/globules in 1 (7%) case.
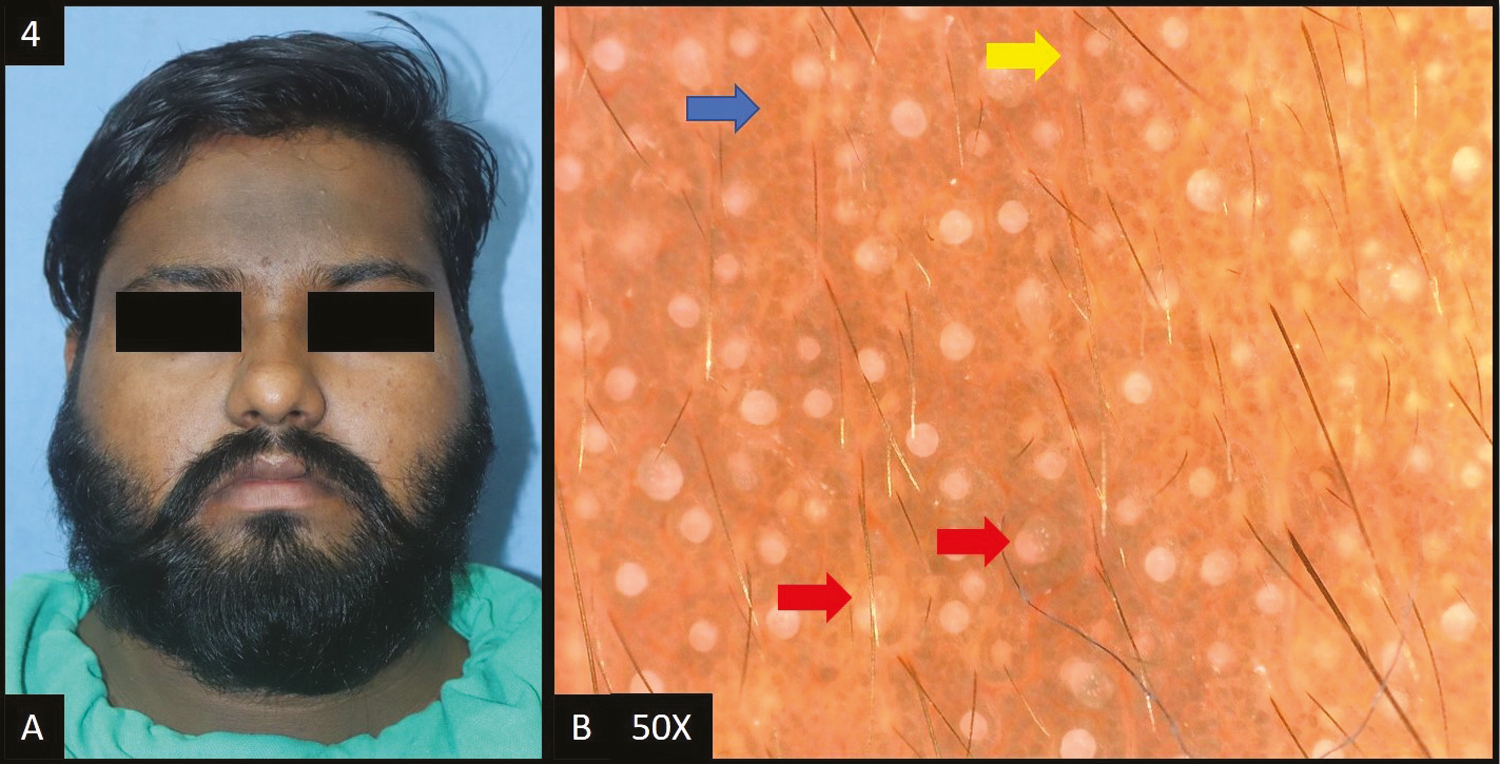
Lichen planus pigmentosus (LPP) was reported in 14 cases (14%) in this study. In all patients, dermoscopic features [Figure 5B] were accentuated pseudo pigment network, slate grey dots/globules followed by dark brown dots/globules in 12 (85%) patients, diffuse hem-like pattern in 11 (78%) patients, blotchy pattern in three (22%) patients and telangiectasia in six out of 14 patients (43%). Ashy dermatosis (AD) is a close differential diagnosis of LPP, both clinically as well as histologically. However, dermoscopically pigmented globules in LPP are more brownish and larger than those in AD, where they are more bluish gray and smaller.[5]
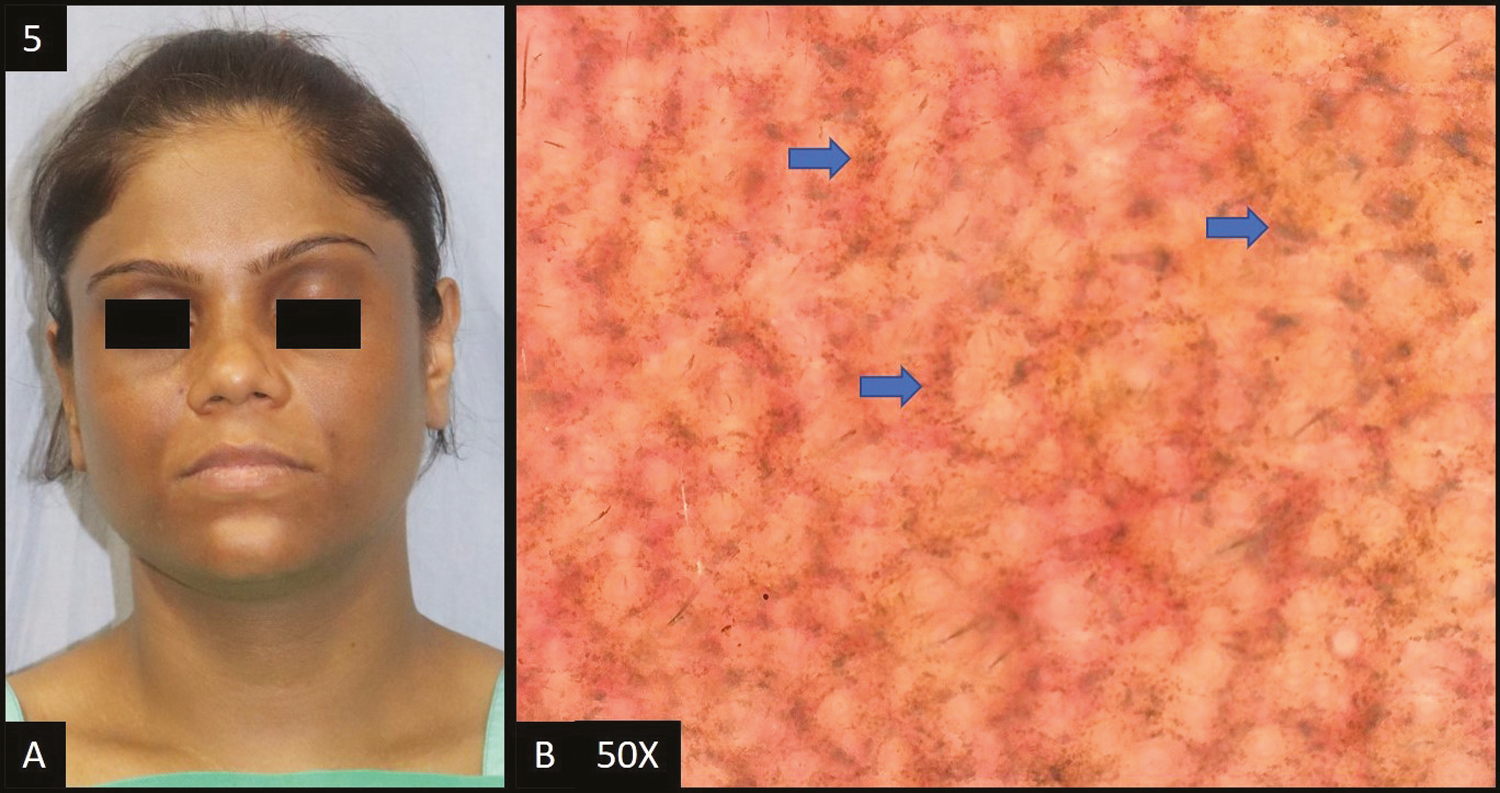
- (A) Lichen planus pigmentosus—diffuse slate grey hyperpigmentation over entire face and neck. (B) Dermoscopy (50×) shows accentuated pseudopigment network with slate grey dots deposited in the network giving a diffuse “hem-like” pattern (blue arrows)
Periorbital hyperpigmentation (POH) was present in 7 (7%) cases [Figure 6A].
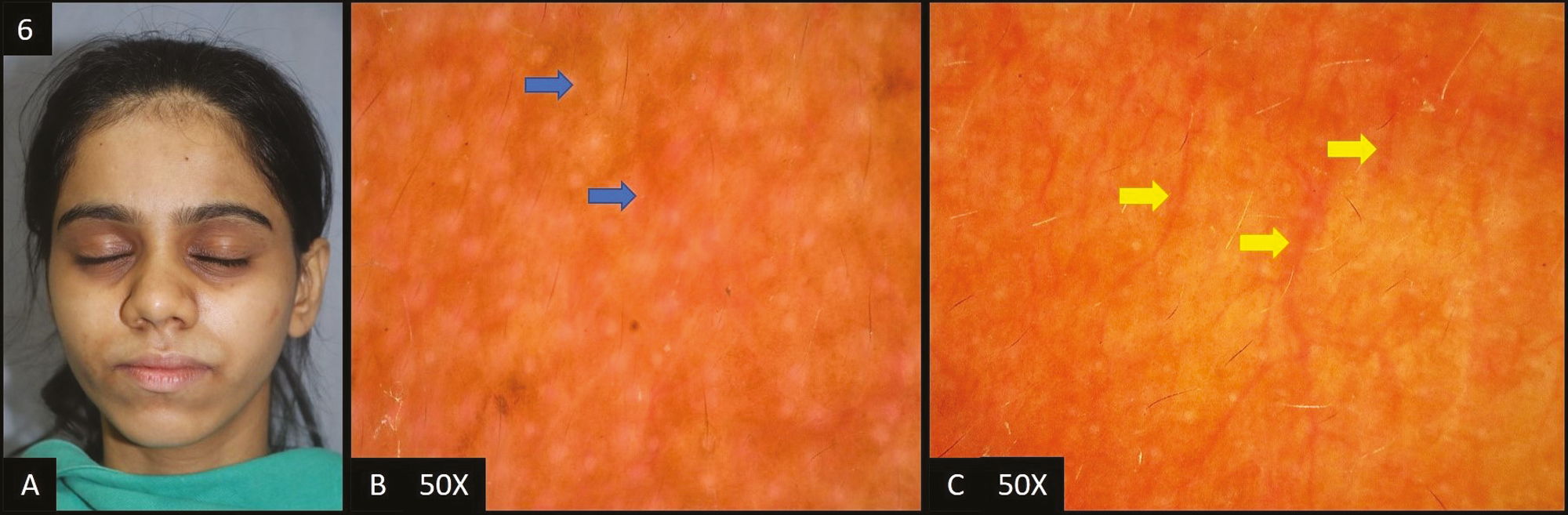
- (A) Periorbital hyperpigmentation—hyperpigmentation present in the periorbital area. (B) Pigmented type—dermoscopy (50×) shows brown colored dots and globules in the accentuated pseudopigment network (blue arrows). (C) Vascular type—dermoscopy (50×) shows diffuse vascular network (yellow arrows)
In all patients, dermoscopic features [Figure 6B and C] were brown dots/globules followed by accentuated pseudo pigment network and telangiectasia in 6 (85.71%) patients each, dark brown dots/globules in two (28.57%) patients and light brown dots/globules in only one out of seven patients (14.29%).
Post-inflammatory hyperpigmentation was seen in four cases (4%) [Figure 7A].
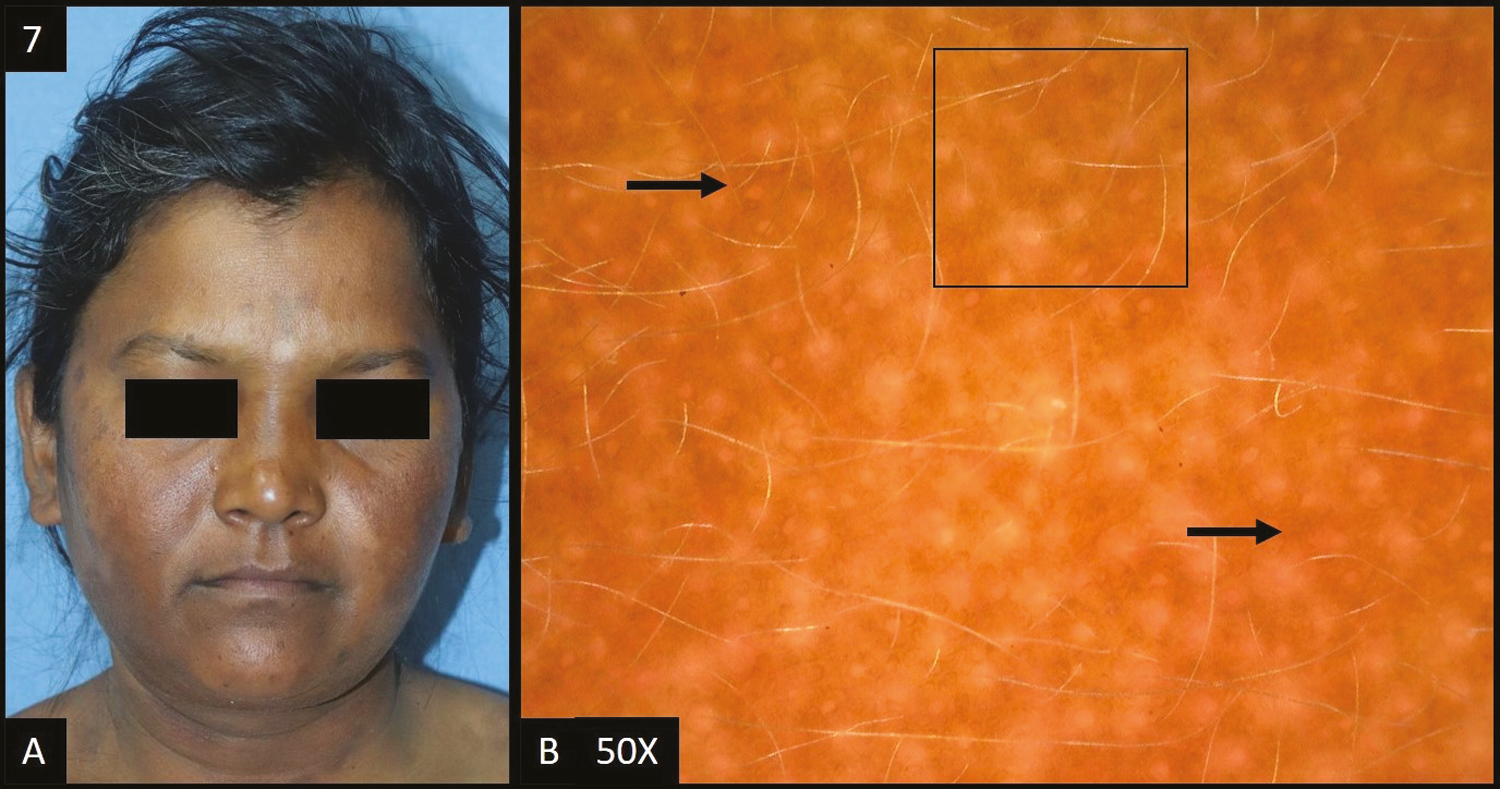
- (A) Post-inflammatory hyperpigmentation—multiple, well defined, brown to dark brown colored, hyperpigmented patches over both the cheeks and neck. (B) Dermoscopy (50×) shows brown to dark brown dots and globules (black arrows) deposited in an accentuated pseudopigment network with perifollicular and perieccrine sparing (black square)
In all patients, dermoscopic features [Figure 7B] were accentuated pseudopigment network and brown dots/globules each followed by dark brown dots/globules in three (75%) patients, telangiectasia in two (50%) patients, and light brown dots/globules in only one out of four patients (25%).
Exogenous ochronosis accounted for two of the total cases [Figure 8A]. In both patients, dermoscopy [Figure 8B] showed accentuated pseudo pigment network, dark brown to slate grey dots/globules, telangiectasia, worm-like pattern, pigment obliterating follicular openings.
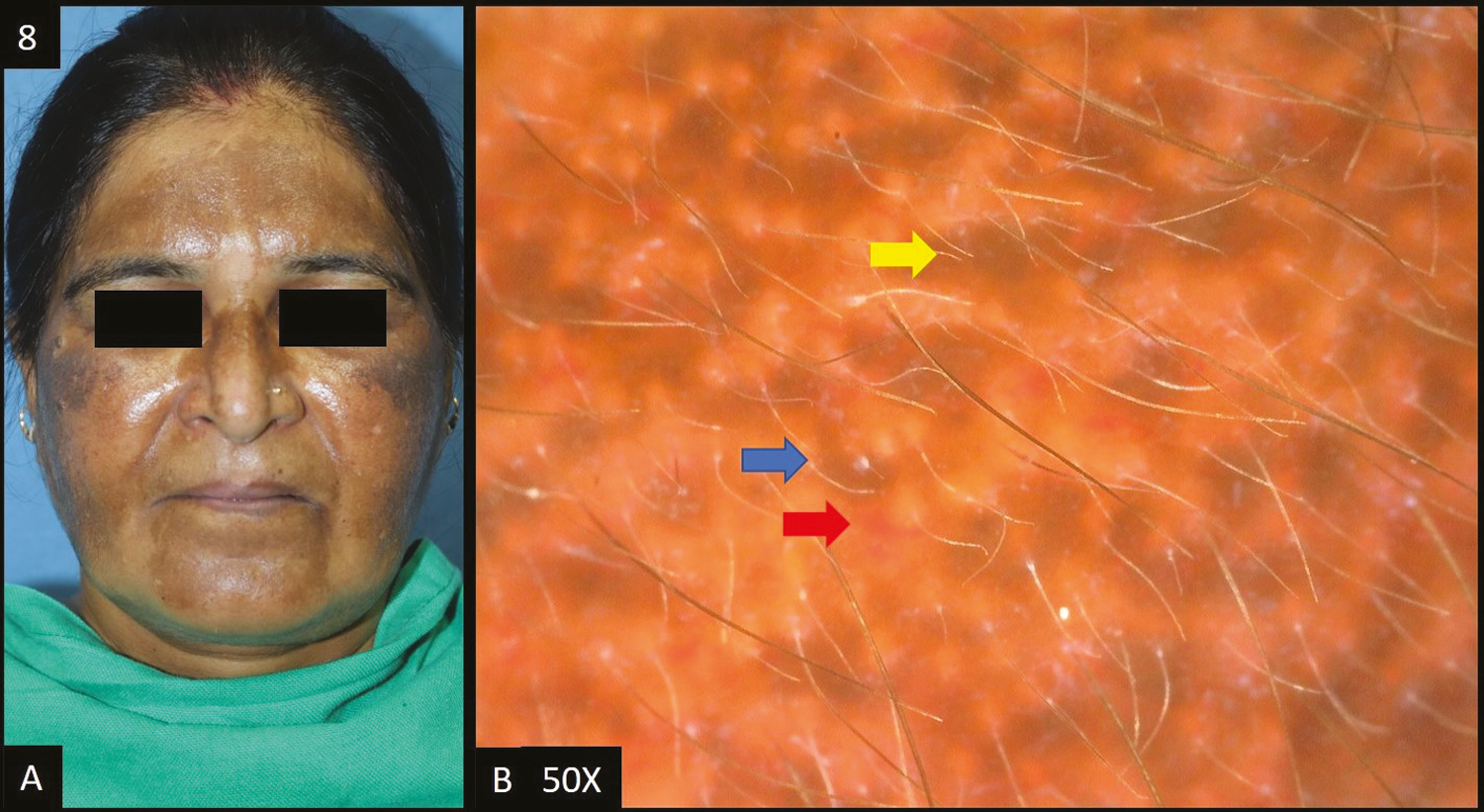
- (A) Exogenous ochronosis—multiple dark brown to black-brown hyperpigmented patches over bilateral malar area, forehead and nose. (B) Dermoscopy shows (50×) accentuated pseudopigment network, dark brown to slate grey dots and globules, telangiectasia (red arrow), worm-like pattern (blue arrow) and pigment obliterating follicular openings (yellow arrow)
Two cases of lentigines were reported in this study. In both patients, dermoscopy [Figure 9A] showed accentuated pseudopigment network, brown to dark brown dots/globules, and telangiectasia (50%) and speckled appearance (50%) was seen in one patient each.
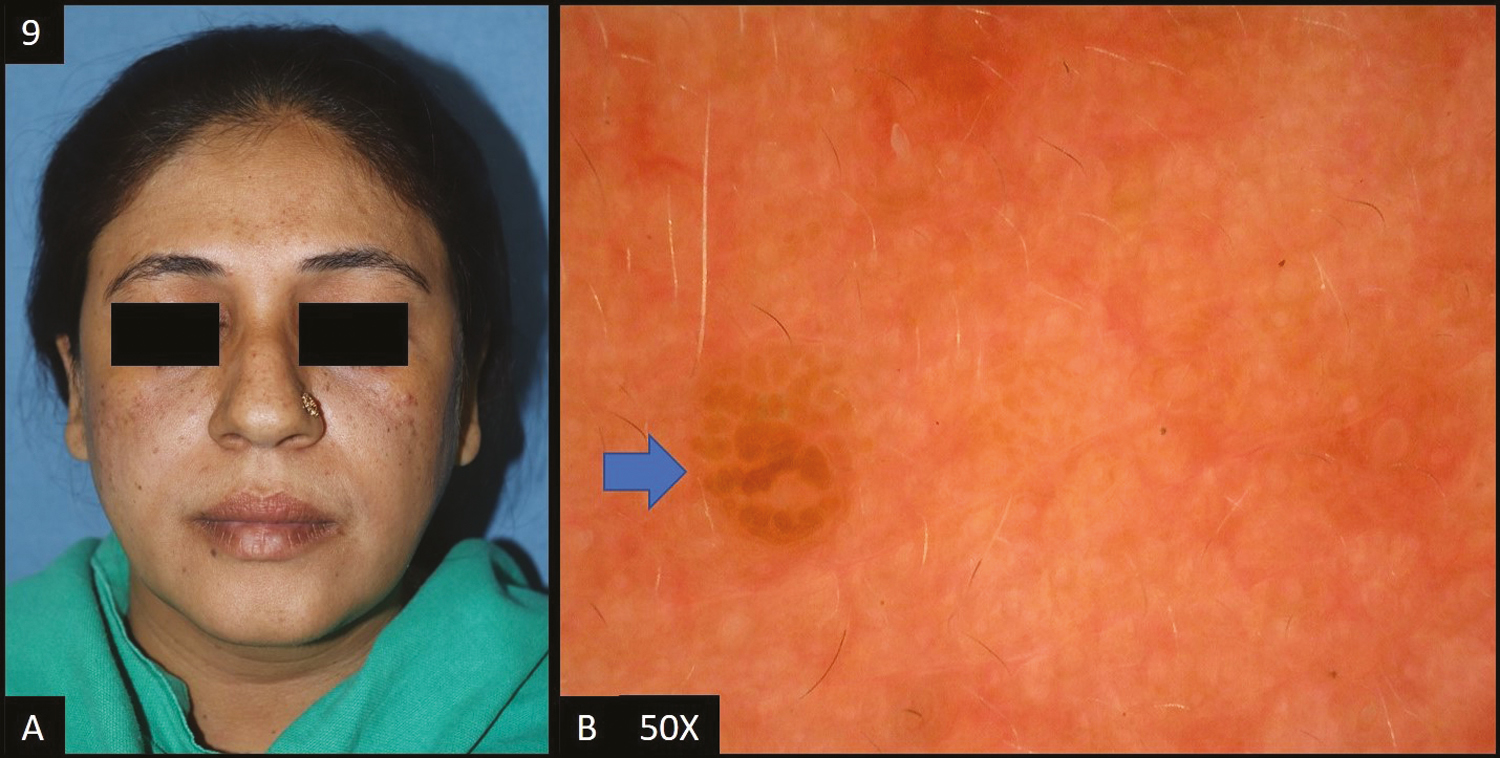
- (A) Lentigines—multiple brown colored macules over malar area, forehead and nose. (B) Dermoscopy (50×) shows brown to dark brown colored globules (blue arrow) with perieccrine sparing, focally
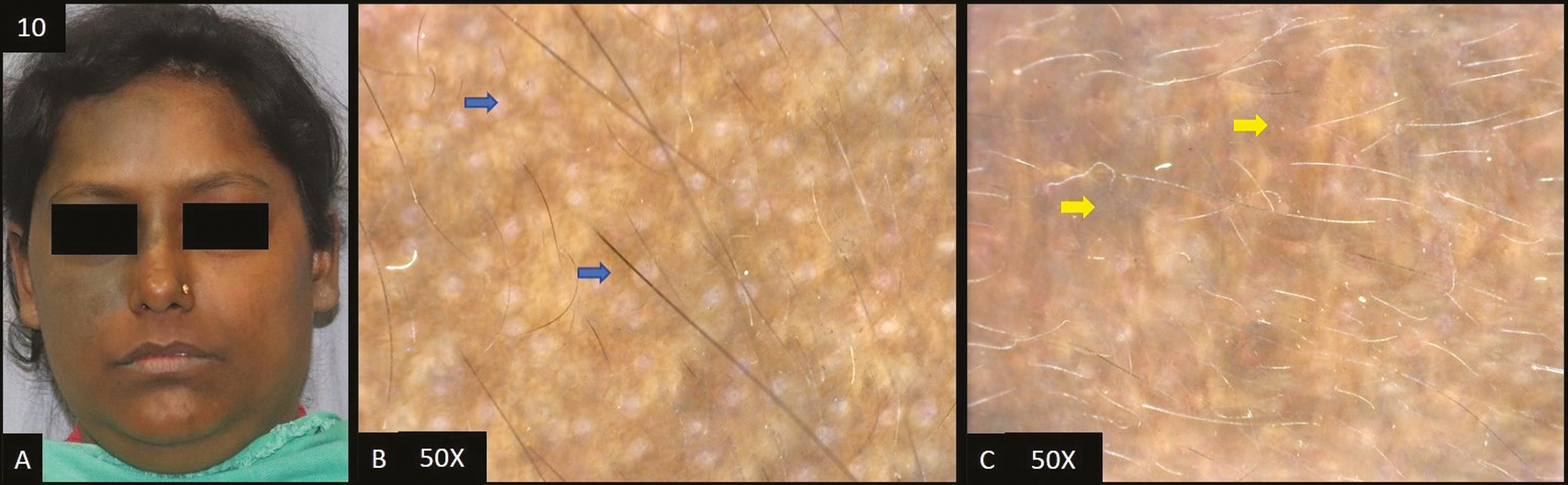
Two cases of frictional melanosis were studied [Figure 10A]. Dermoscopy [Figure 10B] revealed accentuated pseudopigment network in both patients followed by brown and dark brown dots/globules in only 1 out of 2 patients (50%) each.
- (A) Frictional melanosis—multiple brown to dark brown hyperpigmented patches over forehead and cheeks. (B) Dermoscopy (50×) shows brown to dark brown dots (blue arrow) deposited in perifollicular area with accentuated pseudopigment network
Other conditions of facial hyperpigmentation seen in few patients include nevus of Ota [Figure 11], Becker’s nevus [Figure 12], olanzapine-induced hyperpigmentation [Figure 13], Riehl’s melanosis [Figure 14], tanning [Figure 15] and macular amyloidosis [Figure 16], and their dermoscopic features are described in Table 1. Table 2 depicts the distribution of different dermoscopic patterns among study subjects in facial melanosis.
| Clinical diagnosis | Dermoscopy features | Frequency | Percentage |
|---|---|---|---|
| Acanthosis nigricans (n = 14) | Accentuated pseudo pigment network | 9 | 64.29% |
| Brown dots/globules | 9 | 64.29% | |
| Dark brown dots/globules | 13 | 92.86% | |
| Slate grey dots/globules | 1 | 7.14% | |
| Telangiectasia | 2 | 14.29% | |
| Sulcus cutis/Crista cutis | 9 | 64.29% | |
| Becker’s nevus (n = 1) | Accentuated pseudo pigment network | 1 | 100% |
| Brown dots/globules | 1 | 100% | |
| Dermal melasma (n = 4) | Accentuated pseudo pigment network | 4 | 100% |
| Brown dots/globules | 2 | 50% | |
| Light brown dots/globules | 4 | 100% | |
| Telangiectasia | 1 | 25% | |
| Epidermal melasma (n = 22) | Accentuated pseudo pigment network | 21 | 95.45% |
| Brown dots/globules | 21 | 95.45% | |
| Dark brown dots/globules | 19 | 86.36% | |
| Light brown dots/globules | 2 | 9.09% | |
| Telangiectasia | 11 | 50% | |
| White structureless areas | 1 | 4.55% | |
| Exogenous ochronosis (n = 2) | Accentuated pseudo pigment network | 2 | 100% |
| Dark brown dots/globules | 2 | 100% | |
| Slate grey dots/globules | 2 | 100% | |
| Telangiectasia | 2 | 100% | |
| Worm-like pattern | 2 | 100% | |
| Pigment obliterating follicular openings | 2 | 100% | |
| Frictional melanosis (n = 2) | Accentuated pseudo pigment network | 2 | 100% |
| Brown dots/globules | 1 | 50% | |
| Dark brown dots/globules | 1 | 50% | |
| Light brown dots/globules | 1 | 50% | |
| Slate grey dots/globules | 1 | 50% | |
| Lentigines (n = 2) | Accentuated pseudo pigment network | 2 | 100% |
| Brown dots/globules | 2 | 100% | |
| Dark brown dots/globules | 2 | 100% | |
| Telangiectasia | 2 | 100% | |
| Speckled appearance | 1 | 50% | |
| Lichen planus pigmentosus (n = 14) | Accentuated pseudo pigment network | 14 | 100% |
| Dark brown dots/globules | 12 | 85.71% | |
| Slate grey dots/globules | 14 | 100% | |
| Telangiectasia | 6 | 42.86% | |
| Diffuse hem-like pattern | 11 | 78.57% | |
| Blotchy pattern | 3 | 21.43% | |
| Macular amyloidosis (n = 1) | Accentuated pseudo pigment network | 1 | 100% |
| Dark brown dots/globules | 1 | 100% | |
| Mixed melasma (n = 23) | Accentuated pseudo pigment network | 23 | 100% |
| Brown dots/globules | 22 | 95.65% | |
| Dark brown dots/globules | 4 | 17.39% | |
| Light brown dots/globules | 19 | 82.61% | |
| Slate grey dots/globules | 1 | 4.35% | |
| Telangiectasia | 15 | 65.22% | |
| Worm-like pattern | 1 | 4.35% | |
| White structureless areas | 1 | 4.35% | |
| Nevus of Ota (n = 1) | Slate grey dots/globules | 1 | 100% |
| Worm-like pattern | 1 | 100% | |
| Dark brown to grey structureless areas | 1 | 100% | |
| Olanzapine induced hyperpigmentation (n = 1) | Accentuated pseudo pigment network | 1 | 100% |
| Brown dots/globules | 1 | 100% | |
| Dark brown dots/globules | 1 | 100% | |
| Periorbital hyperpigmentation (n = 7) | Accentuated pseudo pigment network | 6 | 85.71% |
| Brown dots/globules | 7 | 100% | |
| Dark brown dots/globules | 2 | 28.57% | |
| Light brown dots/globules | 1 | 14.29% | |
| Telangiectasia | 6 | 85.71% | |
| Speckled appearance | 1 | 14.29% | |
| Post-inflammatory hyperpigmentation (n = 4) | Accentuated pseudo pigment network | 4 | 100% |
| Brown dots/globules | 4 | 100% | |
| Dark brown dots/globules | 3 | 75% | |
| Light brown dots/globules | 1 | 25% | |
| Telangiectasia | 2 | 50% | |
| Riehl’s melanosis (n = 1) | Accentuated pseudo pigment network | 1 | 100% |
| Dark brown dots | 1 | 100% | |
| Slate grey dots | 1 | 100% | |
| Pigment obliterating follicular openings | 1 | 100% | |
| Follicular keratotic plugs | 1 | 100% | |
| Perifollicular whitish halo | 1 | 100% | |
| Scaling | 1 | 100% | |
| Tanning (n = 1) | Accentuated pseudo pigment network | 1 | 100% |
| Brown dots/globules | 1 | 100% | |
| Dark brown dots/globules | 1 | 100% |
| Dermoscopic features | Frequency | Percentage |
|---|---|---|
| Accentuated pseudopigment network | 92 | 92% |
| Brown dots/gobules | 71 | 71% |
| Dark brown dots/gobules | 62 | 62% |
| Light brown dots/gobules | 28 | 28% |
| Slate grey dots/gobules | 21 | 21% |
| Telangiectasia | 47 | 47% |
| Sulcus cutis/Crista cutis | 9 | 9% |
| Hem-like pattern | 11 | 11% |
| Worm-like pattern | 4 | 4% |
| Pigment obliterating follicular openings | 3 | 3% |
| White structureless areas | 2 | 2% |
| Dark brown to grey structureless areas | 1 | 1% |
| Speckled appearance | 2 | 2% |
| Follicular keratotic plugs | 1 | 1% |
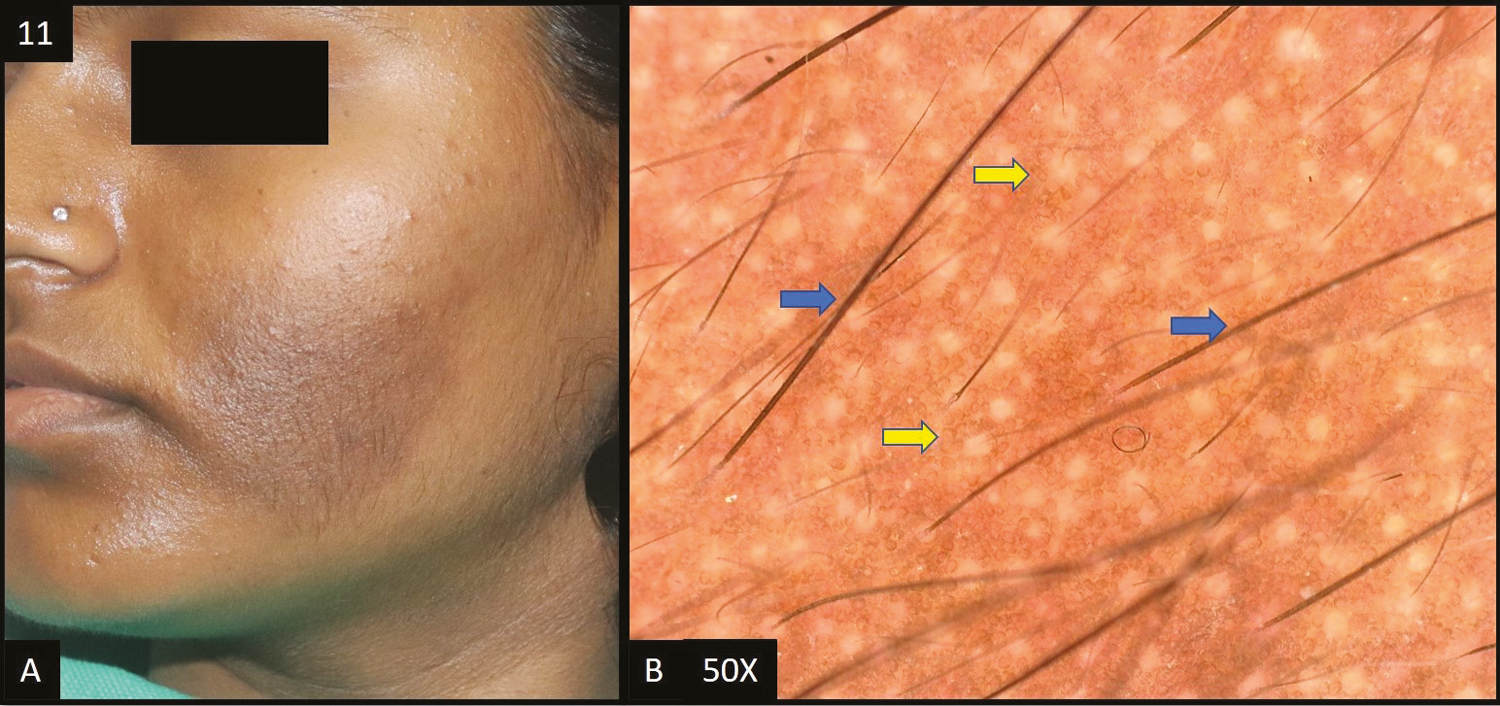
- (A) Nevus of Ota—single, well-defined slate grey colored hyperpigmented patch over right forehead, extending to right temple area, periorbital area, and right cheek. (B, C) Dermoscopy (50×) shows slate grey dots/globules, worm-like pattern (blue arrow) and dark brown to grey structureless areas (yellow areas)
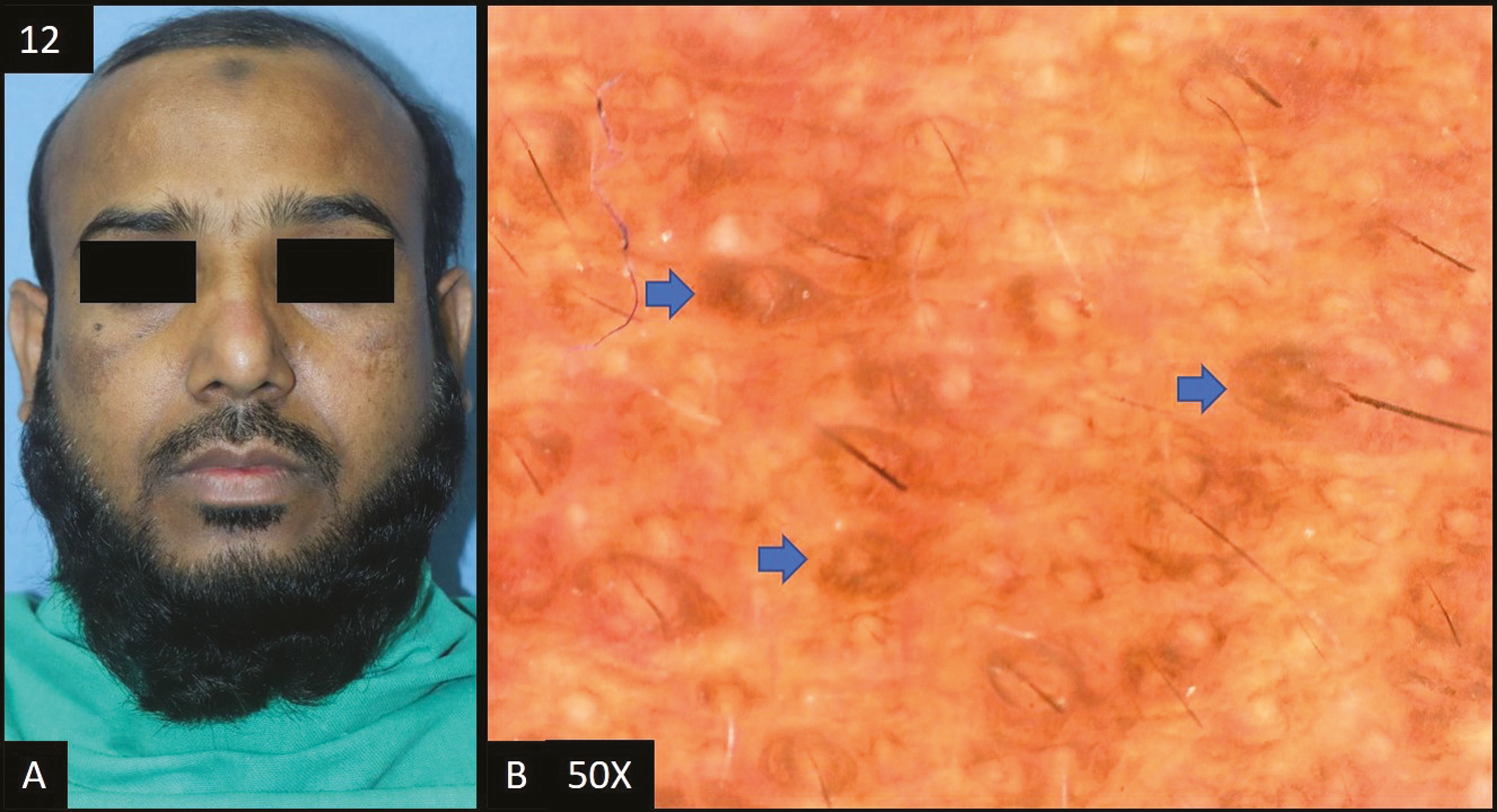
- (A) Becker’s nevus—single, well defined, hyperpigmented tan to brown patch with hypertrichosis. (B) Dermoscopy (50×) shows accentuated pseudopigment network with brown colored dots and globules (yellow arrows). It also shows increase number of terminal (blue arrows) and vellus hairs
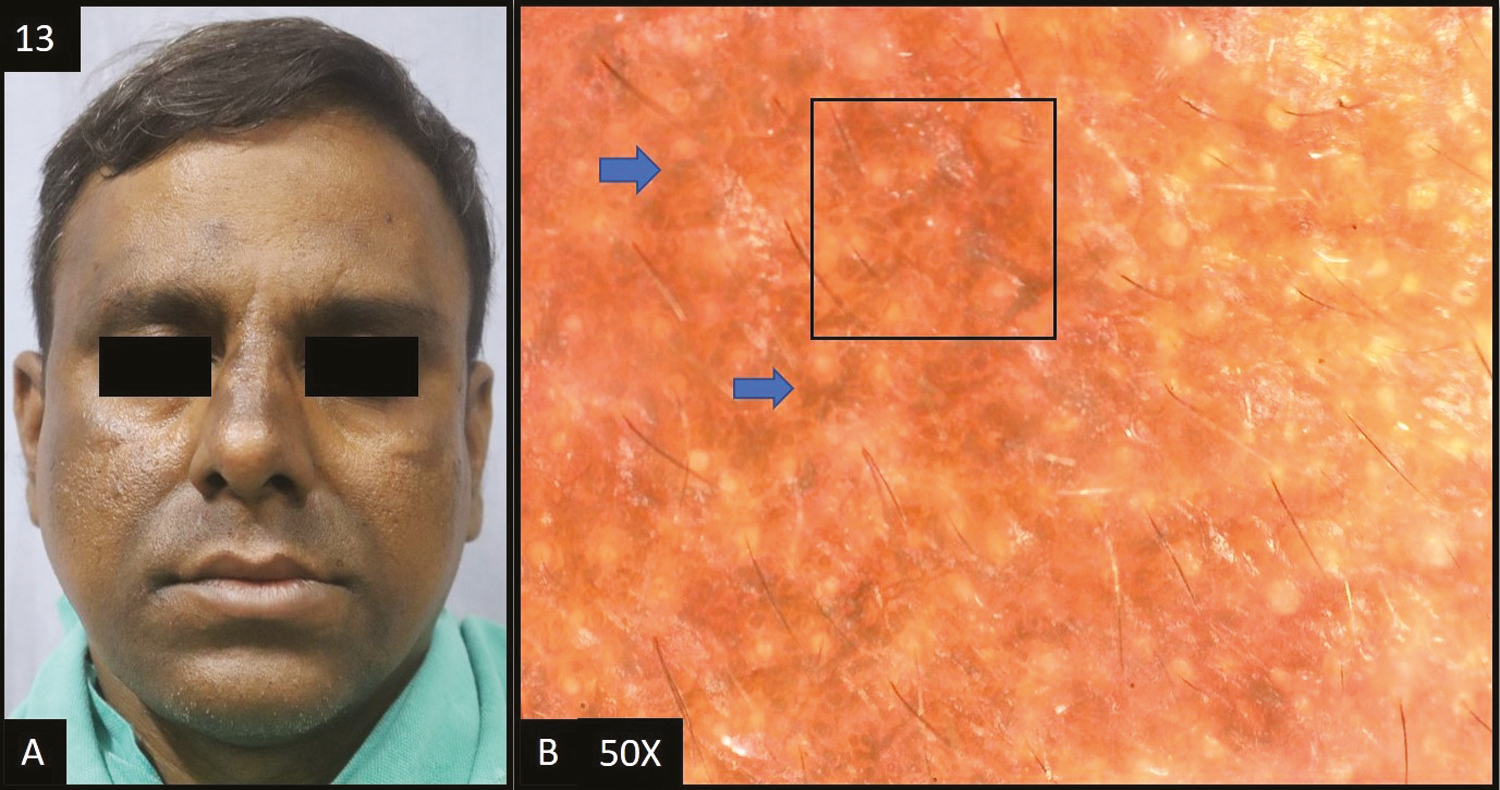
- (A) Olanzapine-induced hyperpigmentation—multiple dark brown hyperpigmented patches over forehead, nose, perioral and temple area of the face. (B) Dermoscopy (50×) shows brown to dark brown dots and globules (blue arrows) deposited in the accentuated pseudopigment network (black square)
![(A–C) Riehl’s melanosis—Diffuse dark brown to slate grey hyperpigmentation over the face more prominent on the lateral rather than the central area of the face. [D (50×), E (200×)] Dermoscopy shows dark brown to slate grey dots (blue arrow) deposited in the perifollicular area, perifollicular whitish halo (green arrow), pigment obliterating follicular openings (black arrow), follicular keratotic plugs (black circle) and slight scaling (blue circle)](/content/173/2024/17/2/img/JCAS-17-112-g014.png)
- (A–C) Riehl’s melanosis—Diffuse dark brown to slate grey hyperpigmentation over the face more prominent on the lateral rather than the central area of the face. [D (50×), E (200×)] Dermoscopy shows dark brown to slate grey dots (blue arrow) deposited in the perifollicular area, perifollicular whitish halo (green arrow), pigment obliterating follicular openings (black arrow), follicular keratotic plugs (black circle) and slight scaling (blue circle)
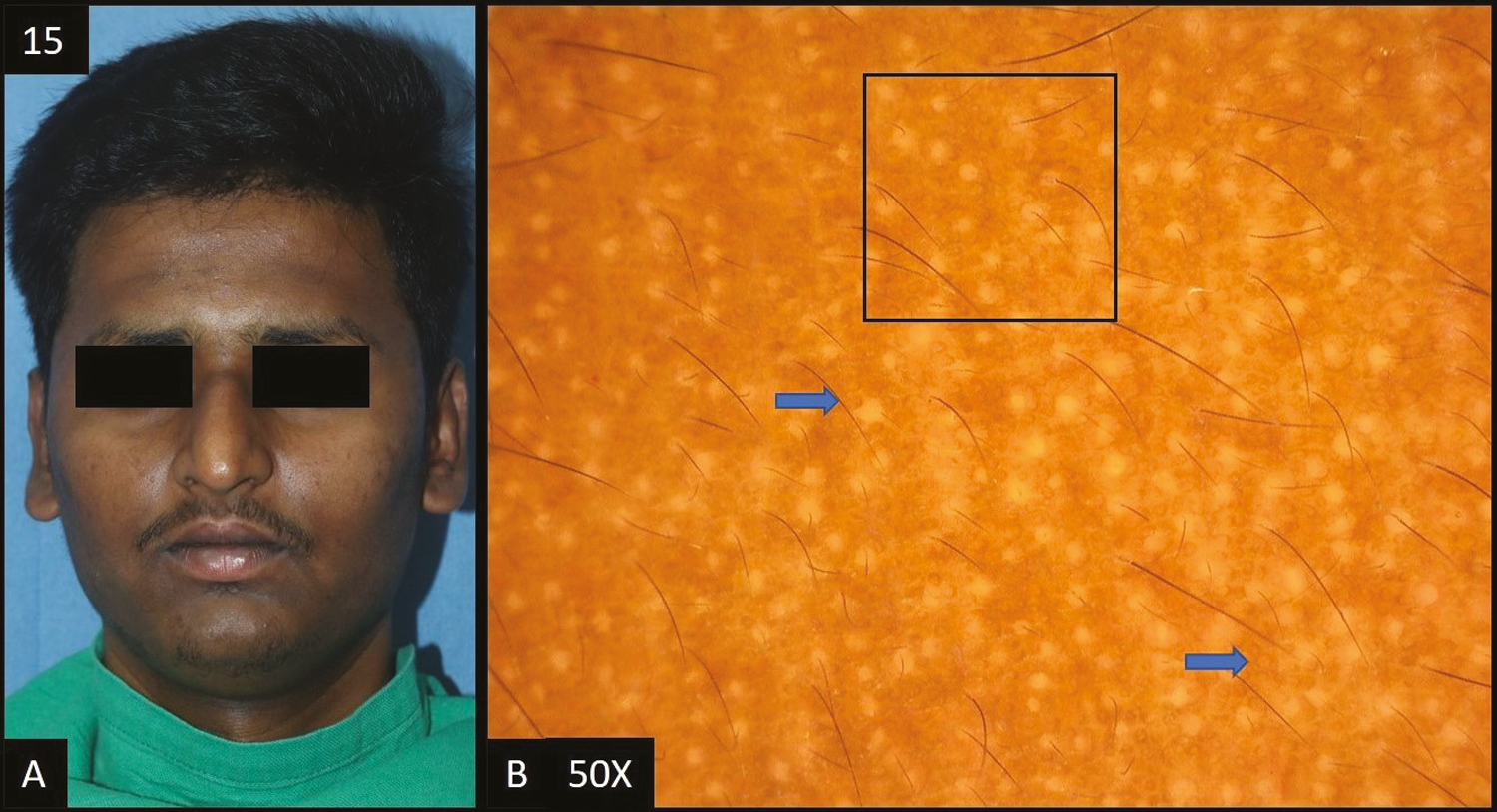
- (A) Tanning—diffuse, ill defined, dark brown hyperpigmentation over forehead extending to temple area of face. (B) Dermoscopy (50×) shows accentuated pseudopigment network (black square) with brown to dark brown dots and globules (blue arrows) deposited in the network
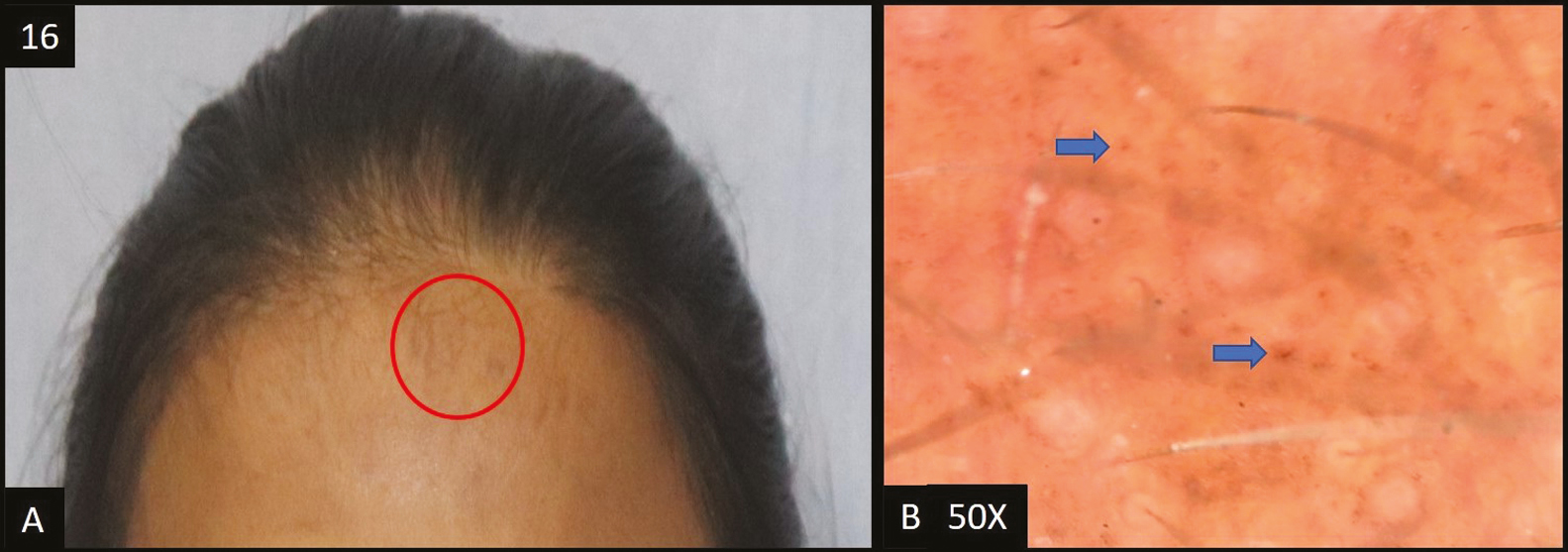
- (A) Macular amyloidosis—multiple hyperpigmented patches over forehead, shoulders and neck. (B) Dermoscopy (50×) multiple scattered brown dots (blue arrow) in the pseudopigment network
DISCUSSION
Facial melanosis is a group of heterogeneous entities, sharing a common clinical feature of altered pigmentation of the face and the easily visible cosmetic disfigurement has significant psychosocial consequences. Because of the increased patient awareness, greater use of cosmetics, and over-the-counter drugs, their incidence and importance are growing rapidly. It is a common presentation in Indian patients, with complex diagnostic and therapeutic problems consisting of well-defined clinical entities. Dermoscopy is a non-invasive technique combining digital photography and light microscopy for in vivo observation and diagnosis of pigmented skin lesions.
In our study, the most common age group affected was 21–40 years in 53% cases, similar to other studies.[6,7,8] Female patients accounted for 62 cases (62%) in our study, which was comparable with other similar studies.[8,9,10] The female-to-male ratio was 1.63:1 in this study. This was seen probably due to hormonal influences and female patients being more conscious about their appearance in comparison to males.
Melasma was reported as the most common cause of facial melanosis constituting 49% of the total cases, which correlated well with five previous studies, all of which concluded that melasma was the most common cause of facial hyperpigmentation.[6,7,8,9,10] Female preponderance was seen in this study, that is, 34 out of 49 cases (69%) and the female-to-male ratio was 2.26:1. Melasma is found to be more common in the age group of 31–40 years, that is, 21 out of 49 cases (43%) in this study which was consistent with the study conducted by Nanjundaswamy et al.[11] Thus, it is statistically proven that among the middle-aged women, the incidence of melasma is more, which was attributed to increased sun exposure, variations in internal estrogen hormone profile and age-related changes in dermis. History of prolonged photo exposure was present in nine out of 49 cases (18%) of melasma. In two cases, the implicated agent was imatinib. Out of the total melasma cases, epidermal melasma constituted 45% (22) cases, dermal melasma constituted 4% (4) of cases and mixed melasma constituted 47% (23) cases. In a study conducted by Nanjundaswamy et al.,[11] the epidermal pattern was seen in 46% cases, dermal melasma in 36% cases and mixed melasma was seen in 18% cases. This finding has therapeutic implications as mixed and dermal melasma are difficult to treat. However, histopathology will be required to exactly classify the type of melasma and dermoscopy remains an indicator till larger studies with dermoscopy and histopathological correlation are conducted.
Accentuated pseudopigment network was the most common dermoscopic pattern seen in all cases of dermal and mixed melasma, with 22 cases (95.45%) of epidermal melasma. Accentuated pseudopigment network was also found to be the most common dermoscopic pattern in other similar studies.[11,12,13] Second most common finding was brown dots and globules seen in 45 out of 49 cases (92%) of melasma. Telangiectasia was present in 26 out of 49 cases (53%). It is not a characteristic finding of melasma but the detection of telangiectasia on dermoscopy has therapeutic implications as it suggests steroid abuse or concomitant erythematotelangiectatic rosacea. In our study, 12 out of 49 cases (24%) gave a history of application of triple combination cream (hydroquinone, steroid, and retinoid) and five out of 49 cases (10%) gave a history of topical steroid use. Mahar et al.[14] showed 11.77% of patients misused topical corticosteroids whereas a study by Sendrasoa et al.[15] showed as high as 49.8% of patients had misused topical corticosteroids. It is important to perform dermoscopy in all patients of melasma, because many a times patients do not reveal the complete history and one can rapidly screen patients in whom triple combination needs to be avoided and thus, they can be educated regarding the misuse of topical steroids and the side effects of the same can be averted.
Lichen planus pigmentosus was reported in 14 cases (14%) in this study. Female cases [9 of 14 (64%)] outnumbered men [5 of 14 (36%)] with female-to-male ratio of 1.8:1 similar to Bhat et al. and Sindhura et al.[1617] Itching was present in eight out of 14 cases (57%) which correlated well with a study by Bhat et al., where itching was present in 50% of cases. Itching is considered by few authors to be a marker of activity and progression. Among the patients with LPP, two out of 14 patients (14%) were known cases of hypothyroidism. Neck was the most common site of extra facial involvement in LPP cases, present in four out of 14 cases (28%). On dermoscopy, accentuated pseudopigment network with perifollicular and peri-eccrine sparing was seen in all the 14 cases (100%). In LPP, pigmented dots and globules varies from slate grey to dark brown in color, among which slate grey dots and globules were seen in all the cases and dark brown colored dots and globules were seen in 12 of 14 cases (85%) in this study. Hem-like pattern was seen in 11 out of 14 cases (78%), which was comparable to 83.3% cases seen by Patel et al.[3]
FAN was reported in 14 cases (14%) in the current study. Male predilection was seen with 10 out of 14 cases being male, with male to female ratio of 2.5:1 which was similar to the study conducted by Verma et al.[18] Age group 21–40 years, was the most commonly affected age group constituting 11 out of 14 cases (78%). Two of them were known diabetics and were on treatment. Two of the 14 cases (14%) gave history of prolonged photo exposure which was comparable with study conducted by Verma et al.[18] Neck and axilla were the most common extra facial site of involvement in this study, both accounting for five cases (37%) each. On dermoscopy, dark brown dots and globules were seen in 13 of 14 cases (93%) and was the most common dermoscopic finding in cases of FAN. Sulcus cutis and crista cutis, which is a characteristic pattern in acanthosis nigricans, was seen in 9 out of 14 cases (64%). Other findings include accentuated pseudopigment network (64%), brown (64%) and slate grey (7%) colored dots and globules and telangiectasia (14%).
POH was present in seven cases (7%). All the cases were women in this study. Female preponderance was seen in other similar studies.[1920] In the present study, the most common age group was 21–40 years. History of atopic tendency was present in four out of seven cases (57%). POH in atopics can be understood by excessive rubbing and scratching of the periorbital region. The presence of brown dots and globules was the most common finding dermoscopically, seen in all the cases. Accentuated pseudopigment network and telangiectasia was present in six out of seven cases (86%) each. Light brown colored dots and globules was seen in one of 14 cases (14%) in this study.
Post-inflammatory hyperpigmentation was seen in four cases (4%). Burn, chickenpox, and pemphigus vulgaris were the causes of post inflammatory hyperpigmentation in three cases. On dermoscopy, the most common finding seen was accentuated pseudopigment network and brown dots and globules, seen in all the cases, followed by dark brown dots and globules in three of four cases (75%) and telangiectasia in two of four cases (50%).
Two cases of frictional melanosis were studied. A history of constant rubbing with handkerchief was present in both patients. In all patients, dermoscopy revealed accentuated pseudopigment network. Dermoscopy feature was brown dots/globules and dark brown dots/globules in only 1 out of 2 patients (50.00%) each.
Exogenous ochronosis accounted for two cases of the total cases. One patient applied hydroquinone 2% for two years duration intermittently and the other one applied triple combination cream consisting of hydroquinone, mometasone and tretinoin for a period of 15 months. Dermoscopy showed accentuated pseudo pigment network, dark brown to slate grey dots/globules, telangiectasia, worm-like pattern, pigment obliterating follicular openings.
Two cases of lentigines were reported in this study. In both patients, dermoscopy showed accentuated pseudopigment network, brown to dark brown dots/globules, and telangiectasia each. A speckled appearance was seen in one patient.
CONCLUSIONS
Facial hypermelanosis is a clinical manifestation of a wide spectrum of disorders. Most commonly seen in middle-aged women, the most common being melasma. Clinically, these spectrums of disorders have variable but overlapping features. Dermoscopy can be useful in differentiating various conditions causing facial melanosis. The study is very useful in understanding the various demographic details with different clinical and dermoscopic patterns of facial melanosis, thus helping the physician to effectively manage the conditions and reduce the need of biopsy.
Limitations
A larger sample size would have given results with greater validity. It is a single center hospital-based cross-sectional study, so observations which were seen in this study need not be the general trend in the population. Histopathological correlation was not done in all the cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Acquired dermal macular hyperpigmentation: An update. Indian Dermatol Online J. 2021;12:663-73.
- [Google Scholar]
- Pigmentary disorders, prevention, treatment and cosmetics contributions. In: Srinivas C, Verschoore M, eds. Basic Science for Modern Cosmetic Dermatology. New Delhi: Jaypee Brothers, Medical Publishers Pvt. Limited; 2015. p. :75-90.
- [Google Scholar]
- Clinico-dermatoscopic study of facial melanosis at a tertiary care hospital. Pigment Int. 2022;9:115-21.
- [Google Scholar]
- A single descriptive observational cross-sectional study of clinic-epidemiological characteristics of facial melanosis in a tertiary care centre. Indian J Appl Res. 2017;7:200-2.
- [Google Scholar]
- Dermoscopy and Trichoscopy in Diseases of the Brown Skin Atlas and Short Text (1st ed). New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2012. p. :60-1.
- Clinico-epidemiological study of facial hypermelanoses. Int J Res Dermatol. 2014;2:1621-6.
- [Google Scholar]
- Clinico-epidemiological study of facial hyper-melanoses among patients attending dermatology outpatient department at a tertiary care hospital at Pondicherry. Int J Res Dermatol. 2020;6:48-56.
- [Google Scholar]
- A study of dermatoscopic features in facial melanosis and its clinical co-relation—An observational study. Int J Dermatol Cosmetol. 2016;1:17-26.
- [Google Scholar]
- Study of clinical patterns of facial pigmentation. Int J Biomed Res. 2015;6:869-73.
- [Google Scholar]
- A clinic-dermoscopic study of melasma in a tertiary care center. Pigment Int. 2017;4:98-103.
- [Google Scholar]
- A clinic-dermatoscopic study of 100 cases of melasma in a tertiary care hospital. Int J Res Dermatol. 2018;4:41-5.
- [Google Scholar]
- Clinicoepidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- [Google Scholar]
- Topical corticosteroid misuse: The scenario in patients attending a tertiary care hospital in New Delhi. J Clin Diagn Res. 2016;10: FC16-20.
- [Google Scholar]
- Misuse of topical corticosteroids for cosmetic purpose in Antananarivo, Madagascar. Biomed Res Int. 2017;2017:9637083.
- [Google Scholar]
- Lichen planus pigmentosus: A retrospective clinic-epidemiologic study with emphasis on the rare follicular variant. J Eur Acad Dermatol Venereol. 2016;30:e142-4.
- [Google Scholar]
- Clinical, histopathological characteristics and immunohistochemical findings in lichen planus pigmentosus. Indian J Dermatol. 2017;62: 612-7.
- [Google Scholar]
- A descriptive study of facial acanthosis nigricans and its association with body mass index, waist circumference and insulin resistance using HOMA2 IR. Indian Dermatol Online J. 2016;7:498-503.
- [Google Scholar]
- Periorbital hyperpigmentation: A study of its prevalence, common causative factors and its association with personal habits and other disorders. Indian J Dermatol. 2014;59:151-7.
- [Google Scholar]
- Clinical and dermoscopic evaluation of periorbital hyperpigmentation. Indian J Dermatopathol Diagn Dermatol. 2018;5:42-7.
- [Google Scholar]






