Translate this page into:
Combination and Rotational Therapy in Androgenetic Alopecia
Address for correspondence: Dr. Muthuvel Kumaresan, Cutis Skin Clinic & Hair Transplant Center, R S Puram, Coimbatore 641002, Tamil Nadu, India. E-mail: dr_kumaresh@yahoo.co.in
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Management of androgenetic alopecia is a challenge because of its long course, need for continuous treatment, and potential adverse effects of the therapies. In order to enhance efficacy, minimize side effects, and ensure patient compliance, the authors propose a scheme for using combination treatments with a rotational scheme, based on current evidence for efficacy, pharmacokinetic properties, convenience of administration over long term, side effect profile, and patient acceptance.
Keywords
AGA
combination therapy
rotational therapy
INTRODUCTION
Combinational and rotational therapy is a concept that has been widely used in medicine and dermatology, and cancer treatment, particularly in chronic and unresponsive conditions.[1] Rotational therapy consists of using drugs and procedures or their combinations at different periods, with the following objectives: minimize the side effects of the different modalities, optimize the efficacy of the modalities, and prevent resistance, ensure maximum patient comfort and benefit, reduce the cost of treatment, and enhance patient compliance.
These objectives are relevant for any long-term treatment of a chronic condition, and when multiple modalities with different mechanisms of actions and pharmacological properties are available to treat. The concept is based on the following:
-
a.
Efficacy profile
-
b.
Mechanisms of actions of different drugs
-
c.
Side effect profile
-
d.
Pharmacokinetics and dynamics
-
e.
Duration of action and duration for which such treatments are needed
-
f.
Ease and cost of treatments
-
g.
Course of disease and its outcome
The concept of combination and rotational therapy is therefore useful and hence has been adapted to several diseases such as psoriasis, pemphigus, and Lupus erythematosus.[234]
Androgenetic alopecia (AGA) is a disease that is chronic, often with a variable course, with multiple modalities of treatment, and hence fits into this category. It has some additional features––It is a cosmetic disease, affects the younger patients more, and affects them socially and psychologically. These patients are very internet savvy and information hungry, are very conscious of adverse effects, and very often are reluctant to take medicines. It is a problem where exaggeration and misinformation are prevalent on social media––a case in point is the adverse publicity on finasteride, particularly post-finasteride syndrome.[567] In this field, often new off-label treatments get introduced ahead of good evidence and achieve internet publicity. It is also a field that is practiced by different specialties such as dermatology, and plastic surgery.
Hence, a concept such as rotational therapy would benefit these patients, due to the advantages mentioned above, help in enhancing long-term compliance, and achieve clarity for the practitioners. This paper explains how existing modalities can be adapted to this concept.
The different modalities that can be part of such systematic combinational and rotational approaches include the following:
-
a.
Minoxidil: Topical formulation with/without modifications; minoxidil finasteride combinations, minoxidil boosters such as tretinoin and dermaroller, and oral minoxidil,
-
b.
Finasteride, oral and topical, alternate regimes of oral administration, oral dutasteride, and topical dutasteride
-
c.
Hair transplantation
-
d.
Platelet-rich plasma (PRP)
-
e.
Low-level laser therapy (LLLT)
-
f.
Vitamins, peptides, and other treatments
-
g.
Diet and lifestyle
This paper will discuss how these modalities can be used either together or rotated sequentially or at intervals based on the current knowledge of the course and pathogenesis of AGA, mechanisms, and duration of actions of each treatment. As the scheme is based on the pathophysiology of AGA, the mechanisms of actions, pharmacokinetic data, and side effect profile it is important to understand these aspects and are discussed first.
Pathogenesis of androgenetic alopecia
AGA is characterized by continuous and progressive, but variable androgen-mediated miniaturization due to decreasing number of stem cells, with exacerbations and remissions. There are often variable periods of rapid progress, and stability. Other factors such as systemic diseases nutrition, lifestyle, stress, etc., may play a role. The key pathophysiological features of AGA are alteration in hair cycle development, follicular miniaturization, and inflammation. The anagen phase decreases with each cycle, while the length of telogen remains constant or is prolonged; this results in a reduction of the anagen to telogen ratio.[8910] Prolongation of the kenogen phase, the lag phase or the delayed replacement of telogen hair, seems to last longer in AGA leaving a higher percentage of empty hair follicles contributing to balding.[10111213] This further reduces hair numbers contributing to the balding process.[1011] Ultimately, anagen duration becomes so short that the growing hair fails to achieve sufficient length to reach the surface of the skin, leaving an empty follicular pore. Hair follicle miniaturization is hence the histological hallmark of AGA. Once the arrector pili muscle, which attaches circumferentially around the primary follicle, has detached from all secondary follicles and primary follicles have undergone miniaturization and detachment, hair loss is irreversible.[8] Traditional models of AGA show follicular miniaturization occurring in a stepwise fashion. This has recently been contested, and it is now believed that the transition from terminal to vellus hair can also occur as an abrupt, large-step process.[13] Thus follicular miniaturization occurs between anagen cycles rather than within the anagen phase. This short window of androgen effect may also explain the lengthy delay experienced between clinical response and the commencement of therapy, as any pharmacological intervention will only have an effect at the point of miniaturization.[13]
Hence, effective, therapy would need treatments that affect androgen, stem cells, growth factors, and anagen prolongers, which are discussed below. Trichoscopy can be used at every visit to provide an objective evaluation of the various treatments.
Different treatment approaches which are useful for rotational therapy
The medical management of AGA consists of different agents shown in Table 1 which also shows their mechanisms of action, their evidence levels, and side effects and limitations. The combination of these modalities helps us to counter the different underlying processes in AGA simultaneously. The authors consider finasteride, topical minoxidil, PRP, and LLT to be core modalities, while others are alternative options. The authors consider PRP to be a primary core treatment in the management of AGA, in view of recent guidelines which advocate Level 2 evidence by Sharma et al.,[17]; level 1 by Gentile et al.,[18] Level of evidence: 2 Category of Recommendation: B by Mysore et al.,[19]
| Type | Evidence S3 guidelines[14] | Mechanism of action | Side effects and limitations |
|---|---|---|---|
| CORE MODALITIES | |||
| Minoxidil 5% topical solution/ foam twice daily Finasteride 1mg once daily dosage Low dose intermittent therapy advocated[15] |
Level 1 Level 1 |
Minoxidil is a nonspecific anagen stimulator Finasteride acts on basic etiology due to the action on 5Alpha reductase - |
Minor side effects, in the initial weeks. Long term compliance is an issue. It does not affect the course of thinning and plateau effect is seen after 1–3 years in different patients. Contraindications to minoxidil include patients with a history of sensitivity of minoxidil or its components like propylene glycol. It is not recommended in pregnant and breastfeeding mother. Topical minoxidil also must be avoided in patients with acutely inflamed or infected scalp. Sexual and central side effects, gynecomastia. poor patient acceptance because of fear of side effects. Contraindicated in children, pregnant women. |
| PRP | Level 3–S3 guidelines evidence Level 2 by other authors[171819] |
Biological regenerative treatment which may affect the basic pathophysiology at the level of stem cells | Popular with great patient acceptability, inconvenient due to frequent treatments and expensive |
| Hair transplantation Low level laser therapy (LLLT |
Level 2 Level 2 |
Surgical hair restoration LLT may induce cytokines for growth |
Surgery is becoming popular, expensive Safe, easy to administer, but of low efficacy and relatively expensive |
| ALTERNATIVE OPTIONS | |||
| Topical finasteride 1%,[16] Minoxidil 5% with finasteride 0.1%, Microneedling, Azelaic acid, Higher strength minoxidil, tretinoin, Oral amino acids, Oral Vitamins(Biotin, Niacin),Oral Trace elements (Zinc and Copper), proteins Peptide solutions |
Level of evidence 3 No recommendation No recommendation |
Tretinoin, azelaic acid microneedling may affect sulphotransferase and enhance absorption Vitamins and minerals may correct micronutrient deficiency Claimed to affect metabolism in hair |
Absorption doubtful, but good acceptability Peptides are cosmetically pleasant and acceptable |
| Oral Minoxidil 1.25–5mg/day . | level of evidence 4 | Prolongs anagen phase | Off label, safety concerns of systemic toxicity To be used in selected cases only |
| DUTASTERIDE O.5 MG per day; intermittent twice weekly doses can be given | Not yet approved | •acts on basic etiology due to their action on 5Alpha reductase - more potent than finasteride.[2021] | • elimination half-life of dutasteride is longer- 5 weeks at steady state.[2223] •no statistical difference between finasteride and dutasteride in prevalence of side effects[24] |
There are specific aspects of each modality that are relevant in combinational and rotational therapy and are considered below.
demonstrated in a study
Finasteride
Finasteride is perhaps the most important treatment modality because of its proven action, long-term efficacy, ease of administration, and its action on the basic pathophysiology. However, it is also the most difficult of the treatments to prescribe over long term not only because of its side effect profile, but also the fear of its side effect profile. Minimum period of 3 months of therapy is required for clinical response.[25] Finasteride needs to be continued indefinitely and efficacy seems to improve with time and consistent use prevents deterioration of miniaturization[26272829] The effects of the drug are reversed within 12 months after treatment cessation.[25]
The mean terminal elimination half-life of 1 mg daily finasteride after repeated administration is 4.8 h and tissue binding is 4–5 days.[2530] Steady-state trough plasma finasteride concentrations are reached within 3 days.[31]
Serum DHT levels returned to baseline values within 14 days after drug withdrawal irrespective of the dosage.[31]
Doses of 0.2 mg are adequate to suppress both scalp skin and serum DHT levels.[30]
Suppression of DHT levels persists for 14 days after drug withdrawal irrespective of the dosage administered before returning to normal.[31]
Intermittent therapy has been shown to be effective. Intermittent discontinuation of the drug does not lead to loss of efficacy.[32] Hence drug holidays to create drug washout is feasible.
Side effects of finasteride include lowered libido, erectile dysfunction, reduced ejaculatory volume, temporary reduction in sperm count, testicular pain, depression, and gynecomastia.[33] Sexual events resolved in many patients who reported them but remained on therapy.[33] Central side effects include depression, mood changes. These need to be considered before starting treatment.
An important predictor of development of side effects was use of the drug for at least 7 months suggesting that administration of several months was necessary for the development of side effect.[34]
Existence of post-finasteride syndrome (PFS) has been questioned[353637]
Current FDA guidelines stress that patient needs to be given full information about finasteride side effects and the final decision to take the drugs rests with the patient.
Based on these findings it can be summarized that:
Finasteride is a highly effective, easy-to-administer, cheap drug
Its effect is sustained over long duration
It increases hair counts and reverses miniaturization
It may work at does lower than FDA recommended dose of 1mg daily
It may also work if discontinued and reintroduced, and used intermittently
The onset of side effects is after about 7 months
Since, its side effective profile is of concern to many patients, and is a deterrent in acceptance of this drug, alternative strategies are necessary to enhance its acceptance. These include topical therapy. Administration of lower doses, intermittent therapy with drug holidays
Minoxidil
Is a safe and proven topical drug in 5% concentration.
Minoxidil causes frequent though minor side effects such as irritation, headache, initial transient hair loss, dryness, etc., leading to poor compliance. These can be minimized by initiating once-daily administration alongside the use of cosmetically acceptable peptide therapy.
Minoxidil boosters such as 0.01% tretinoin, dermaroller elevate the level of follicular sulfotransferase enzyme levels which remain unchanged throughout even up to 8 weeks post-therapy after administration of boosters.[3839] Hence, the boosters can be used intermittently, particularly in nonresponsive cases.
Does not affect the primary miniaturization process which continues unabated and hence its effects taper off when used for 3–4 years continuously.[40414243]Thus minoxidil cannot be an effective solo treatment for AGA.
If treatment is ceased, any positive effect on hair growth is lost in 4-6 months.[4445]
Initial effects are noticed by approximately 8 weeks while the maximum effects after 4 months.[46] The effects peak by the 12th month.[4748]
Non-responders may have a clinical response with higher concentration of minoxidil.[4950]
Thus, the above summary shows that minoxidil has a high quality of evidence, is safe, but has compliance issues in the early phase of therapy and efficacy issues in later phases of therapy. It is also not an effective solo treatment when used long term. There may be an additional role for use of minoxidil boosters in nonresponder and when plateau effect is reached.
PRP
PRP is a well-accepted and safe modality and is backed by 19 systematic reviews, 9 meta-analysis, and 12 clinical trials.[18] However, there is variability in its administration and preparation and hence levels of evidence. PRP has received level 3 evidence in 2018 European S3 guidelines on the treatment of AGA, because of the heterogeneity of methods of preparation of PRP.[14] Recently, the Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) has come out with recommendations on the preparation of PRP which will pave the way for a more standardized PRP preparation.[51] The authors consider PRP to be a primary core treatment in the management of AGA, in view of recent guidelines which advocate Level 2 evidence by Sharma et al.,[17]; level 1 by Gentile et al.,[18] Level of evidence: 2 Category of Recommendation: B by Mysore et al.,[19]
The onset of effect of PRP in hair growth is quick, about 2–3 weeks, with some effects seen as early as 5 days.[525354]
Most studies have recommended three sessions at one month apart.[55] The maintenance of sessions of PRP has been advised to be once in six months.[56]
Duration of action of one single session of PRP may persist till 6 months after the procedure.[5657]
Thus, there is an opinion that minimum three-five sessions are required prerequisite for a significant increase in hair density.[58]
Maintenance treatments have been carried at intervals of 3–6 months[17] beyond six months of therapy.
Long-term effects beyond 16 months are not known.[54]
In summary, it can be said that PRP has been shown to have increasingly better evidence and hence is included as a core treatment by authors. Its onset of action is once in 2-3 weeks and is given in 3-5 monthly sessions. The actions can persist for many months and hence can be repeated once in six months. Longer-term data beyond 16 months is not yet available.
Low-level laser therapy
LLLT is typically administered through home-use devices that are available in the forms of combs, helmets, and caps and hence are an acceptable treatment, particularly to patients who are apprehensible to drug treatments.
The mechanism of action is not completely elucidated, but is believed to be increased ATP production, reactive oxygen species modulation, and transcription factor induction, protein synthesis, and NO-related vasodilation.[596061]
LLLT increases the terminal hair count and reduces hair fall with minimal adverse effects.[626364]
The most widely used regimen is alternate days for a period of at least 4 months to start seeing the results and to be continued indefinitely.[656667]
Most reports are in patients with early thinning.
Combining LLLT with other standard medical management has proven to give superior results.[6869]
European S3 guidelines gave evidence level 2 for the device.[14]
LLLT is an option for management in those in whom drug therapy is contraindicated or not feasible. It is also an option for long-term management. It may not work well in patients with significant thinning.
Hair transplantation (HT): HT by either FUT or FUE with scalp or body hair is a safe, acceptable option yielding cosmetically acceptable and lasting results. The choice of HT depends on the stage of baldness, age of the patient, psychological requirement of the patient, availability of donor, etc.
A recent publication provides logic for use of multitherapies sequentially or rotationally. Studies have demonstrated that, hairs could recover their original dimensions if there was a less than two-thirds decrease of the dermal papilla cells; but beyond this, the hairs went into vellus in dimension, suggesting the need for early specific therapeutic intervention.[70]Another publication by Paul Kemp hypothesized that the scalp may contain four types of cells:[70]
Type 1: Hair follicles containing DHT-insensitive DP cells that will not miniaturize.
Type 2: Hair follicles containing DHT-sensitive DP cells that are not miniaturizing yet but probably will in the future.
Type 3: Hair follicles containing DHT-sensitive DP cells that are actively miniaturizing but can be rejuvenated.
Type 4: Hair follicles containing DHT-sensitive DP cells that have miniaturized too far that cannot be rejuvenated.
Most of the therapies in rotational and combinational therapy act on follicle types 2 and 3 which are most susceptible to miniaturization and most amenable for therapeutic intervention. Judicious use of above modalities in combination/ drug holidays/ intermittent usage can therefore help in sustaining results by preventing miniaturization of DP cells beyond the point of recovery over long time, and we propose the following scheme.
Figure 1 shows core treatments (treatments commonly used and with evidence) and noncore treatments (treatments that are off label, and used as an alternative option).
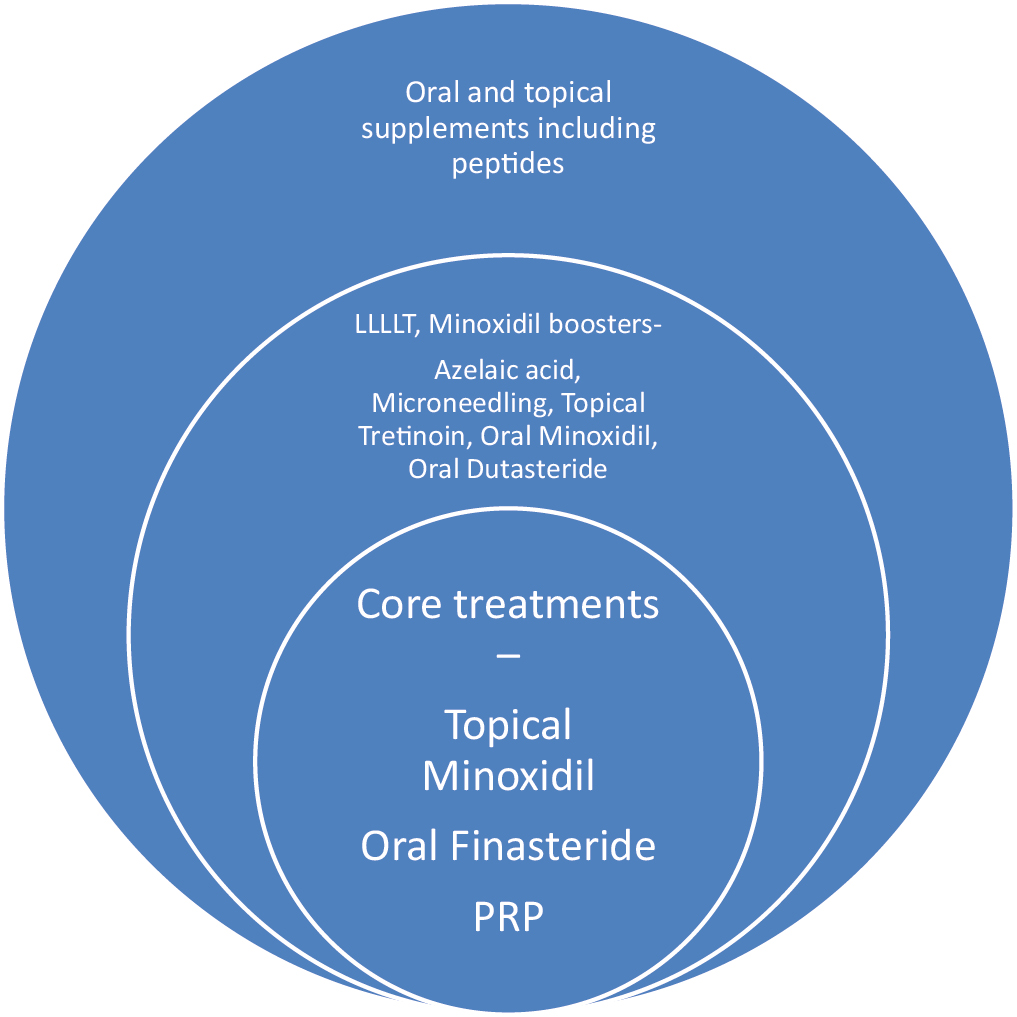
- Treatments divided on the basis of evidence and use. Core treatments are commonly used and with evidence shown in the innermost circle. Peripheral circles show non-core treatments which are either off-label or used as alternative options
These different treatments are used in a combination approach, based on above properties, with treatments entering and exiting at different time zones. Noncore treatments are added on need basis based on the judgment of the physician. The proposed scheme is shown in Figures 2,3–4.
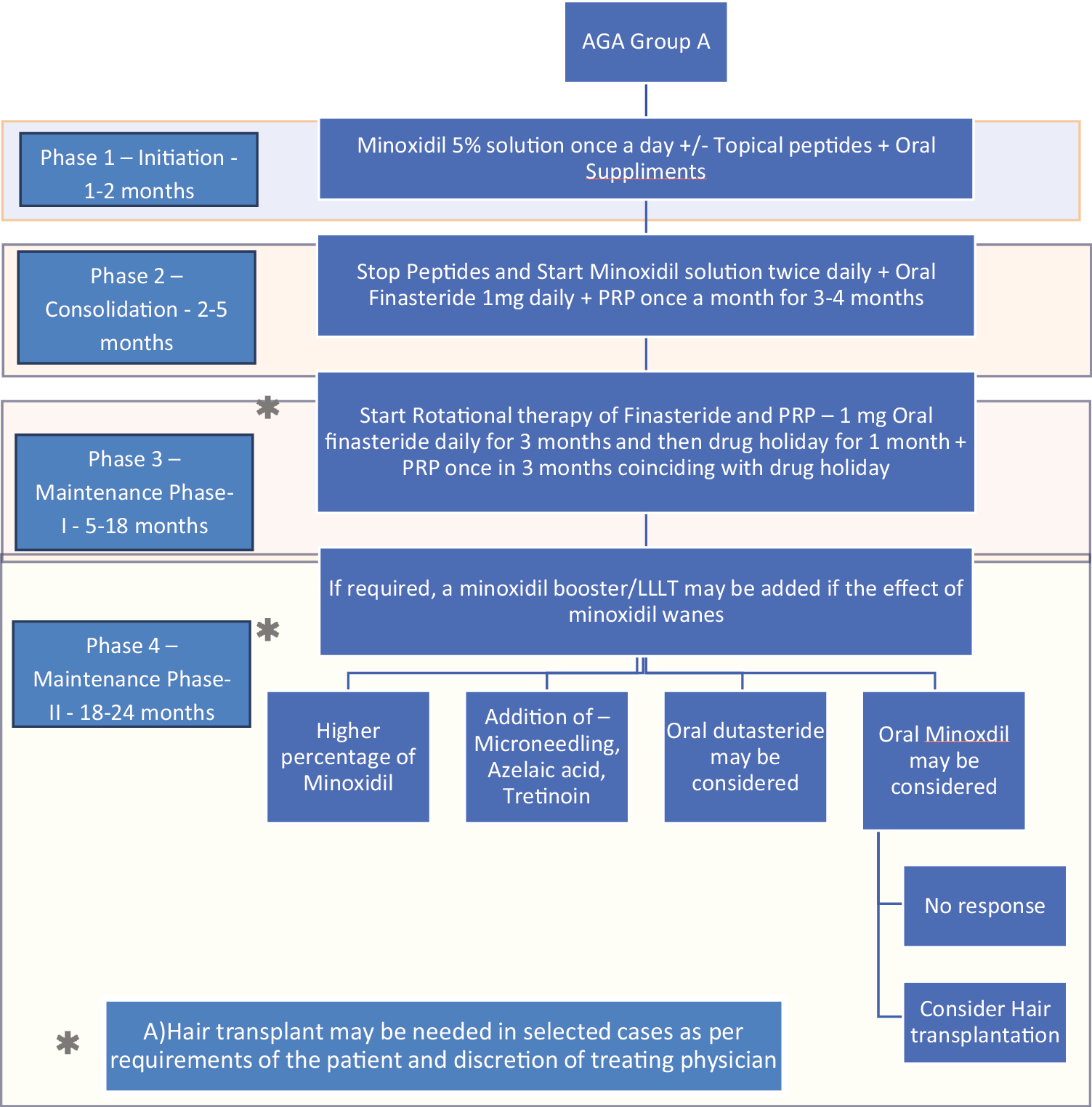
- Treatment algorithm using rotational therapy for androgenetic alopecia group A (patients who accept and tolerate finasteride)
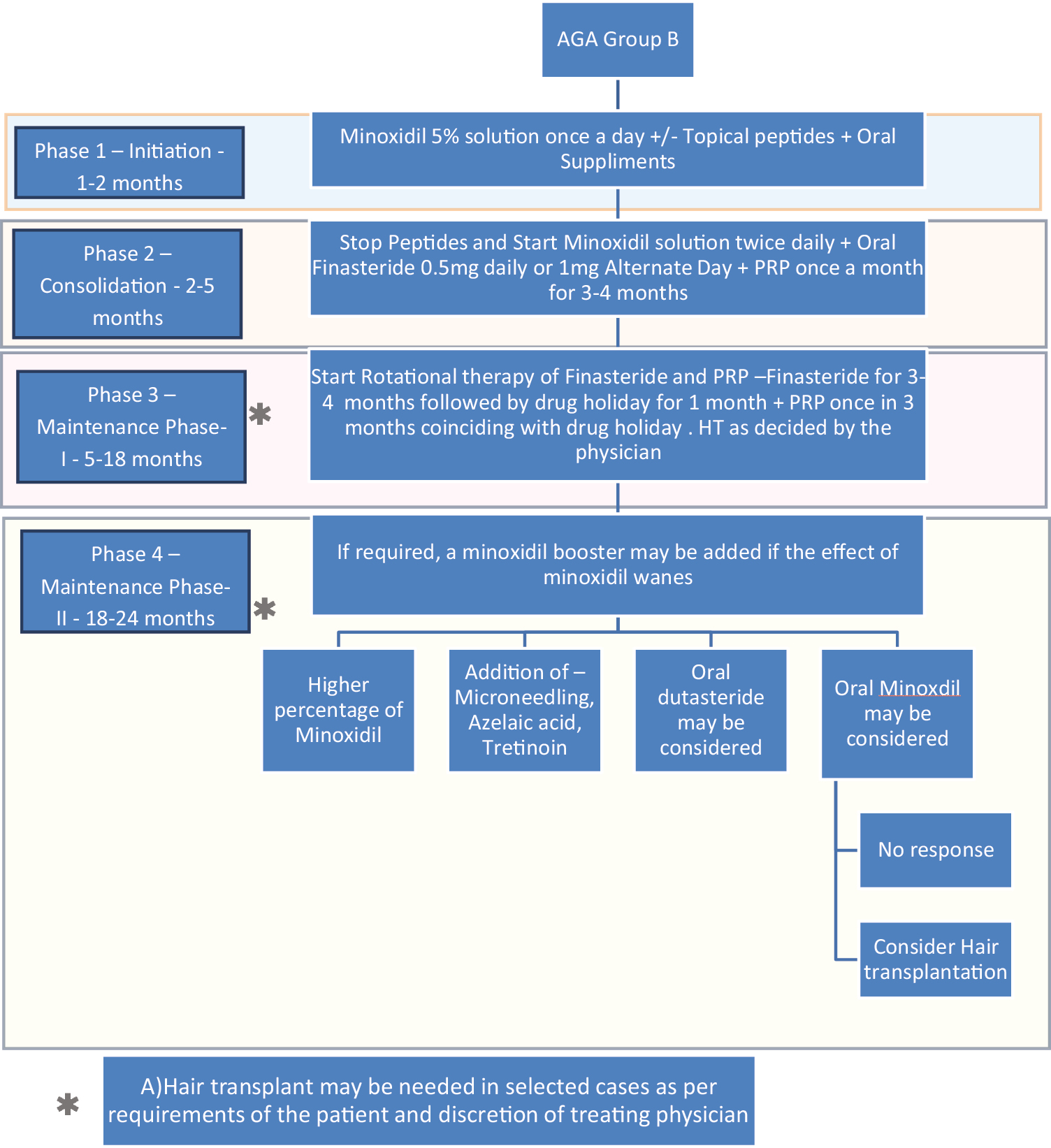
- Treatment algorithm using rotational therapy for androgenetic alopecia group B (patients who are apprehensive about taking finasteride)
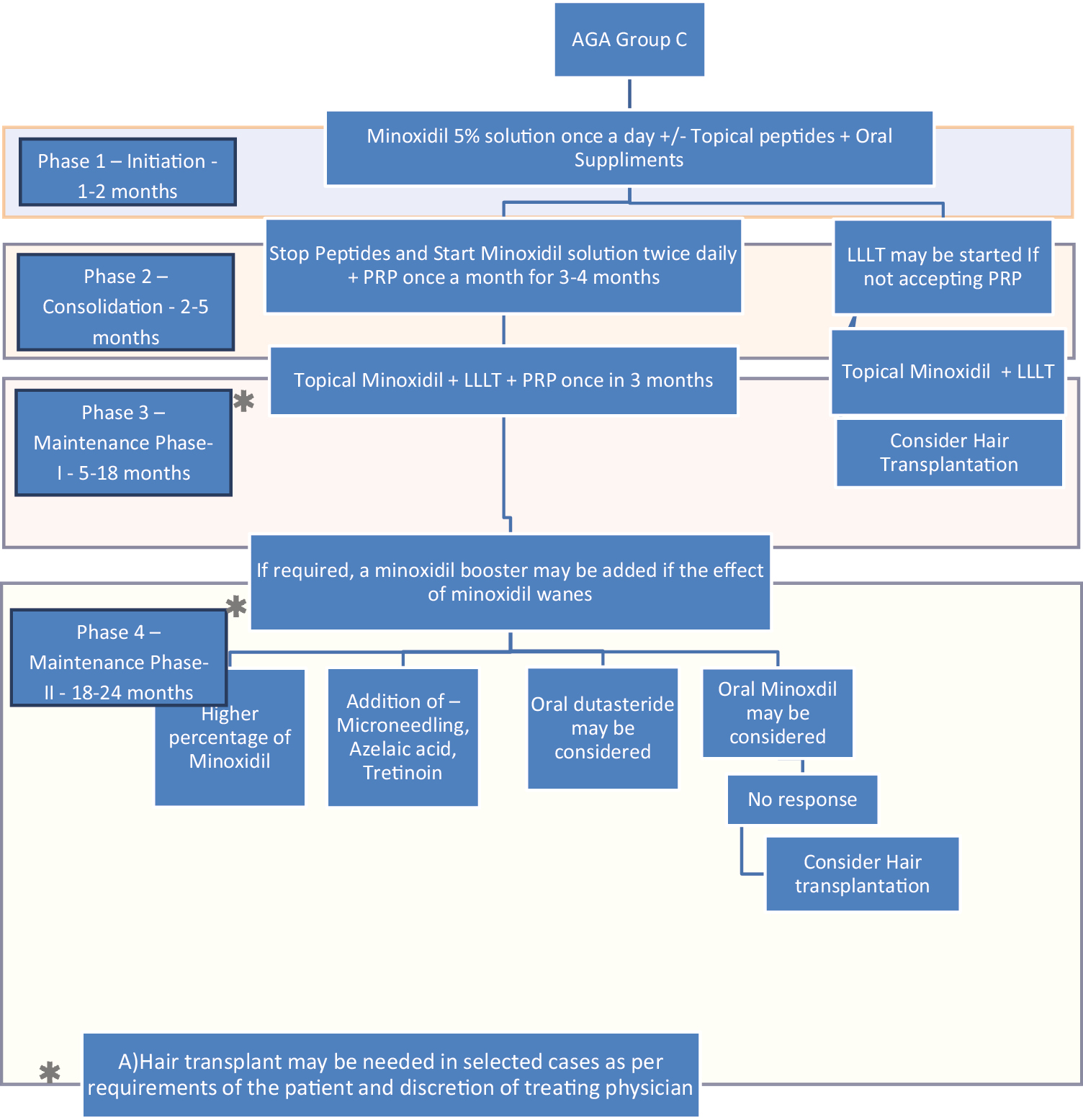
- Treatment algorithm using rotational therapy for androgenetic alopecia group C (patients who either refuse or develop side effects of finasteride and who need alternative treatments)
There are four phases in the management. Table 2 outlines these phases and their options in tabular manner. It needs to be recognized that the principal issues are:
| Phase | Duration | Treatment | Comments |
|---|---|---|---|
| Phase 1 | 0–2 months | Topical minoxidil 5% once a day, +/- peptides, nutrients |
Focus on diet, counseling education, acceptability of treatments |
| Phase 2 | 2–6 months | Group a. Continue Topical minoxidil 5% twice daily Start Finasteride 1mg daily if acceptable Group b. Low dose finasteride 0.5 mg daily/topical finasteride if apprehensive of finasteride Group c. PRP/ LLLT if finasteride is refused |
Focus on diet, counselling, avoiding smoking HT may be needed in special situations based on patient needs and physician assessment |
| Phase 3 | 6–18 months | Group a. Good responders- continue as in phase 2 with Topical minoxidil, Finasteride holiday, Intermittent PRP once in 3–4 months Group b & c. Continue topical minoxidil 5% twice daily, intermittent PRP/LLT HT in selected cases |
Finasteride introduction or finasteride full dose may be possible in those who were apprehensive earlier |
| Poor responders- Oral Minoxidil, Dutaseride & HT to be considered | Proper counselling needed | ||
| Phase 4 | Beyond 18 months | Group a. Continue Topical minoxidil 5% twice daily with, Finasteride holiday, Intermittent PRP once in 3–4 month, HT in selected cases Group b. Topical minoxidil, Intermittent PRP once in 3-4 month4 months/LLLT HT in selected cases Group c. Nonresponders: Minoxidil boosters finesteride, Dutaseride, HT |
Finasteride introduction may be possible in those who were apprehensive earlier Proper counseling is needed |
Acceptability and tolerance of finasteride: Three categories exist.
Group A patients who accept and tolerate finasteride [Figure 2].
Group B patients who are apprehensive but are willing to try low dose and other alternative regimes such as topical finasteride initially. These patients may accept and tolerate finasteride in course of time and hence full dose may be acceptable at a later date [Figure 3].
Group C patients who either refuse or develop side effects of finasteride and who need alternative treatments [Figure 4].
In group A and B, in the longer run in phases 3–4, finasteride will be administered intermittently with 1 month drug holidays to avoid possibilities of side effects.
-
2.
PRP is introduced as core treatment with an intensive phase of 3–5 sessions in phase 2 and later maintenance once in 3–6 months in phases 3–4, particularly in the finasteride holiday month
-
3.
LLLT and HT are needed in addition to above treatments depending on requirements of a given case.
Different phases are given below.
Phase 1: Initial 1–2 months of treatment: Initiation phase
At this stage, primary concern is smooth initiation of treatment, with minimum side effects, preventing any patient dropout due to side effects that may be minor, but may affect quality of life, such as dryness, irritation, temporary shedding, etc., and education of the patient for long term therapy. So, the authors recommend initiation with topical minoxidil 5% local application once daily, with or without a peptide serum once daily with supplements of vitamins, micronutrients and protein-rich diet as necessary in the patient. Finasteride is not recommended at this stage. Patients are encouraged to read and get informed about different modalities with counselling is done about different options for therapy.
Phase 2: 2–5 months of treatment––Consolidation phase
At this stage, as patients are getting accustomed to the schedule and are beginning to see early result, peptide is stopped and topical minoxidil 5%application is increased to twice daily (which is indeed the recommended dose). At this stage, a modality that affects the basic pathogenesis; oral 1mg finasteride (if patient is accepting it) and/or PRP (once a month for 3–4 months) is initiated. If patient is apprehensive of finasteride, he is encouraged to use an alternative regime for finasteride such as alternate days or low dose (0.5mg daily) or topical finasteride till he finds confidence to accept oral finasteride. No effort is made to compel the patient to take finasteride. The emphasis is on education and compliance. If patient refuses to accept finasteride, PRP is initiated once a month. LLLT is an option if both finasteride and PRP are not feasible or acceptable.
Phase 3: Beyond 5/6 months of treatment maintenance phase 1
At this stage, most patients have seen the results and can be shifted on to maintenance regimes;
-
A)
Topical minoxidil+ oral finasteride daily (in finasteride accepting patients. Since there is evidence that drug-induced side effects are seen after 6-7 months of therapy, intermittent therapy as stated earlier, with a drug holiday period of 1 month for washing out the drug is introduced once every 3-4 months. The authors also recommend PRP in the month of drug holiday to compensate for lack of finasteride effects. This is logical since onset of PRP is seen in 2 weeks.as stated above.
-
B)
For patients who do not accept finasteride, Topical minoxidil with LLT and PRP once in 3-4 months.
-
C)
Topical minoxidil 5% with LLT for patients who do not accept Finasteride and PRP is not feasible.
-
D)
HT may be needed in selected cases as per requirements of the patient and discretion of treating physician.
Phase 4: 18–24 months and beyond. Maintenance phase 2
If patient continues to respond well, above treatment as in phase 3 is continued. Currently, the maximum duration for which PRP effect has been followed up is 16 months and hence there is no evidence to state how long PRP can work in the long run––but there is no evidence to state it does not work either. Hence this is left to the discretion of the treating physician. However, this is when effect of minoxidil may begin to wane, and patients may see deterioration of results and hence alternative options may be are needed:
-
A)
Introduce a higher percentage of minoxidil
-
B)
Addition of minoxidil boosters
-
C)
In case of nonresponder, HT may be needed as per the decision of the treating physician.
-
D)
Oral dutasteride and oral minoxidil are options available in this phase in nonresponder who do not want surgery. Both the drugs, however, carry the risk of potentially serious side effects and hence need to be given after proper counseling.
CONCLUSIONS
Thus the scheme provides a framework for physicians to introduce, combine, alternate, and rotate different treatments, to minimize side effects, render the regime acceptable to patients, and to minimize costs. However, as in all regimes, this is a general scheme and may need to be tailored/modified depending on the needs of a given patient. The authors agree that the outlined schema is not necessarily fully perfect (no scheme is) and is not necessarily applicable or can be effective in all cases. Nor is the authors claim that this is an original discovery. It is only an attempt at formulating a systematic approach based on available data and much of what is already in practice by many dermatologists. It renders the approach to patient management simple and systematic, which can be followed easily.
The authors would like to clarify that these recommendations/proposals are an effort to systematize the approach based on currently available evidence and logic, to minimize side effects, and to enhance patient acceptability. These could change as further data become available.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The Advantage of Cyclosporine A and Methotrexate Rotational Therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- [Google Scholar]
- An approach to the treatment of moderate to severe psoriasis with rotational therapy. J Am Acad Dermatol. 1993;28:454-9.
- [Google Scholar]
- Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80:14-25.
- [Google Scholar]
- Therapeutic strategies: Rotational therapy and combinations. Clin Exp Dermatol. 2001;26:356-61.
- [Google Scholar]
- Post-finasteride syndrome: A surmountable challenge for clinicians. Fertil Steril. 2020;113:21-50.
- [Google Scholar]
- Destruction of the arrector pili muscle and fat infiltration in androgenic alopecia. Br J Dermatol. 2014;170:1291-8.
- [Google Scholar]
- Anagen hairs may fail to replace telogen hairs in early androgenic female alopecia. Dermatology. 1996;192:28-31.
- [Google Scholar]
- Androgenetic alopecia: New insights into the pathogenesis and mechanism of hair loss. F1000Res. 2015;4(F1000 Faculty Rev):585.
- [Google Scholar]
- Androgenetic alopecia: Analysis of proliferation and apoptosis. Arch Dermatol. 2002;138:1101-2.
- [Google Scholar]
- Possible mechanisms of miniaturization during androgenetic alopecia or pattern hair loss. J Am Acad Dermatol. 2001;45:S81-86.
- [Google Scholar]
- Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22.
- [Google Scholar]
- Guidelines on the use of finasteride in androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2016;82:128-34.
- [Google Scholar]
- A systematic review of topical finasteride in the treatment of androgenetic alopecia in men and women. J Drugs Dermatol. 2018;17:457-63.
- [Google Scholar]
- Platelet-rich plasma in androgenetic alopecia. Indian Dermatol Online J. 2021;12(Suppl 1):S31-S40.
- [Google Scholar]
- Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil®, finasteride®, and adult stem cell-based therapy. Int J Mol Sci. 2020;21:2702.
- [Google Scholar]
- Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179-84.
- [Google Scholar]
- Dutasteride in androgenetic alopecia: An update. Curr Clin Pharmacol. 2017;12:31-5.
- [Google Scholar]
- A review of current data on a novel dual inhibitor of 5α reductase. Rev Urol. 2005;7:203-10.
- [Google Scholar]
- The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. Br J Clin Pharmacol. 1999;47
- [Google Scholar]
- The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: A systematic review and meta-analysis. Clin Interv Aging. 2019;14:399-406.
- [Google Scholar]
- Long-term treatment with finasteride 1 mg decreases the likelihood of developing further visible hair loss in men with androgenetic alopecia (male pattern hair loss) Eur J Dermatol. 2008;18:400-6.
- [Google Scholar]
- Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-61.
- [Google Scholar]
- Evaluation of long-term efficacy of finasteride in korean men with androgenetic alopecia using the basic and specific classification system. J Dermatol. 2019;46:139-43.
- [Google Scholar]
- Five-year efficacy of finasteride in 801 Japanese men with androgenetic alopecia. J Dermatol. 2015;42:735-8.
- [Google Scholar]
- The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. J Am Acad of Dermatol. 1999;41:550-4.
- [Google Scholar]
- Effects of finasteride (MK-906), a 5α-reductase inhibitor, on circulating androgens in male volunteers. J Clin Endocrinol Metab. 1990;70:1136-41.
- [Google Scholar]
- Intermittent low-dose finasteride is as effective as daily administration for the treatment of hirsute women. Fertil Steril. 2004;82:752-5.
- [Google Scholar]
- Incidence and severity of sexual adverse experiences in finasteride and placebo-treated men with benign prostatic hyperplasia. Urology. 2003;61:579-84.
- [Google Scholar]
- Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:e3020.
- [Google Scholar]
- Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011;8:1747-53.
- [Google Scholar]
- Persistent sexual side effects of finasteride: Could they be permanent? J Sex Med. 2012;9:2927-32.
- [Google Scholar]
- Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:e3020.
- [Google Scholar]
- Efficacy of 5% minoxidil versus combined 5% minoxidil and 0.01% tretinoin for male pattern hair loss: A randomized, double-blind, comparative clinical trial. Am J Clin Dermatol. 2007;8:285-90.
- [Google Scholar]
- Comparative study of efficacy of topical minoxidil 5% and combination of topical minoxidil 5%, topical azelaic acid 1.5% and topical tretinoin 0.01% on the basis of dermoscopic analysis in androgenetic alopecia. MVP Journal of Med Sci. 2015;2:90-9.
- [Google Scholar]
- Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:643-6.
- [Google Scholar]
- Androgenetic alopecia: An evidence-based treatment update. Am J Clin Dermatol. 2014;15:217-30.
- [Google Scholar]
- Long-term efficacy of topical minoxidil in male pattern baldness. J Am Acad Dermatol. 1987;16:711-8.
- [Google Scholar]
- Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J Am Acad Dermatol. 1999;41:717-21.
- [Google Scholar]
- Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:643-6.
- [Google Scholar]
- Topical minoxidil in male pattern baldness: Effects of discontinuation of treatment. J Am Acad of Dermatol. 1987;17:97-101.
- [Google Scholar]
- Relationship between contact time of applied dose and percutaneous absorption of minoxidil from a topical solution. J Pharm Sci. 1990;79:483-6.
- [Google Scholar]
- Topical minoxidil in androgenetic alopecia, how good is it? Indian J Dermatol Venereol Leprol. 1990;56:187-92.
- [Google Scholar]
- Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-6.
- [Google Scholar]
- Minoxidil dose response study in female pattern hair loss patients determined to be non-responders to 5% topical minoxidil. J Biol Regul Homeost Agents. 2016;30:1153-5.
- [Google Scholar]
- Minoxidil and its use in hair disorders: A review. Drug Design, Development and Therapy. 2019;13:2777-86.
- [Google Scholar]
- Preparation of platelet-rich plasma: National IADVL PRP taskforce recommendations. Indian Dermatol Online J. 2021;12(Suppl 1):S12-S23.
- [Google Scholar]
- Promotional effect of platelet-rich plasma on hair follicle reconstitution in vivo. Dermatol Surg. 2013;39:1868-76.
- [Google Scholar]
- As a carrier-transporter for hair follicle reconstitution, platelet-rich plasma promotes proliferation and induction of mouse dermal papilla cells. Sci Rep. 2017;7:1125.
- [Google Scholar]
- The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4:1317-23.
- [Google Scholar]
- Platelet-rich plasma for androgenic alopecia treatment: A comprehensive review. Dermatol Online J. 2018;24:13030/qt8s43026c.
- [Google Scholar]
- Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J CutanAesthet Surg. 2014;7:213-9.
- [Google Scholar]
- Platelet-rich plasma for androgenetic alopecia: A review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2018;5:46-51.
- [Google Scholar]
- Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-7.
- [Google Scholar]
- Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559-67.
- [Google Scholar]
- The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516-33.
- [Google Scholar]
- Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46:144-51.
- [Google Scholar]
- Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: A multicenter, randomized, sham device-controlled, Double-blind Study. Am J Clin Dermatol. 2014;15:115-27.
- [Google Scholar]
- HairMaxLaserComb® laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham devicecontrolled. Multicentre Trial Clin Drug Investig. 2009;29:283-92.
- [Google Scholar]
- Low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-83.
- [Google Scholar]
- Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: A 24-week, randomized, double-blind, sham devicecontrolled trial. Lasers Med Sci. 2019;34:1107-14.
- [Google Scholar]
- Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: A 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med Sci. 2019;34:1107-14.
- [Google Scholar]
- The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-95.
- [Google Scholar]
- The effectiveness of adding low-level light therapy to minoxidil 5% solution in the treatment of patients with androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2018;84:547-53.
- [Google Scholar]
- Role of Low-Level Light Therapy (LLLT) in Androgenetic Alopecia. J CutanAesthet Surg. 2021;14:385-91.
- [Google Scholar]
- The evolution of the promise of hair cloning. How hair cell cloning will fit into your practice. Hair Transplant Forum International. 2022;32:37-43.
- [Google Scholar]






