Translate this page into:
Clinicopathological Profile of Nodular Hidradenoma: A ten year Study in a Tertiary Care Center
Address for correspondence: Dr. K. Arun Kumar, Department of Pathology, Sri Ramachandra Institute of Higher Education and Research, Chennai 600116, Tamil Nadu, India. E-mail: arunkumar.k@sriramachandra.edu.in
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Nodular hidradenoma is a rare skin adnexal tumor of eccrine differentiation with predominant site being scalp and axillae. Due to its variable locations and unusual clinical presentation and no definite radiological criteria, histopathology seems to be the mainstay in diagnosing these tumors. Most of the lesions present as a cystic swelling and was clinically thought to be a sebaceous cyst/metastasis/carcinoma/sarcoma. In our study, we have included 37 cases and compared its varied clinical and radiological presentation.
Keywords
Histopathology
nodular hidradenoma
skin adnexal tumor
INTRODUCTION
Nodular hidradenoma is a benign adnexal tumor that arises from the distal excretory duct of eccrine sweat gland. Nodular hidradenoma resembles the cells and structures of ductal segment of the eccrine sweat gland, hence the name.[1] Most of the cases previously reported as eccrine now have been reclassified as apocrine. The usual presentation of this tumor is slowly enlarging, solitary, freely movable nodule, solid or cystic, measuring on an average 0.5–2 cm in diameter, but may reach 6.0 cm or more.[2] The common locations of the lesion are axilla, face, arms, thighs, trunk, scalp, and pubic region but the most common site is head. Most commonly it is seen in the age group of 20–50 years and is rare in children.[2] It occurs in women twice as commonly as in men.[2]
Histologically, the tumor is composed of lobulated masses located in the dermis and extending into the subcutaneous fat.[3] Epidermal connection is present in up to one-third of the cases. It is composed of two cell types: the polygonal cells, whose glycogen content may give the cytoplasm a clear appearance, and elongated, darker, and smaller cells, which may appear at the periphery. The tumor may be solid or cystic in varying proportions.
Malignant transformation is very rare and if does, takes years to transform in to a malignant neoplasm.[4]
Due to its varied location and cystic nature of the lesion, it poses a clinical and radiological diagnostic challenge.[5]
Objective
The objective is to study the clinical and pathological spectrum of nodular hidradenoma in a tertiary care center.
MATERIALS AND METHODS
This is an observational study done at a tertiary care hospital in South India. All skin adnexal tumor with the histological diagnosis of nodular hidradenoma presented to the Department of pathology from 2011 to 2021 were included in the study. This is a 10-year study, where we got 37 cases. Clinical details of the patient such as age, sex, site, and size were noted. Provisional diagnosis of the lesion was obtained from the histopathological requests. Radiological investigation if done was noted. The lesions were grouped and the results were compared. The clinical and radiological diagnosis was compared with the final histological diagnosis.
RESULTS
A total of 37 cases were studied of which 15 were males and 22 females [Graph 1]. The age distribution ranges from 17-15 years [Graph 2].
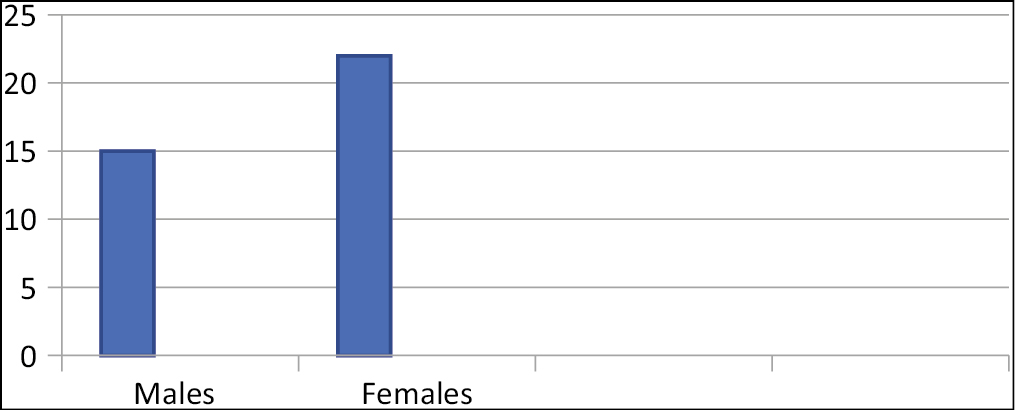
- Male/female distribution
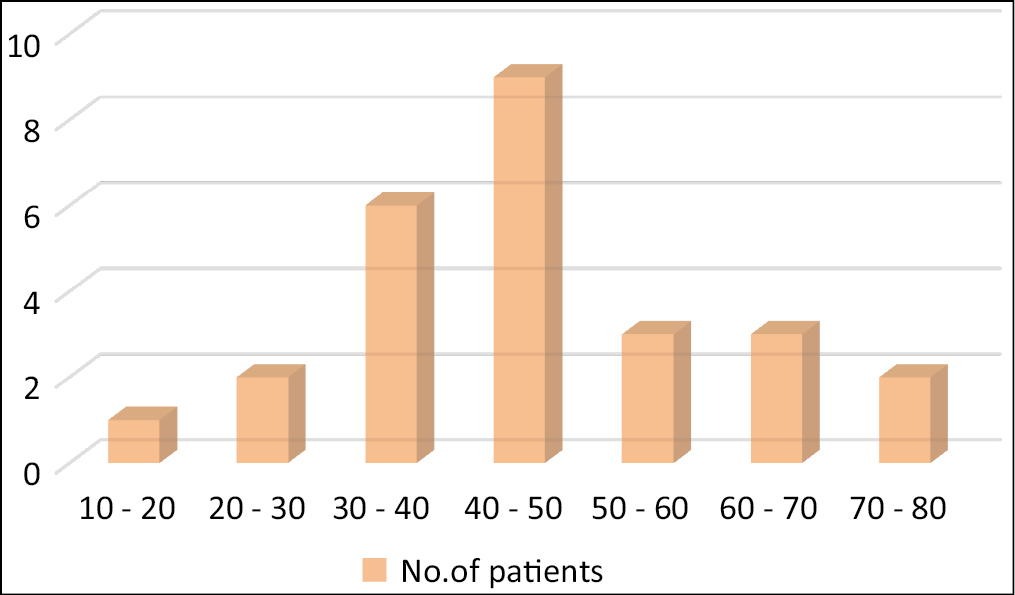
- Age distribution
On gross examination, most of the cases presented as solitary, well-circumscribed, solid, and cystic swelling with the size of the lesion ranging from 3.5 to 7.5 cm [Figure 1]. Clinical diagnosis of dermoid cyst was thought in approximately 50% of the cases. The other diagnoses were lymphatic cyst, metastasis, and sarcoma [Table 1]. Most of the tumors were from the scalp and axillae, and other sites included gluteal region, breast, chest, and vulva.
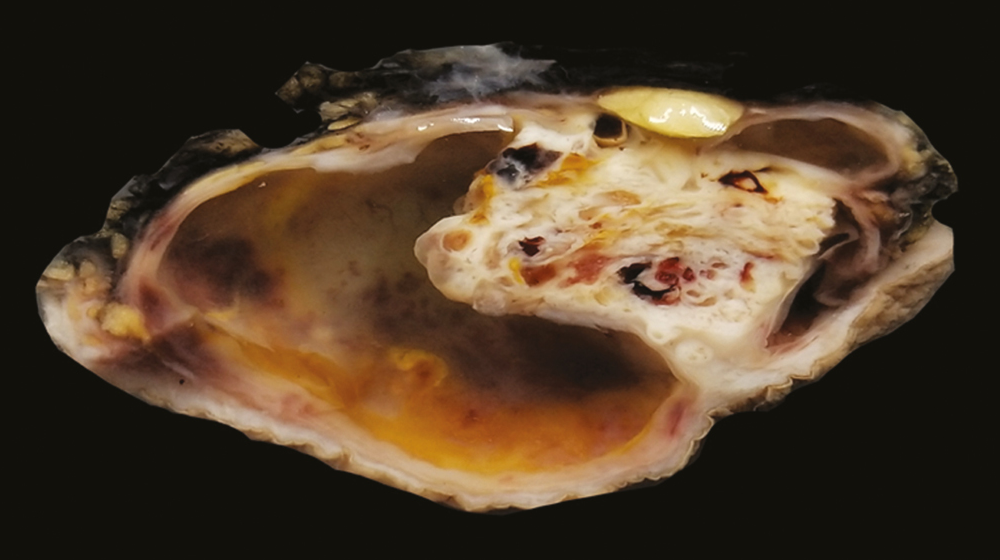
- Gross of nodular hidradenoma showing solid and cystic swelling
| Clinical diagnosis | No. of cases |
|---|---|
| Sebaceous cyst/dermoid | 48.6% (18) |
| Neurofibroma | 5.4% (2) |
| Lymphatic cyst | 8.1% (3) |
| Sarcoma | 2.7% (1) |
| Metastasis | 2.7% (1) |
| Cystic swelling | 13.5% (5) |
| Without clinical diagnosis | 13.5% (5) |
Among the 37 cases, three cases were radiologically diagnosed to be a malignant tumor [Table 2], which after histopathological microscopic features were proven to be nodular hidradenoma.
| Cases | Clinical diagnosis | Radiology | Pathological diagnosis |
|---|---|---|---|
| 1. | Metastatic deposit (K/C/O Ca lung) | FDG avid lesion – possibly metastasis [Figure 2] | Nodular hidradenoma |
| 2. | Dermoid cyst – scalp | Dermoid/dermatofibrosarcoma protruberans [Figure 3] | Clear cell hidradenoma |
| 3. | Soft tissue sarcoma – forearm | Soft tissue sarcoma/ganglion cyst [Figure 4] | Nodular hidradenoma |
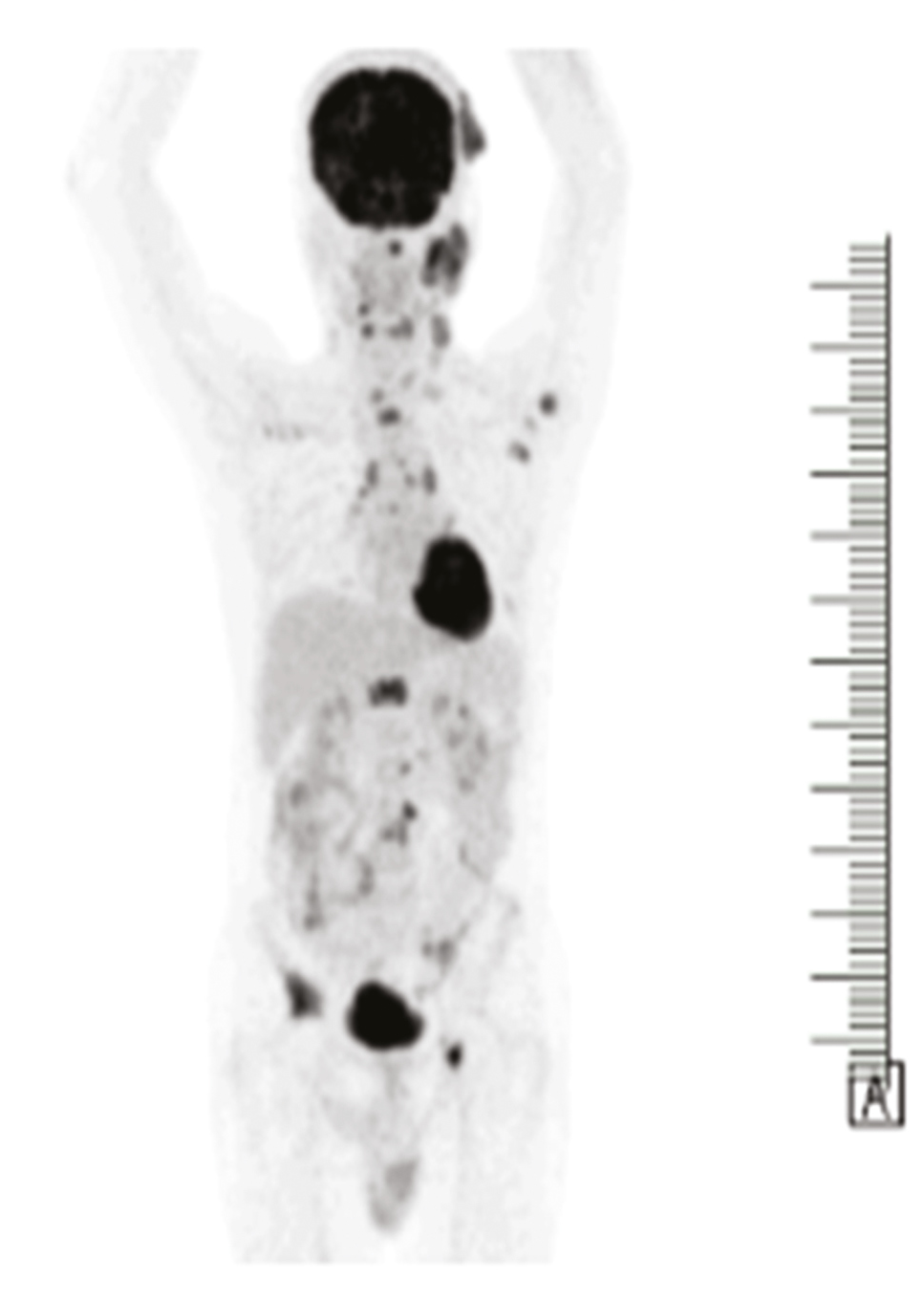
- Case 1 FDG avid PET lesion in scalp
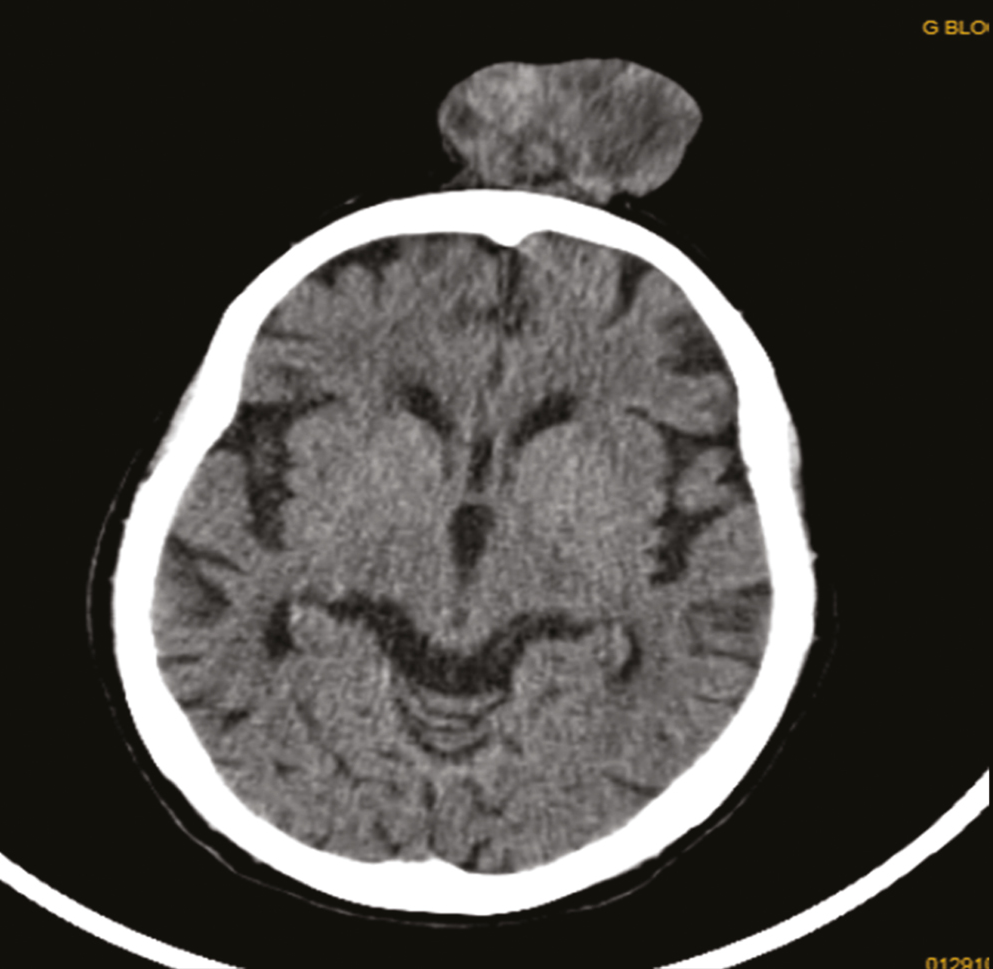
- Case 2 CT scan of scalp lesion
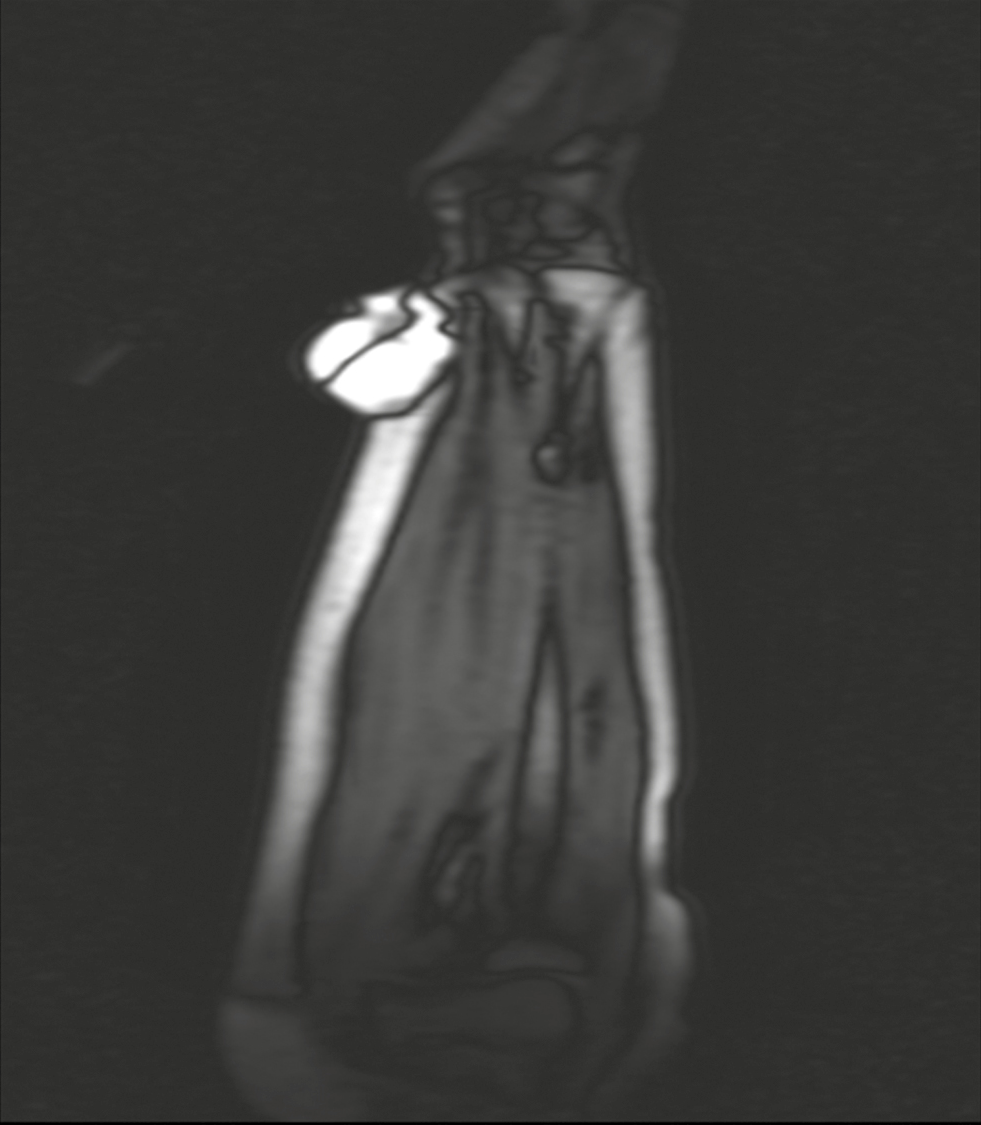
- Case 3 MRI of soft tissue swelling of forearm
One of the cases was diagnosed to be a malignant deposit of scalp in a male patient with a history of known case of lung carcinoma, and in other two patients as sarcoma of one presented in forearm and other a midline mediastinal swelling.
Microscopy of the tumor were solitary, circumscribed [Figure 5], solid and cystic, dermal, symmetrical, lobulated tumors with a sheet-like and papillary architecture [Figure 6]. The solid area was composed of two types of cells: eosinophilic/polygonal cells and clear cells.
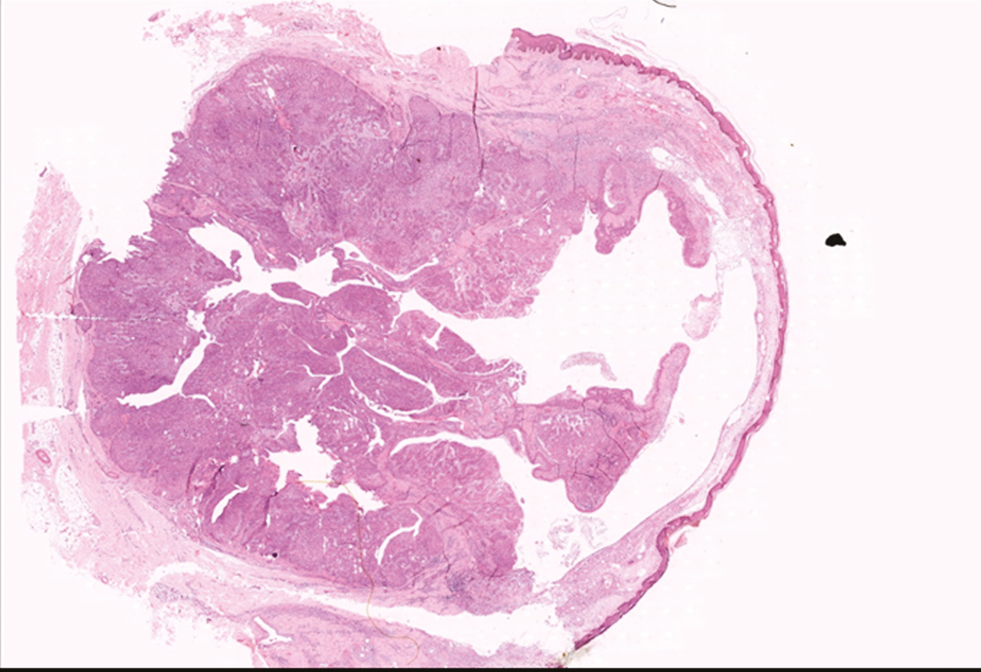
- H&E scanner view showing well circumscribed solid and cystic lesion
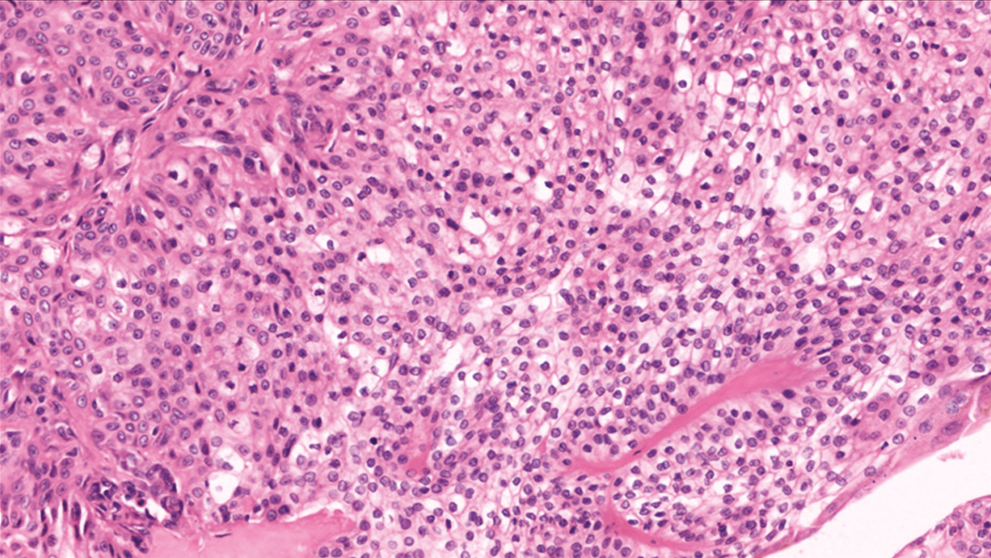
- H&E 400× showing polygonal cells with eosinophilic and clear cytoplasm
The shape of the eosinophilic cells vary from round to polygonal. They showed a rounded to ovoid nucleus, small distinct nucleoli, and faintly eosinophilic cytoplasm surrounded by hyalinized fibrovascular cores.
Clear cells showed abundant clear cytoplasm with a small, round to oval, eccentric nucleus. Some duct-like tubular structures were observed, which were constituted by cuboidal cells and filled with eosinophilic hyaline material. Cystic spaces were also filled with amorphous eosinophilic material. Occasional squamoid morules were also evident. No evidence of atypia, invasion, necrosis, and mitoses were noted in any of the cases.
DISCUSSION
Skin adnexal tumors often cause a diagnostic dilemma due to uncertain origin, varied clinical presentation, nonspecific radiological features, and overlapping morphology. But it is crucial to diagnose them as some of them have high incidence of recurrence and malignant potential. Nodular hidradenoma is one such skin adnexal tumor with varied clinical and morphological pattern, often misdiagnosed clinically and radiologically. It is a benign sweat gland neoplasm that is now reclassified as apocrine, occurs as a single mass in the skin with a nodular or cystic structure.[5] It presents as a solitary, firm nodule with a slight predilection for the head, face, and upper extremities. Hernandez et al.[6] in their study showed a female predominance (1.7:1), with a mean age at presentation of 37.2 years similar to our study, where females showed a predominance and the mean age being 40.1 years.
In our study, the most common site of involvement was scalp (35.5%) similar to that of Nandeesh et al.[5] Grossly, the lesion is solid and cystic with serous fluid-filled cystic areas, mimicking a dermoid cyst.
Histologically, the lesion is well-circumscribed and sharply demarcated.[6] The tumor is composed of solid and cystic spaces. Solid areas shows two types of cells, one being polyhedral cells with more basophilic cytoplasm and the other is round with a clear cytoplasm.[7] The clear cells contain glycogen and periodic acid-Schiff-positive, diastase-resistant material, but no lipid.[8] Clear cell hidradenoma, the most common variant, contains predominantly clear or pale cells with distinct cell borders.[9] The intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Variable numbers of mitotic figures are noted, and the presence does not necessarily indicate malignancy.
Although immunohistochemistry can aid in confirmation of the diagnosis such as epithelial membrane antigen, carcinoembryonic antigen, and cytokeratin AE1/AE3, they are not often necessary.[10]
Since the lesion is dermal origin, although epidermal connection is seen in up to one-third of the cases, they can grow to enormous sizes and radiologically mimic malignancy.[11] Kyung et al. in his study elaborates on no specific radiological criteria to diagnose nodular hidradenoma, hence a radiological misdiagnosis is frequent.[12] In our study, three cases were thought to be malignant radiologically, which eventually proved microscopically as nodular hidradenoma.
Malignant counterpart of hidraenoma is termed hidradenocarcinoma. Infiltrative growth pattern, deep extension, necrosis, nuclear pleomorphism, ≥4 mitoses per 10 high-power fields, and angiolymphatic invasion are specific features of hidradenocarcinomas.[13] Souvatzidis et al. in their study says malignant transformation is rare and histologically characterized by nuclear atypia, necrosis, and abnormal mitoses.[7] Malignant transformation is quite aggressive course with widely disseminated disease and death has been reported.[13]
Wide surgical excision is the mode of treatment to minimize the risk of recurrence, with histological confirmation of the same.[9] Inadequate excision of the tumor results in recurrence and is common, up to 10%.[14] Adequate excision is recommended due to its high recurrence rate and potential malignancy. Complete excision of the nodular hidradenoma with wide margins should prevent local recurrence.[14]
CONCLUSION
Our study describes the clinical, pathological spectrum of the lesion. Although nodular hidradenoma is a benign lesion and excision is the only treatment, due to its varied clinical presentation, histopathology remains the main stay of diagnosis and helps to rule out the other skin appendageal tumors, certain benign and malignant conditions.
Acknowledgement
Nil.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Study on morphological features of nodular hidradenoma. Journal of Evolution of Medical and Dental Sciences. 2015;4:9140-5.
- [Google Scholar]
- Solid-cystic hidradenoma of the skin. Clinical and histopathologic study. Arch Dermatol. 1968;97:651-61.
- [Google Scholar]
- Nodular hidradenoma: A series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-7.
- [Google Scholar]
- A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-70.
- [Google Scholar]
- Dermoscopy of eccrine acrospiroma masquerading as nodular malignant melanoma. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:23-5.
- [Google Scholar]
- Malignant nodular hidradenoma of the skin: Report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-54.
- [Google Scholar]
- Weedon’s Skin Pathology. Vol 33. Edinburgh: Churchill Livingstone/Elsevier; 2016. p. :943-4.
- Fine-needle aspiration cytology of apocrine hidradenoma. Coll Antropol. 2010;34:671-4.
- [Google Scholar]
- Nodular hidradenoma case of rare localization. Asploro Journal of Biomedical and Clinical Case Reports. 2020;2020:18.
- [Google Scholar]
- Malignant hidradenoma–a case report demonstrating insidious histological and clinical progression. Clin Exp Dermatol. 1991;16:474-7.
- [Google Scholar]
- Clear cell hidradenoma of the axilla: A case report with literature review. Korean J Radiol. 2010;11:490-2.
- [Google Scholar]
- Atypical and malignant hidradenomas: A histological and immunohistochemical study. Mod Pathol. 2009;22:600-10.
- [Google Scholar]
- McKee’s Pathology of the Skin with Clinical Correlations (4th ed). China: Elsevier Saunders; 2012. p. :1571-4.






