Translate this page into:
Anaphylactic reaction to hyaluronidase use for a complication of lip augmentation with hyaluronic acid
*Corresponding author: Luiz Felipe Palma, Department of Pathology, Federal University of São Paulo, R. Botucatu, 720 - Vila Clementino, São Paulo, SP, Brazil. luizfelipep@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Seyboth Wild G, Palma LF, Morimoto S, Pereira PA. Anaphylactic reaction to hyaluronidase use for a complication of lip augmentation with hyaluronic acid. J Cutan Aesthet Surg. doi: 10.4103/JCAS.JCAS_99_23
Dear Editor,
Hyaluronidase, an enzyme that degrades hyaluronic acid, has received approval for numerous medical applications by regulatory agencies worldwide. Its most well-known indications (e.g., hypodermoclysis and hematoma absorption) have been established for over 60 years. However, other potential uses of hyaluronidase such as dissolving hyaluronic acid fillers are currently off-label.1
As a result of the growing demand for noninvasive rejuvenation procedures, soft tissue augmentations have gained significant popularity. This trend, however, has also led to an increase in the incidence of undesirable events.2 The use of hyaluronidase for the correction of complications arising from hyaluronic acid fillers, therefore, has become a common practice in daily medical care. Concomitantly, there has been substantial growth in the number of papers on the potential risk of adverse reactions related to the administration of this enzyme.3
Hyaluronidase is thought to be a potential allergen, which can lead to the onset of allergic/hypersensitivity reactions, mainly after many hours from the application (i.e., delayed reactions).4,5 In light of these facts, the current paper aims to briefly present the first report case in which a female patient experienced an anaphylactic reaction to hyaluronidase.
A 40-year-old Caucasian woman underwent lip augmentation, and the hyaluronic acid filler overflowed from an injection site, resulting in an unaesthetic outcome (asymmetry) due to the formation of a small, non- fluctuant nodule of a non-inflammatory nature on the upper lip [Figure 1a]. Following the recommendations of the Global Aesthetics Consensus Group,6 massage and an antibiotic drug were prescribed, but there was no improvement.
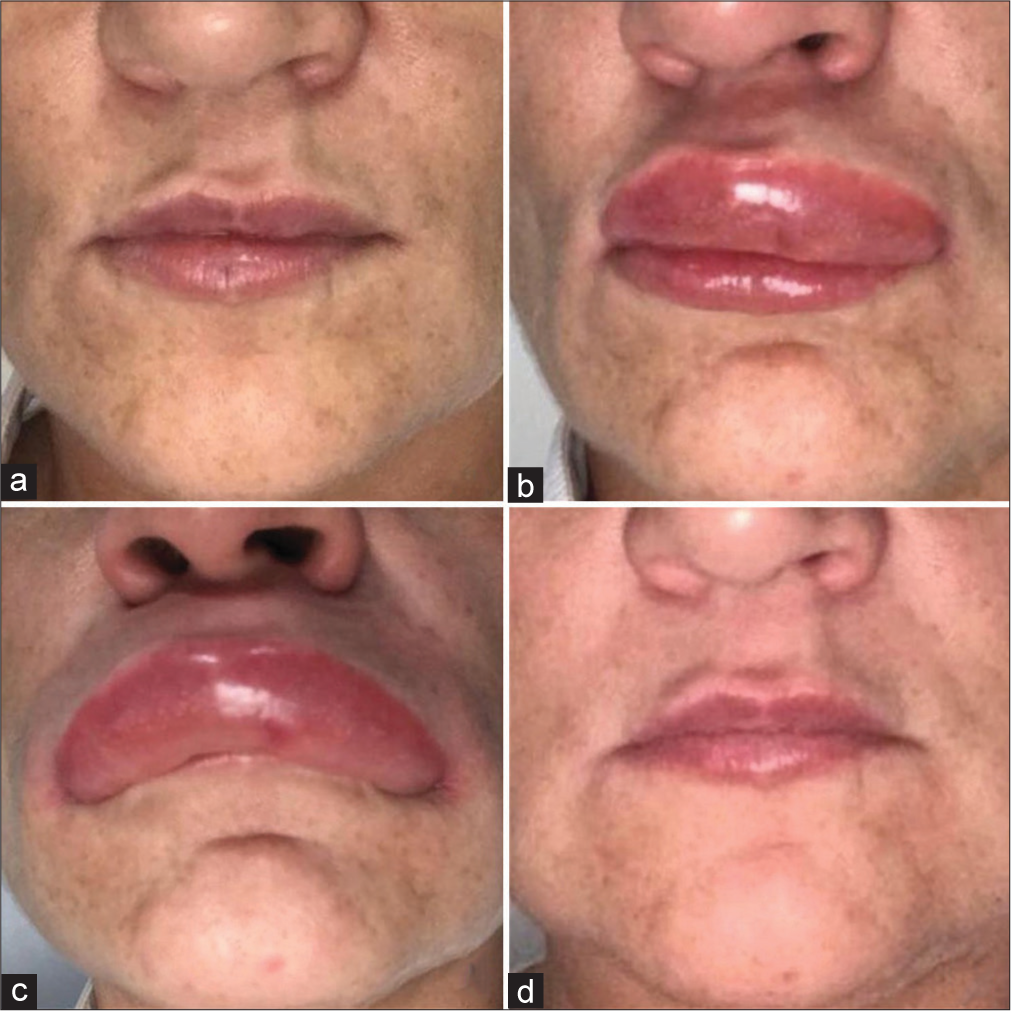
- Clinical Progression. (a) Pretreatment clinical appearance. (b) Clinical appearance shortly after administration of hyaluronidase. (c) Clinical appearance 30 min after administration of hyaluronidase. (d) Clinical appearance 6 months after the anaphylactic reaction.
Seven days later, she received an injection of 0.4 UI of hyaluronidase (Biometil®, Laboratório Biometil, São Bento do Sul, SC, Brazil; source: purified bovine testicle) for the management of the unwanted event. Within a few minutes, the patient experienced breathing difficulty and significant edema affecting the upper lip [Figure 1b and c]. A tablet of bilastine 20 mg was administered, without success. She also did not report any prior allergic event related to animal stings, such as bees and spiders.
The patient was quickly taken to a hospital, where the diagnosis of an anaphylactic reaction to hyaluronidase was confirmed. She received epinephrine, a corticosteroid, and an antihistamine via parenteral administration, which resulted in prompt stabilization of her condition. She was hospitalized for 24 h for observation and further supportive care. Afterward, oral dexamethasone was prescribed every 8 h for 7 days. The facial edema gradually reduced over some months, probably due to concurrent histaminergic angioedema, a drug-induced condition that is usually associated with anaphylaxis reactions and may become chronic, lasting for long periods.7 Unfortunately, she did not agree to undergo a biopsy due to aesthetic concerns regarding a potentially noticeable scar, which may be considered a major limitation in the diagnosis process. However, after a period of 6 months, there were no longer any visible signs of an allergic reaction to hyaluronidase [Figure 1d].
It is important to highlight that allergy skin testing should always be conducted before using hyaluronidase; however, only a few actually do that. For example, in Canada, 9% of plastic surgeons who have experience using this enzyme for the management of hyaluronic acid fillers-associated complication performs hypersensibility tests.8
History of allergic reactions or anaphylaxis to animal stings is another crucial information to be obtained before using hyaluronidase. Hyaluronidase is also present in the venoms of many animals (e.g., snakes, leeches, scorpions, bees, caterpillars, and spiders), which is responsible for the spread of other substances and toxins through the tissues.9 Thus, based on the patient’s past medical history, the use of hyaluronidase must be contraindicated in these specific cases.10
In conclusion, while hyaluronidase is generally considered a safe and efficient agent for treating some complications resulting from aesthetic orofacial procedures with hyaluronic acid, clinicians must be highly attentive to the potential risk of allergic or anaphylactic reactions associated with its use. Obtaining an in-depth medical history of the patient and performing allergy skin testing are cornerstone strategies to mitigate the risk of undesirable events from the application of hyaluronidase.
Authors’ contributions
All the authors contributed to the research study. Gisela Seyboth Wild: Concepts, Design, Definition of intellectual content, Literature search, Manuscript preparation, Manuscript Editing, and Manuscript review. Luiz Felipe Palma: Concepts, Design, Definition of intellectual content, Literature search, Manuscript preparation, Manuscript Editing, and Manuscript review. Susana Morimoto: Concepts, Design, Definition of intellectual content, Literature search, Manuscript preparation, Manuscript Editing, and Manuscript review. Priscilla Aparecida Pereira: Concepts, Design, Definition of intellectual content, Literature search, Manuscript preparation, Manuscript Editing, and Manuscript review.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has given her consent for her images and other clinical information to be reported in the journal. The patients understand that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Hyaluronidase: An overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic reaction to hyaluronidase use after hyaluronic acid filler injection. J Cosmet Laser Ther. 2015;17:283-5.
- [CrossRef] [PubMed] [Google Scholar]
- Use of hyaluronidase in plastic surgery: A review. J Plast Reconstr Aesthet Surg. 2021;74:1610-4.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed allergic hypersensitivity to hyaluronidase during the treatment of granulomatous hyaluronic acid reactions. J Cosmet Dermatol. 2018;17:991-5.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed hypersensitivity of hyaluronidase: A case report. J Eur Acad Dermatol Venereol. 2022;36:e691-3.
- [CrossRef] [Google Scholar]
- Global aesthetics consensus: Avoidance and management of complications from hyaluronic acid fillers-evidence-and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137:961e-71e.
- [CrossRef] [PubMed] [Google Scholar]
- Angioedema [Internet] 2023. StatPearls. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30220453 [Last accessed on 25 Mar 2023]
- [Google Scholar]
- Hyaluronidase for treating complications related to HA fillers: A national plastic surgeon survey. Plast Surg (Oakville). 2022;30:233-7.
- [CrossRef] [PubMed] [Google Scholar]
- A biological overview of hyaluronidase: A venom enzyme and its inhibition with plants materials. Mater Today Proc. 2018;5:6406-12.
- [CrossRef] [Google Scholar]
- This month’s guideline: The use of hyaluronidase in aesthetic practice (v24) J Clin Aesthet Dermatol. 2018;11:E61-8.
- [Google Scholar]





