Translate this page into:
A Clinicopathological Study to Assess the Role of Intralesional Sclerotherapy Following Propranolol Treatment in Infantile Hemangioma
Address for correspondence: Dr Preeti Tiwari, Department of Oral and Maxillofacial Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. E-mail: drtiwaripreeti@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Context:
As propranolol has emerged as first-line therapy for problematic infantile hemangioma, the number of non-responders and partial responders to propranolol therapy is also increasing.
Aims:
The study was conducted to evaluate the response of intralesional bleomycin, triamcinolone, and a combination of both as second line of treatment for the residual hemangioma following propranolol therapy
Settings and Design:
A prospective comparative study was conducted in patients who were either non-responders or partial responders to previous propranolol treatment.
Materials and Methods:
The patients randomly received injection bleomycin, injection triamcinolone, and combination of both bleomycin and triamcinolone. The response to treatment was recorded clinically by using photographs. The pathological response was assessed by calculating pre-treatment and post-treatment microvessel density in biopsy of lesion from the non-cosmetic sites using immunohistochemistry.
Statistical Analysis Used:
χ2 test was used to test the association between the variables. The utility of microvessel diameter (MVD) in terms of clinical response to the therapy was predicted by using receiver operating characteristic (ROC) curve.
Results:
Out of the 134 patients, 42 received bleomycin and 44 received triamcinolone and were treated with a combination of both. The overall clinical response was better in the combination group compared with the bleomycin group (P = 0.018) and triamcinolone group (P = 0.0005), respectively, after 6 months of follow-up. There was no difference in clinical response between the triamcinolone and bleomycin groups. Change in MVD correlated with the clinical response.
Conclusion:
The combination of bleomycin and triamcinolone is effective and safe for the treatment of residual hemangioma.
Keywords
Bleomycin
infantile hemangioma
sclerotherapy
triamcinolone
Intralesional sclerotherapy with a combination of bleomycin and triamcinolone sclerotherapy of residual hemangioma following propranolol therapy is safe and more effective than each of the agents alone. The modality can decrease the need of surgical excision of these lesions and associated anesthetic and surgical complications.

INTRODUCTION
Infantile hemangioma (IH) is the most common vascular tumor in children and is frequently encountered in clinical practice.[1] Parents of these children generally present with lot of apprehension. As most of these cases have a self-limiting course, a proper counseling is sufficient in majority of these.[2] Only children with problematic IHs, i.e. cases with the functional problems (like hampered vision or respiratory distress) or complications (like ulceration or bleeding), require some kind of intervention.[3] Diverse treatment modalities such as steroids, bleomycin sclerotherapy, vincristine, and LASER have been utilized in the past to manage these cases.[456] Out of these, propranolol has emerged as the first line of management of IHs following the accidental discovery of its efficacy in IHs.[78] As more and more cases are being managed by propranolol, the number of partial and non-responders to propranolol is also increasing.[9] This has prompted investigation for the search of the most effective second line of treatment. Intralesional therapy like triamcinolone and bleomycin has been found to be effective in different reports.[510] Studies have shown that the efficacy of one is more over the other, as the two are having different mechanisms of actions. We conducted this study with the purpose of evaluating the response of the combination of bleomycin and triamcinolone in children with residual hemangioma following propranolol therapy.
SUBJECTS AND METHODS
A prospective comparative study was conducted in the Department of Pediatric surgery in collaboration with the Oral surgery Department from September 2016 to December 2019. After ethical approval from the Institutional Review Board, all the children with problematic IH, who were either non-responders or partial responders to previous propranolol treatment with a refusal to surgical excision, were included in the study. All the cases were diagnosed clinically and on color Doppler. Non-responders were defined as patients who had received propranolol at a dose of 2–3 mg/kg body weight in three divided doses for at least 3 months and who had regression of less than 25%. Partial responders were defined as those who had taken propranolol for at least 6 months and had a clinical response between 25% and 50%. The patient’s guardians were informed about the nature of the procedure, expected number of treatments, and also expected side effect of the procedure. Written consent was taken from parents before inclusion in the study. The patients were randomly divided into three groups with the help of a computer-generated random number table: Group A: received injection bleomycin, Group B: received injection triamcinolone and Group C: received a combination of both bleomycin and triamcinolone.
Group A received intralesional bleomycin at a dose of 0.5 IU/kg (maximum of 10 IU in a single dose), Group B received intralesional triamcinolone at a dose 2 mg/kg (maximum of 60 mg in a single dose), and Group C received an intralesional injection of bleomycin (0.5 IU/kg weight) followed by intralesional injection of triamcinolone (2 mg/kg), repeated after 4 weeks on an outpatient basis. The calculated dose was diluted to 1–1.5 mL. A 30-gauge needle was used. The needle was inserted from the edge of lesion toward the center; the drug was instilled while the needle was withdrawn. The same procedure was repeated in two to three directions, depending on the size of the lesion. Compression was applied by a gauze piece for 2 min. After each injection, the patients were observed for 24 h for any adverse reactions.
Patients who did not attend follow-up at 6 months after the completion of treatment were excluded from the study. Patients in all three groups received at least four doses and a maximum of six doses of the assigned drug.
Response of treatment
The response was assessed as clinical and pathological response. The clinical response was assessed by the clinical photographs taken at the onset of treatment and by comparing it to the follow-up photographs taken at 3 months and after 1 month of completion of treatment. Clinical response was assessed by a senior resident and one consultant at each visit, who was blinded for the group. The response was categorized as <25% reduction in size (no response), 25–50% reduction (partial response), 50–75% reduction (good response), and >75 reduction in size (excellent response).
Histopathological response assessment
Histopathological assessment was done for microvessel density by immunochemical staining and was performed using the primary monoclonal antibody of CD34 (Biogenex Life Sciences Pvt. Ltd., CA, USA). The microvessel diameter (MVD) assessment was done by counting the blood vessels.[11] The stained sections were first screened at (4× and 20×) to determine the areas of most intense staining for CD34 [Figure 1A and B]. Blood vessel counting was then performed under (40×) magnification [Figure 2A and B]. The blood vessel density was recorded as a mean ± standard deviation (SD). Those endothelial cells colored with brown (CD34-positive) that formed a cluster of endothelial cells with a lumen were considered as blood vessels. Single CD34-positive endothelial cells were also included in the count. Blood vessels with muscle wall were excluded. Three high-power fields (HPF) with the highest number of blood vessels (hot spots) were chosen. The representative areas were carefully scanned from left to right of every slide to avoid recounting of same areas. The endothelial cells for each case were the average number of blood vessels in these three chosen HPFs and expressed as the number of endothelial cells per HPF (endothelial cells/HPF). The mean of three values was calculated and expressed as mean ± SD. All IHC-stained slides along with the corresponding H and E slides were evaluated by experienced pathologist blinded for the groups. The mean MVD was compared with a response.
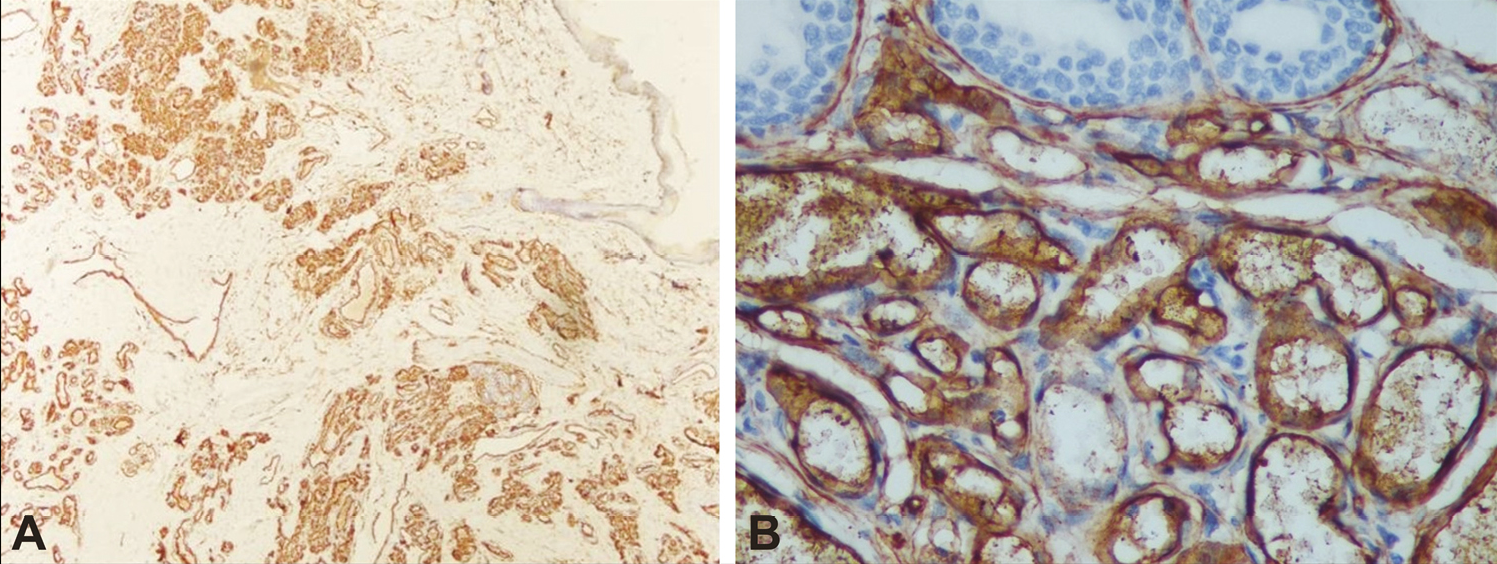
- Microphotograph showing numerous positive blood vessel expression of CD34-positive of IH at 4× (A) and 40× (B)
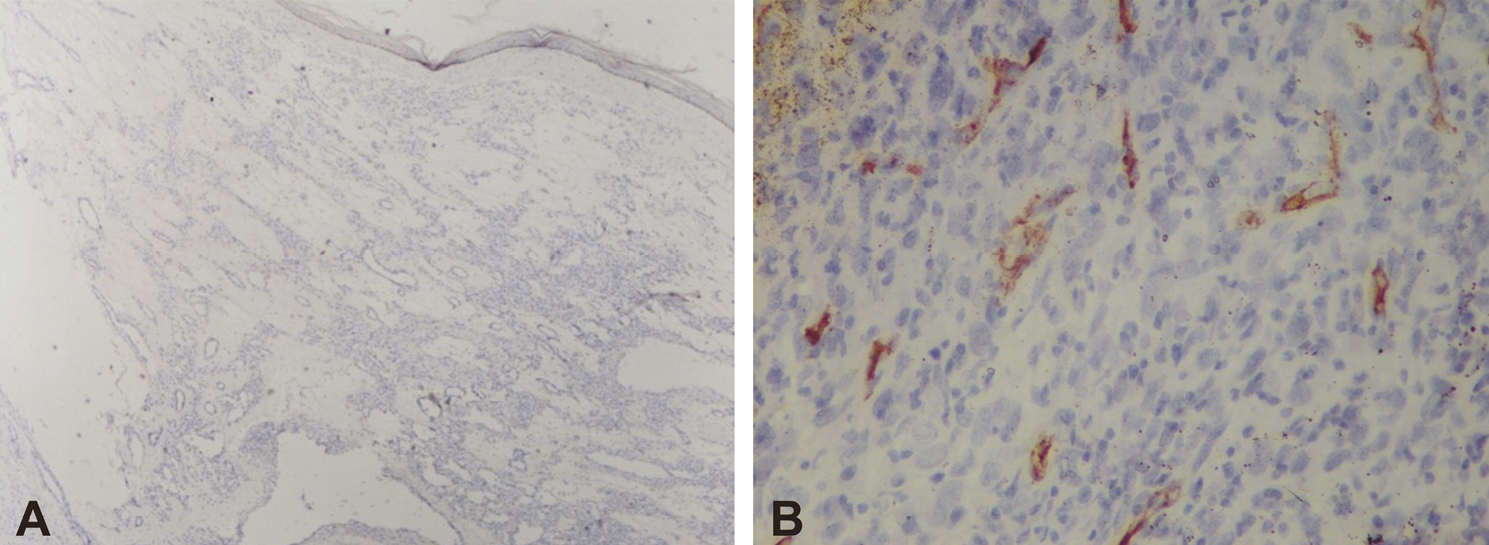
- Microphotograph showing scanty positive blood vessel expression of CD34-positive of IH at 4× (A) and 40× (B)
Statistical analysis
Statistical analyses were performed using the IBM SPSS 19.0 Statistics for Windows software (IBM Corp., Armonk, NY, USA). Data are expressed as mean±SD. Data were tested for normality by using the KS test. Paired t-test was used for making pre–post comparison. χ2 test was used to test the association between the variables. The utility of MVD in terms of clinical response to the therapy was predicted by using the receiver operating characteristic (ROC) curve. P-value less than 0.05 was considered as statistically significant.
RESULTS
The study included 144 cases of residual infantile hemangioma following propranolol treatment. Ten patients were excluded as they lost to follow-up. Finally, 134 patients were included in the study. The bleomycin group had 42 cases, the triamcinolone group had 44 children, and the combination group had 48 cases.
The mean age of the bleomycin group was 34.3±10.6 months, that of the triamcinolone group was 35.2±11.8 months, and that of the combination group was 34.9±11.5 months. Both males and females were equally distributed in all the three groups. All the three groups were comparable in respect of age and sex (P = 0.93 and P = 0.383, respectively). Head and neck were the most common site (63, 47.0%), followed by limbs (36, 26.9%) and trunk (35, 26.1%). Distribution of site of the hemangioma was similar in all the three groups: the mean duration of treatment was 4.50±1.04 months in the bleomycin group, 4.86±1.02 months in the triamcinolone group, and 4.85±1.03 months in the combination group, which was also comparable in all the three groups [Table 1]. The mean age at the start of propranolol treatment was 9.65±3.03 months. The mean duration of treatment was 6.50±1.04 months in the bleomycin group, 7.1±0.92 months in the triamcinolone group, and 6.85±1.03 months in the combination group, which was comparable in all the three groups.
| Characteristics | Group A N=42 (%) | Group B N=44 (%) | Group C N=48 (%) | P-value |
|---|---|---|---|---|
| Men age (months) | 34.3±10.6 | 35.2±11.8 | 34.9±11.5 | 0.932 |
| Gender | ||||
| Male | 24 (57.1) | 28 (63.6) | 34 (70.8) | 0.399 |
| Female | 18 (42.8) | 16 (36.3) | 14 (29.1) | |
| Number of lesions | 0.271 | |||
| Single | 37 (88.0) | 42 (95.4) | 41 (85.4) | |
| Multiple | 5 (11.9) | 2 (4.5) | 7 (14.5) | |
| Site of lesion | 0.576 | |||
| Head and neck | 24 (57.1) | 19 (43.1) | 20 (41.6) | |
| Trunk | 8 (19.0) | 13 (29.5) | 15 (31.2) | |
| Limbs | 10 (2.3) | 12 (27.2) | 13 (27.0) | |
| Mean duration of previous propranolol therapy (months) | 6.50±1.04 | 7.1±0.92 | 6.85±1.03 | 0.022 |
In the bleomycin group, 16 (38.1%) cases had excellent response, 15 (35.7%) had a partial response, and 11 (26.2%) cases had no response. In the triamcinolone group, 11 (25.0%) cases had excellent response, 19 (43.2%) had partial response, and 14 (31.8%) cases had no response. In the combination (bleomycin + triamcinolone) group, 29 (60.0%) cases had excellent, 16 (33.3%) had partial, and three (6.2%) had no response [Figures 3A and B and 4A and B]. The overall clinical response was better in the combination group when compared with the bleomycin group (P = 0.018) and triamcinolone group (P = 0.0005), respectively, after 6 months of follow-up. There was no difference in clinical response between the triamcinolone and bleomycin groups [Table 2].
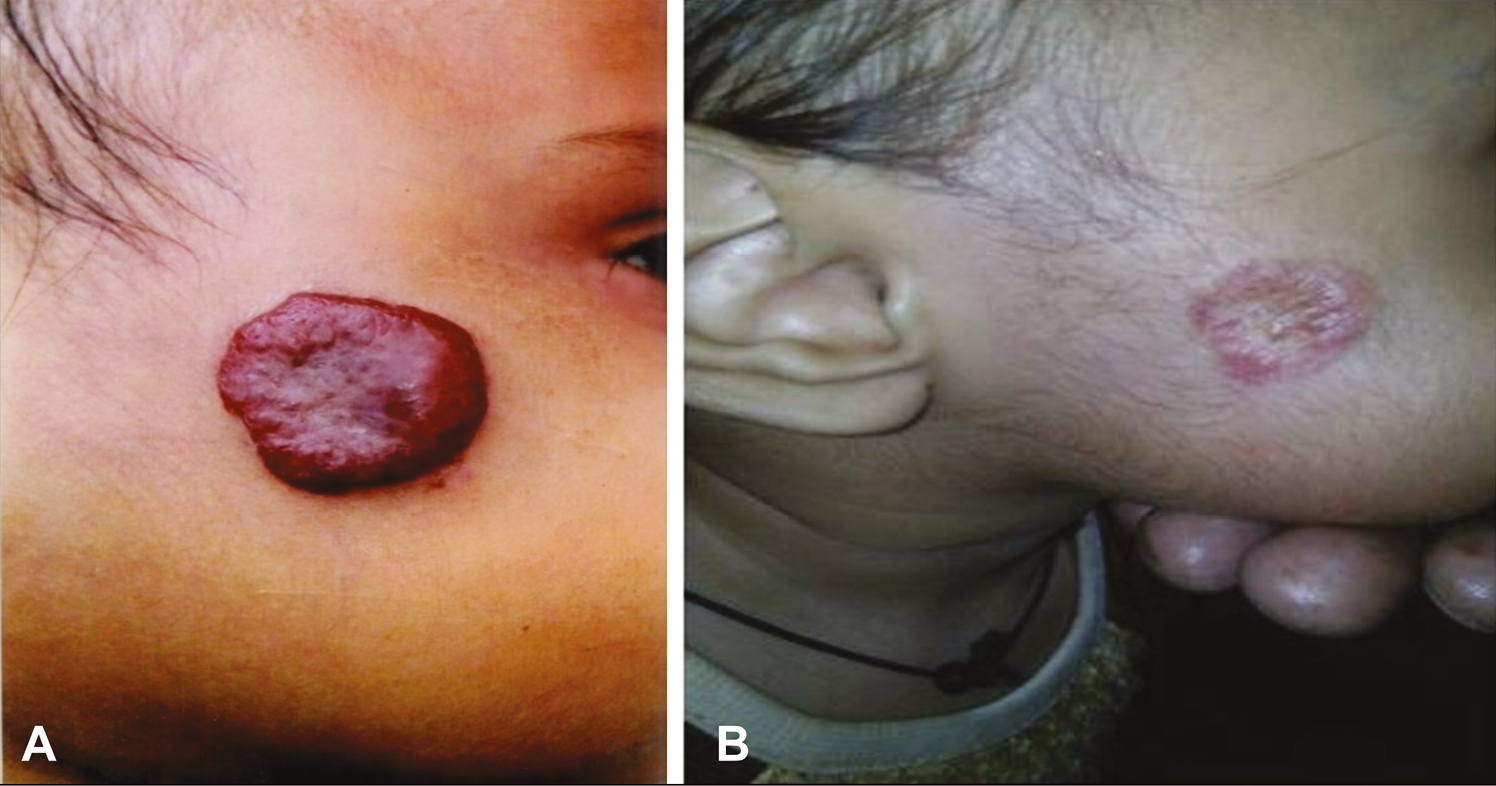
- Pre-treatment (A) and post-treatment (B) lesions following combination treatment in an excellent responder
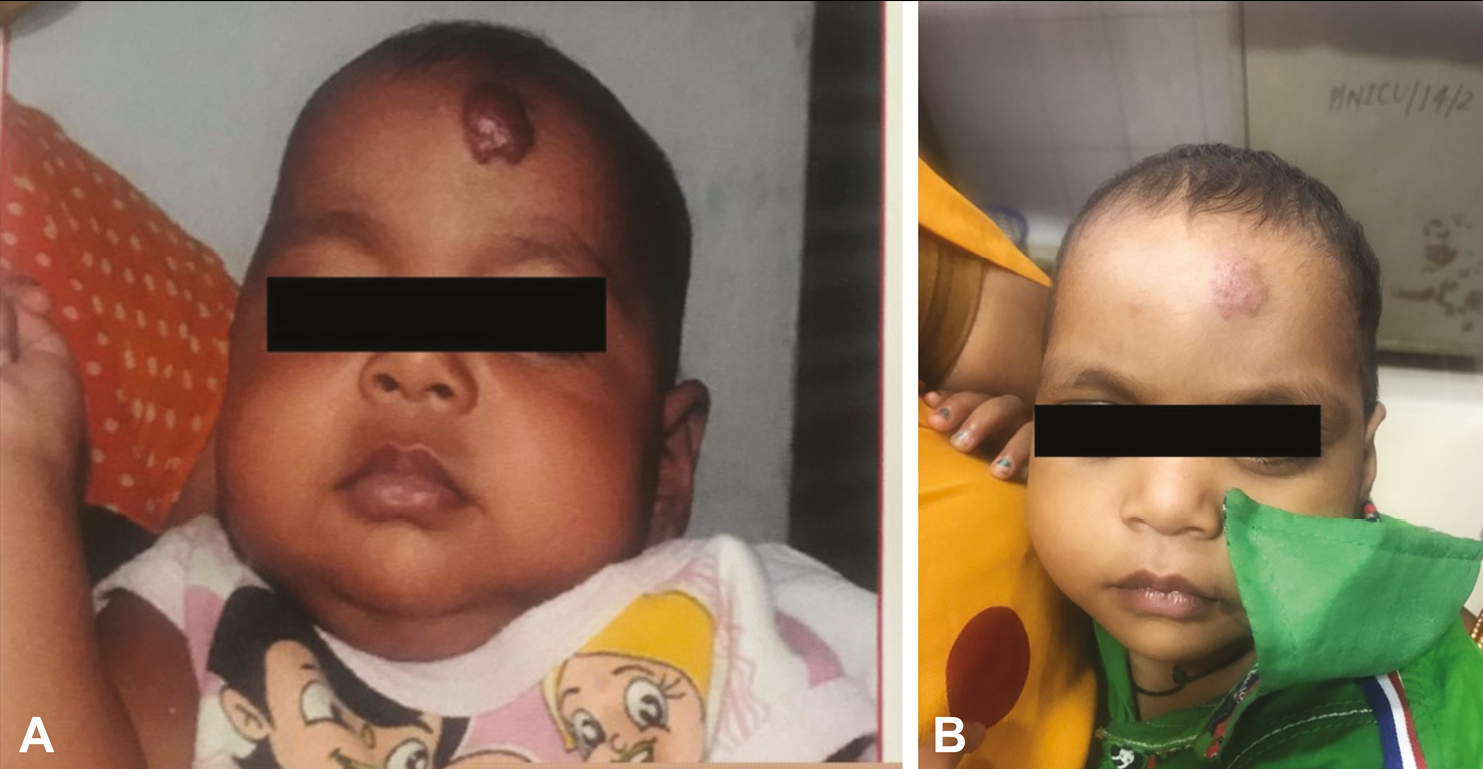
- Pre-treatment (A) and post-treatment (B) lesions following combination treatment in an excellent responder
| Group | Response | ||
|---|---|---|---|
| Bleomycin (n=42) | Triamcinolone (n=44) | Combination (n=48) | |
| No. (%) | No. (%) | No. (%) | |
| Excellent | 16 (38.1) | 11 (25.0) | 29 (60.0) |
| Partial | 15 (35.7) | 19 (43.2) | 16 (33.3) |
| No response | 11 (26.2) | 14 (31.8) | 3 (6.2) |
| Total | 42 (100) | 44 (100) | 48 (100) |
P-value:
Bleomycin vs. triamcinolone: 0.425;
Bleomycin vs. combination: 0.018;
Triamcinolone vs. combination: 0.0005.
MVD assessment was performed in 22 cases of the bleomycin group, 19 cases of the triamcinolone group, and 23 cases of the combination group. The mean pre-treatment MVD in the bleomycin group was 33.86±6.25, in the triamcinolone group was 35.57±4.32 and in the combination group was 33.17±5.71 and was comparable in all the three groups. This decreased significantly to 21.51±7.269, 25.57±8.237, and 19.03±4.711, respectively, in three groups at 6 months (P = 0.001, P = 0.0005, and P = 0.001, respectively). On overall comparison, the MVD decrease was higher in the combination group when compared with the bleomycin or triamcinolone group [Table 3].
| Group | Pre-treatment MVD | Post-treatment MVD |
|---|---|---|
| Bleomycin group (n=22) | 33.86±6.25 | 21.13±7.63 |
| Triamcinolone group (n=19) | 35.57±4.32 | 26±8.31 |
| Combination group (n=23) | 33.17±5.71 | 18.17±5.56 |
Bleomycin vs. triamcinolone: P = 0.005, bleomycin vs. combination: P = 0.039, triamcinolone vs. combination: P = 0.001.
In the bleomycin group, among the excellent responders, the mean MVD decreased from 35.43±6.02 to 13.57±3.59, in partial responders it decreased from 33.50±6.07 to 22.12±4.76 and in non-responders it decreased from 32.71±7.29 to 27.57±6.9. The decrease was significant in excellent and partial responders (P = 0.001) but was not in non-responders (P = 0.057). The results of the triamcinolone and combination groups are detailed in [Table 4].
| Response | |||
|---|---|---|---|
| Excellent | Partial | No response | |
| Bleomycin group (n=22) | |||
| Pre-treatment MVD | 34.09±5.33 | 35.87±6.04 | 33.33±6.48 |
| MVD after 6 months | 13.91±4.25 | 23.13±4.22 | 28.11±6.11 |
| Excellent vs. partial: <0.001, excellent vs. no response: <0.001, partial vs. no response: 0.057 | |||
| Triamcinolone group (n=19) | |||
| Pre-treatment MVD | 34.33±2.44 | 35.75±4.61 | 37.10±4.65 |
| MVD after 6 months | 16.56±2.12 | 24.50±4.84 | 35.40±4.74 |
| Excellent vs. partial: <0.001, excellent vs. no response: <0.001 partial vs. no response: 0.001. | |||
| Combination group (n=23) | |||
| Pre-treatment MVD | 32.38±5.79 | 35.25±5.17 | 30.00±2.0 |
| MVD after 6 months | 14.15±2.60 | 22.25±2.44 | 28.00±2.0 |
| Excellent vs. partial: <0.001, excellent vs. no response: <0.001, partial vs. no response: 0.067. | |||
ROC analysis of the MVD as a predictor of clinical response was performed to evaluate a cut-off value of MVD in responders (excellent and partial) and non-responders. The value of area under curve (AUC) (95% CI) was 0.926 (0.0869–0.983) with P = 0.0001. The cut-off point was calculated to be 24.5 with max sensitivity and specificity of 0.904 and 0.848, respectively [Figure 5].
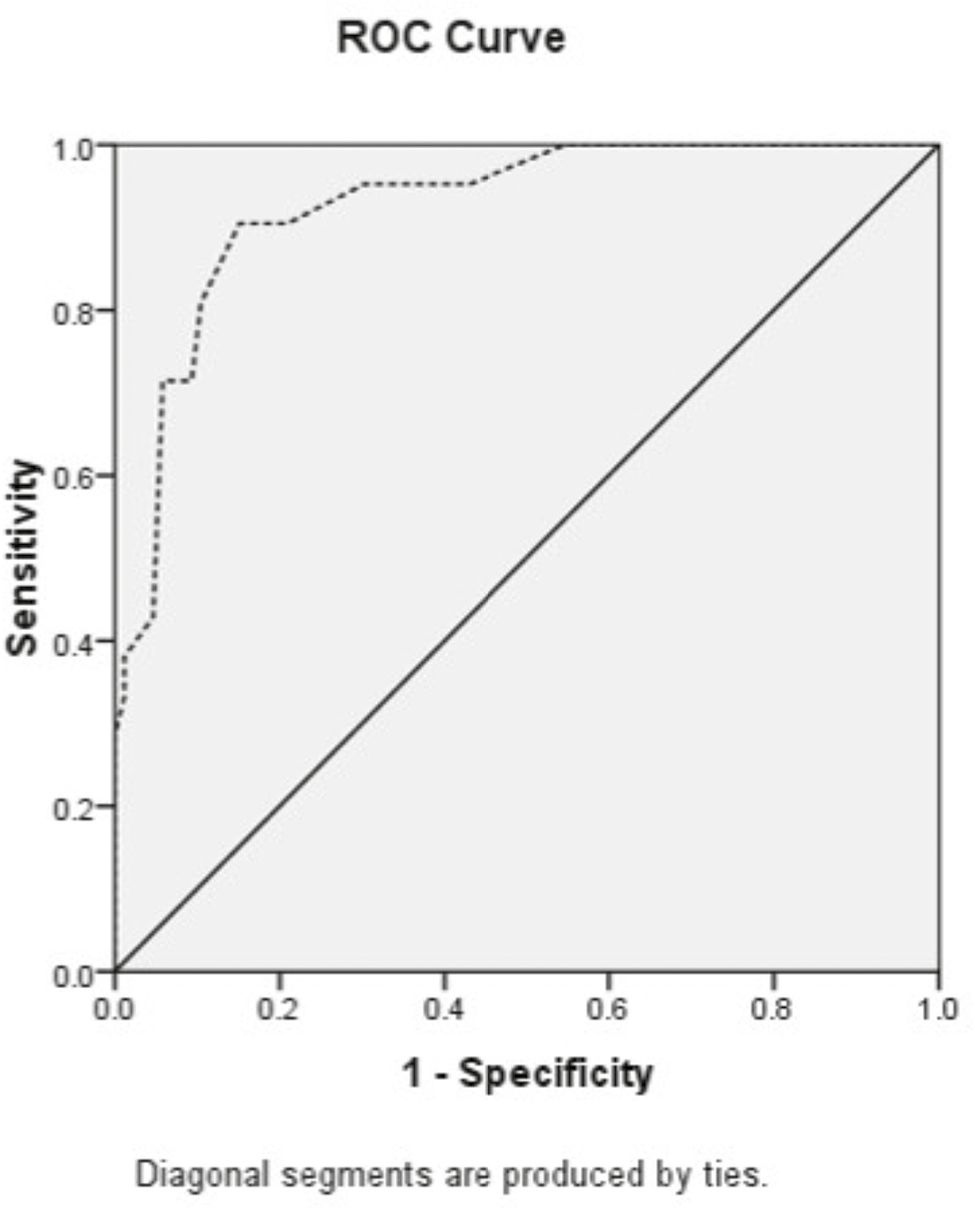
- ROC curve of MVD to clinical response
Five patients developed ulceration of the lesion: three in the bleomycin group and one each in triamcinolone and combination group. All ulcerations were reported within 1 week of injection and were managed with oral anti-inflammatory drugs. Two children in the triamcinolone group had an infection of the lesion. Both were managed by oral antibiotics and did not require drainage. Five patients in the bleomycin group have peripheral pigmentation involving both ventral and dorsal surfaces. The lesions resolved on discontinuation of the injection.
DISCUSSION
The treatment of problematic hemangioma has evolved from steroids as first line to beta-blockers. Beta-blockers have been used in different forms and routes with good efficacy and minimal adverse effects.[1213] We know that if propranolol is initiated after 12 months of age, the effectiveness of therapy will be diminished because the hemangioma is no longer in the proliferative phase. The mean age of initiation of propranolol therapy was higher in our series when compared with other reports, as our patients usually present late.[14] This may be the reason for a comparatively lower response to primary propranolol therapy when compared with other reports. Surgical excision with simple closure is a useful option in these children, but surgery in children is still associated with a lot of stigma in our society and most parents refuse it as a treatment option. Further, surgery is associated with complications of anesthesia and procedural complications like wound infection and dehiscence.[15] Intralesional sclerotherapy as an alternative approach to surgical excision has shown a lot of promise, with excellent results. Bleomycin and triamcinolone have been used with good effects.[5] The mechanism of action of bleomycin and triamcinolone is different. Bleomycin has a local sclerosing effect on endothelial cells, and it causes strong non-specific inflammatory reaction.[16] The mechanism of action of triamcinolone in hemangioma is not very well understood but it is known to sensitize the vascular bed to vasoconstricting agents in the presence of heparin.[17]
Masiha et al.[18] evaluated the synergistic effect of bleomycin, triamcinolone, and epinephrine in the treatment of hemangioma and arteriovenous malformations in 32 patients. By the end of the follow-up time, the success rates among hemangiomas, low-flow arteriovenous malformation, and mixed lesions were excellent. They proposed that the combination has a synergistic effect.
Similarly, Camacho-Martínez et al. had successfully managed keloids with a combination of bleomycin and triamcinolone. They concluded that the combination had a synergistic effect.[13] Pandey et al. have also proposed that both triamcinolone and bleomycin are equally effective in the management of residual infantile hemangioma and they have a different mechanism of action as found on the analysis of subgroups of their subjects.[19]
In this study, we found that the response in the combination group was better. The assessment of the MVD and its decrease following intralesional therapy in our study provide an objective assessment of treatment response. The anti-CD34 antibody is a sensitive endothelial marker which stains small and large vessels in normal and tumor tissues equally.[20] On ROC analysis, we found that MVD of 24.5 is a predictor of good response.
We could not perform a power calculation to have the desired number of patients in each group as this work was performed during a stipulated time period. We acknowledge this as our limitation.
CONCLUSION
The combination of bleomycin and triamcinolone is effective and safe for the treatment of residual hemangioma. The combination is more effective than either alone and can be used as the first/second-line therapy for the residual hemangioma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Current treatment and management of infantile hemangiomas. Surv Ophthalmol. 2019;64:608-18.
- [Google Scholar]
- Infantile hemangiomas: Our current understanding and treatment options. Dermatol Online J. 2018;24:13030/qt5jt8q9km.
- [Google Scholar]
- Diagnosis and management of infantile hemangiomas in the neonate. Pediatr Clin North Am. 2019;66:437-59.
- [Google Scholar]
- Laser treatment of infantile hemangioma: A systematic review. Lasers Surg Med. 2016;48:221-33.
- [Google Scholar]
- Role of intralesional bleomycin and intralesional triamcinolone therapy in residual haemangioma following propranolol. Int J Oral Maxillofac Surg. 2018;47:908-12.
- [Google Scholar]
- Pharmacologic interventions for infantile hemangioma: A meta-analysis. Pediatrics. 2016;137:e20153896.
- [Google Scholar]
- Propranolol for infantile haemangiomas: Experience from a tertiary center. J Cutan Aesthet Surg. 2014;7:37-41.
- [Google Scholar]
- Efficacy and adverse effects of oral propranolol in infantile hemangioma: A meta-analysis of comparative studies. World J Pediatr. 2019;15:546-58.
- [Google Scholar]
- Safety of intralesional injection of lauromacrogol combined with triamcinolone for infantile hemangiomas. J Dermatol. 2019;46:770-6.
- [Google Scholar]
- Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169-80.
- [Google Scholar]
- A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-46.
- [Google Scholar]
- A meta-analysis on the effectiveness of propranolol for the treatment of infantile airway haemangiomas. Int J Pediatr Otorhinolaryngol. 2011;75:455-60.
- [Google Scholar]
- Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475.
- [Google Scholar]
- Individualized treatment for infantile hemangioma. J Craniofac Surg. 2018;29:1876-9.
- [Google Scholar]
- Efficacy of bleomycin treatment for symptomatic hemangiomas in children. Pediatr Surg Int. 1997;12:526-8.
- [Google Scholar]
- Efficacy of intralesional steroid injection in head and neck hemangioma: A systematic review. Ann Plast Surg. 2011;66:98-106.
- [Google Scholar]
- The synergistic effect of bleomycin, triamcinolone and epinephrine in treatment of hemangioma and arteriovenous malformations. World J Plast Surg. 2012;1:83-90.
- [Google Scholar]
- Results of a combination of bleomycin and triamcinolone acetonide in the treatment of keloids and hypertrophic scars. An Bras Dermatol. 2013;88:387-94.
- [Google Scholar]
- CD34 as a marker for evaluating angiogenesis in cervical cancer. Pathol Res Pract. 2005;201:313-8.
- [Google Scholar]





