Translate this page into:
Comparative analysis of various hair peptide serums in managing telogen effluvium in females: Efficacy, safety, and patient satisfaction
*Corresponding author: B. S. Chandrashekar, Department of Dermatology, CUTIS Academy of Cutaneous Sciences, Bengaluru, Karnataka, India. cutisclinic@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chandrashekar BS, Roopa MS, Kusuma MR, Madura C, Shenoy C, Narayna NL, et al. Comparative analysis of various hair peptide serums in managing telogen effluvium in females: Efficacy, safety, and patient satisfaction, J Cutan Aesthet Surg. doi: 10.25259/JCAS_138_2024
Abstract
Objectives
The aim of this study was to evaluate the efficacy and safety of three hair serums containing different active ingredients Capilia longa and combinations, cytokines and peptides, and redensyl and aminexil in managing Telogen effluvium (TE).
Material and Methods
An open-label, triple-arm, single-center, and comparative study was conducted over 90 days, enrolling 45 adult females with TE. Subjects were randomized into three groups (n = 15 each): Group 1 (plant stem cell-based serum), Group 2 (cytokine-based serum), and Group 3 (redensyl and aminexil-based serum). Hair shedding was assessed through a hair wash test, trichoscopy, and clinical evaluations, and safety was monitored throughout.
Results
All groups demonstrated hair shedding reduction, with Group 2 showing the most significant decrease (54.6% reduction in hair fall) compared to Group 1 (23.9%) and Group 3 (26.3%). Trichoscopy analysis revealed improvements in hair count, density, and anagen percentage across groups, with Group 2 showing the highest increases in anagen percentage (+6.16%) and hair density (64.74%). No adverse events were reported, indicating good tolerability.
Conclusion
All three hair serum formulations were effective in reducing shedding and promoting hair regrowth in TE. Among them, cytokine-based hair serum (Group 2) showed the greatest efficacy followed by the other formulations. Hair serums can serve as a valuable adjunctive treatment for TE.
Keywords
Cytokine-based serum
Hair regrowth
Hair serum
Telogen effluvium
INTRODUCTION
Telogen effluvium (TE) is a scalp disorder characterized by the diffuse thinning or shedding of hair due to the premature entry of hair into the telogen phase. The condition is one of the most common causes of diffuse hair loss and typically occurs 3 months after a triggering event, such as stress or illness. Although generally self-limiting, TE lasts about 6 months, with hair shedding ceasing within 3–6 months after the removal of the trigger. However, cosmetically significant regrowth may take 12–18 months. Management focuses on identifying and addressing underlying causes and counseling patients about the natural course of the disorder. Potential therapeutic options are formulations that promote anagen and inhibit catagen and exogen.1-3
In TE, the general pharmacological treatments for hair regrowth include both topical and low-dose oral minoxidil.4 Corticosteroids may also be prescribed to reduce inflammation and promote regrowth, particularly in cases where inflammation is a contributing factor to hair loss.2 Alternative therapies like botulinum toxin A, administered in a single session, have been effective in improving various hair parameters.5 In addition, dietary supplements containing Boswellia serrata, Curcuma longa, and Vitis vinifera have demonstrated potential benefits for hair health, although further studies are necessary to confirm their efficacy.6
Hair serums play a supportive role in managing TE by targeting multiple aspects of hair health, primarily through ingredients that nourish the scalp, strengthen the hair shaft, and promote follicle activity.7 In TE, a significant proportion of hair follicles prematurely shift to the telogen phase, leading to excessive hair shedding.8 The active ingredients in hair serums such as peptides, vitamins (e.g., biotin and niacinamide), botanical extracts, and antioxidants are designed to improve scalp circulation, enhance the strength and resilience of existing hair, and support a healthy environment for new growth.7,9 Peptides and plant extracts encourage follicles to enter the anagen (growth) phase more rapidly, while vitamins and hydrating agents help repair the hair shaft, minimizing breakage and the appearance of thin, weak hair that is common with TE. In addition, antioxidants protect the scalp from environmental stressors, which may further disrupt hair cycles in TE.10,11 These combined actions make hair serums a valuable adjunct in managing TE.
This study aims to assess the efficacy and safety of three different hair serum products: Group 1: Capilia longa and its combinations; Group 2: A combination of cytokines and peptides; and Group 3: A combination of redensyl and aminexil in the treatment of TE.
MATERIAL AND METHODS
Ethical conduct of the study
The study was approved by the Institutional Ethics Committee CUTIS Institutional Ethics Committee, which is registered at the Central Drugs Standard Control Organization registration #ECR/930/Inst/KA/2017/RR-20. This clinical study was registered with the Clinical Trial Registry of India (CTRI) with CTRI/2024/10/075602. The study was conducted in accordance with the International Council for Harmonization of Technical Requirements of Pharmaceuticals for Human Use (ICH)-Good Clinical Practice E6 (R3) guideline, 2023.
Study design
This was an open-label, triple-arm, single-center, prospective, comparative, safety, and efficacy study in adult subjects having TE. A total of 45 subjects aged 18–45 years were enrolled. The subjects were randomly divided into three groups of fifteen each. Group A-plant stem cells-based hair serum, Group B-cytokine-based hair serum, and Group C-redensyl formula with aminexil argan stem cells and biotin serum. The total duration of the study was 90 days (+2 days) which includes a total of four visits. Visit 1 was scheduled for day 01, followed by Visit 2 on day 20, Visit 3 on day 60 (+2 day), and Visit 4 on day 90 (+2 day).
Patient selection
Potential subjects were screened on the basis of inclusion-exclusion criteria only after obtaining signed written informed consent from them.
Inclusion criteria
Participants aged 18–45 years, in good general health, willing to use the test product and comply with study requirements, including maintaining hairstyle, length, and color throughout. Only treatment-naïve individuals who can comprehend study protocols and are available for scheduled visits were included in the study.
Exclusion criteria
Participants were excluded if they were on medications that could affect study outcomes, had known sensitivity to the test product ingredients, or participated in similar trials within the past 12 weeks. Those with active or prior scalp skin diseases, a history of malignancy, or prior procedures within protocol-specified durations were also excluded from the study. In addition, participants who had used hair weaving, extensions, texturizers, relaxers, occlusive wigs, or non-study hair growth products/procedures within 30 days before screening were not eligible.
Test product
Three different hair serum products with different active ingredients were considered as test products. Group 1: The test product contained peptides and plant stem cells from C. longa. It also contains niacinamide, caffeine, C. longa callus conditioned media, sodium gluconate, phenoxyethanol, ethylhexylglycerin, lecithin, glycerin, biotin, citric acid, Nasturtium officinale extract, Tropaeolum majus extract, apigenin, phytic acid, oleanolic acid, and biotinoyl tripeptide-1.
Group 2: The test product Q- sera™ hair serum is researched and developed by Palsons Derma Pvt. Ltd. It contains butylene glycol, dextran, acetyl tetrapeptide-3, Trifolium pratense flower extract, panthenyl ethyl ether, milk-based anti-inflammatory cytokines-insulin-like growth factor 1 (IGF1), basic fibroblast growth factor (BFGF), platelet-derived growth factor (PDGF)-keratinocyte growth factor (KGF), lactose, inositol, acetyl cysteine, acetyl methionine, alcohol, Foeniculum vulgare (Fennel) fruit, Humulus lupulus (Hops), Melissa officinalis leaf, Viscum album leaf extract, Chamomilla mecutita (Matricaria) flower, Achillea millefolium extract, Caprylhydroxamic Acid, and Glycerin.
Group 3: The test product had dihydroquercetin glucoside, epigallocatechin gallate, glucoside (derived from redensyl), aminexil, argan stem cells, xylishine, biotin, promois WJ, and calcium pantothenate.
Dose
The enrolled subjects were advised to apply the hair serum of 2 mL once daily in the evening for 12 weeks.
Hair wash test
On the test day, the patient washed their hair over a filtering cloth in the sink to collect shed hair. A mild or normal shampoo was used, and the hair was washed thoroughly, following the patient’s usual routine.
After washing, the hairs caught in the filter cloth were collected and air-dried. For longer hair, not all shed hairs were rinsed off during washing due to inter-hair friction. Therefore, shed hairs that fell during drying with an instant dry towel or low-speed hairdryer were also collected. The dried hairs were counted manually for quantification. This test was conducted on each visit to observe changes in shedding patterns over time.12
Trichoscopy assessment
The study was performed at three sites on the scalp, that is, center, frontal, and vertex/occipital area. The microscopic images were captured using FotoFinder© dermoscope with ×20 magnification by medicam 800HD. The FotoFinder© software version v2.1 automatically calculated general hair count, general hair density, anagen%, telogen%, terminal%, vellus%, mean hair thickness, and total follicular units.
Assessment of physician’s global evaluation
Clinical photographs were captured for each patient using global photography at baseline and on the 90th day of treatment. A blinded investigator rated changes in scalp appearance relative to baseline (immediately before treatment commencement) using a standardized 7-point rating scale as follows: greatly decreased (score of −3), moderately decreased (−2), slightly decreased (−1), unchanged (0), slightly increased (+1), moderately increased (+2), and greatly increased (+3).13 Investigator assessments were performed at 0–90 days.
Subject’s satisfaction
Subject satisfaction was validated based on the hair growth satisfaction scale, a self-7-point assessment of the appearance of scalp hair (overall, scalp appearance, coverage, amount of hair in the thinning areas, and hair growth in the thinning areas).
Assessment of subject’s satisfaction of condition at the end of the study and by the point assessment questionnaire – decreased (−3, −2, −1), no change (0), and increased (+3, +2, +1)
Safety assessments
Subjects were enquired about adverse events (AE) and serious adverse events (SAE) such as erythema, edema, and pain at each clinical visit and were informed to contact the investigator at any time to report the possible AE/SAE.
Statistical analysis
Statistical analysis was performed using IBM Statistical Package for the Social Sciences Statistics 27.0 software. Quantitative variables were summarized using means and analyzed through descriptive statistics. Group differences were assessed with a one-way analysis of variance, setting a significance threshold at P < 0.05. Where significant differences were observed, Tukey’s post hoc analysis was conducted for pairwise comparisons.
RESULTS
Demographic details
The demographic characteristics were comparable across the three groups (n = 14 per group after one dropout each). All participants were female. Group 1 had an average age of 30.3 years, Group 2 was 30.6 years, and Group 3 was slightly younger at 28.9 years. Average weights were highest in Group 1 (65.6 kg), followed by Group 2 (61.9 kg) and Group 3 (58.0 kg). Heights were similar: Group 1 (158.9 cm), Group 2 (159.2 cm), and Group 3 (158.1 cm). Body mass index was highest in Group 1 (25.9 kg/m2), with Group 2 at 24.4 kg/m2 and Group 3 at 23.2 kg/m2. Hair loss duration averaged 2.1 years in Group 2, 1.8 years in Group 1, and 1.6 years in Group 3, reflecting the chronic TE stage in the patients [Table 1].
| Average | |||||
|---|---|---|---|---|---|
| Age (y) | Weight (cm) | Height (kg) | BMI (kg/m2) | Hair loss history (y) | |
| Group 1 | 30.3 | 65.6 | 158.9 | 25.9 | 1.8 |
| Group 2 | 30.6 | 61.9 | 159.2 | 24.4 | 2.1 |
| Group 3 | 28.9 | 58.0 | 158.1 | 23.2 | 1.6 |
BMI: Body mass index
Hair wash test
The hair wash test revealed significant hair fall reduction differences among groups (P = 0.001). Group 2 exhibited greatest average decrease of 54.6% (mean reduction of 78.1) from visit V1 to V4, followed by Group 3 at 26.3% (mean reduction: 39.9), and Group 1 at 23.9% (mean reduction: 38.8). Post hoc analysis confirmed Group 2’s superior efficacy, while Group 3 had significantly higher hair fall than Groups 1 and 2. Group 2 consistently achieved the most effective hair fall reduction, while Groups 1 and 3 showed moderate improvements [Figure 1].
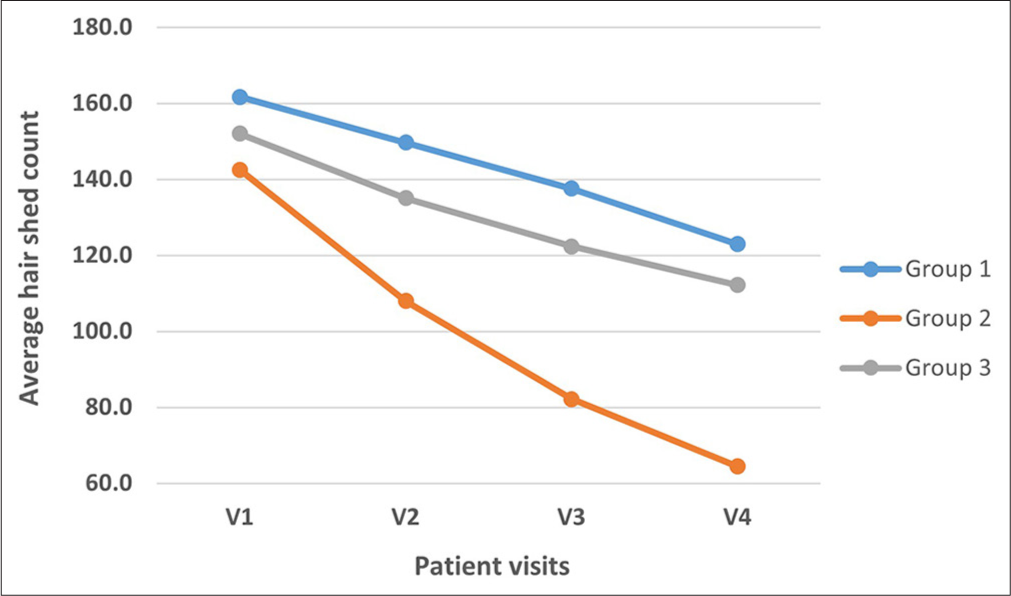
- Comparison of average hair shed count in hair wash test (y-axis) across different groups in various visits (x-axis) where V1, V2, V3, V4 is visit 1, visit 2, visit 3, and visit 4, respectively.
Trichoscopy assessment
The trichoscopy analysis results demonstrate noticeable improvements in average hair count, density, and various other hair parameters across all groups after treatment [Table 2].
| Average | Group 1 | Group 2 | Group 3 | Statistical significance (ANOVA ) | |||
|---|---|---|---|---|---|---|---|
| V1 | V4 | V1 | V4 | V1 | V4 | ||
| Hair count | 125.33 | 171.57 | 113.17 | 171.64 | 118.60 | 166.74 | 0.333 |
| Hair density (/cm2) | 138.75 | 189.94 | 125.28 | 190.02 | 131.29 | 184.59 | 0.279 |
| Anagen (%) | 75.74 | 79.25 | 79.09 | 85.25 | 79.93 | 81.65 | 0.020 |
| Telogen (%) | 24.26 | 20.75 | 20.91 | 14.75 | 20.08 | 18.35 | 0.020 |
| Terminal (%) | 75.99 | 80.10 | 71.75 | 81.55 | 78.23 | 83.38 | 0.125 |
| Vellus (%) | 24.02 | 19.91 | 28.25 | 18.45 | 21.77 | 16.62 | 0.126 |
| Mean thickness | 0.07 | 0.06 | 0.07 | 0.06 | 0.07 | 0.06 | <.001 |
| Total follicular units | 76.90 | 99.19 | 72.19 | 100.17 | 75.24 | 98.62 | 0.660 |
P<0.05, statistically significant, ANOVA: Analysis of variance
Average hair count
Group 1 saw an increase from 125.33 to 171.57, Group 2 from 113.17 to 171.64, and Group 3 from 118.60 to 166.74. The average changes were 46.24, 58.48, and 48.14, respectively, indicating significant hair regrowth in all groups, with Group 2 showing the largest improvement.
Average hair density
Group 1 improved from 138.75/cm2 to 189.94/cm2, Group 2 from 125.28/cm2 to 190.02/cm2, and Group 3 from 131.29/cm2 to 184.59/cm2. The corresponding increases in hair density were 51.19, 64.74, and 53.30, respectively, with Group 2 showing the highest improvement. However, the differences in hair density among the groups were not statistically significant (P > 0.05).
Average anagen percentage
All groups showed an increase in anagen hair (growth phase), with Group 1 rising from 75.74% to 79.25%, Group 2 from 79.09% to 85.25%, and Group 3 from 79.93% to 81.65%. Group 2 demonstrated the most significant improvement in anagen percentage with a 6.16% increase (P = 0.020).
Average telogen percentage
The telogen (resting phase) percentage decreased across all groups, indicating less hair loss (P = 0.02). Group 1 reduced from 24.26% to 20.75%, Group 2 from 20.91% to 14.75%, and Group 3 from 20.08% to 18.35%. Group 2 had the most significant reduction (−6.16%).
Average terminal hair percentage
Terminal hair (thicker and longer hair) improved in all groups, with Group 1 increasing from 75.99% to 80.10%, Group 2 from 71.75% to 81.55%, and Group 3 from 78.23% to 83.38%. The largest increase was seen in Group 2 (+9.80%).
Average vellus hair percentage
Vellus hair (thin and short hair) decreased across all groups, reflecting healthier hair regrowth. Group 1 reduced from 24.02% to 19.91%, Group 2 from 28.25% to 18.45%, and Group 3 from 21.77% to 16.62%. The most significant decrease was in Group 2 (−9.80%).
Average mean hair thickness
There was no change in mean hair thickness across all groups and it remained approximately the same (0.07–0.06).
Average total follicular units/cm2
Total follicular units increased across all groups, with Group 1 rising from 76.90 to 99.19, Group 2 from 72.19 to 100.17, and Group 3 from 75.24 to 98.62. The largest increase was seen in Group 2 (+27.98).
Variables such as hair count, hair density, terminal percentage, and total follicular units showed no statistically significant differences, suggesting uniformity in these aspects across treatment groups. Overall, Group 2 consistently demonstrated the most significant improvements across parameters, with significant differences compared to Groups 1 and 3, especially in hair fall reduction, hair density, anagen percentage, and terminal hair count. Post hoc analysis also confirmed that Group 2 significantly outperformed Groups 1 and 3 in several metrics, suggesting its greater efficacy in promoting hair regrowth and improving hair quality.
Physician’s global evaluation
A minimal decrease in hair growth was noted in one subject each from Groups 1 and 2, while none from Group 3 showed this result. Most subjects in all groups showed improvement, with eight subjects in Groups 1 and 2, and ten subjects in Group 3 demonstrating a “minimally increased” response. A “moderately increased” response was noted in one subject in Group 1 and two in Group 2. No subjects showed a “greatly increased” response in any group [Figure 2]. Figures 3-5 illustrate the improvement in hair density.
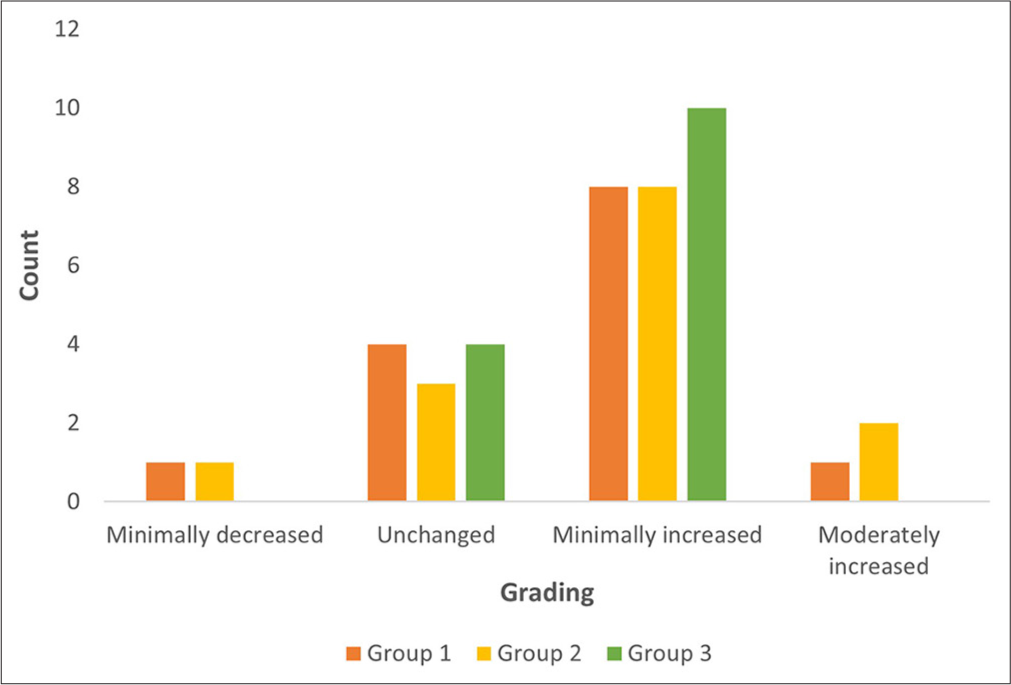
- Physician global evaluation in various groups.
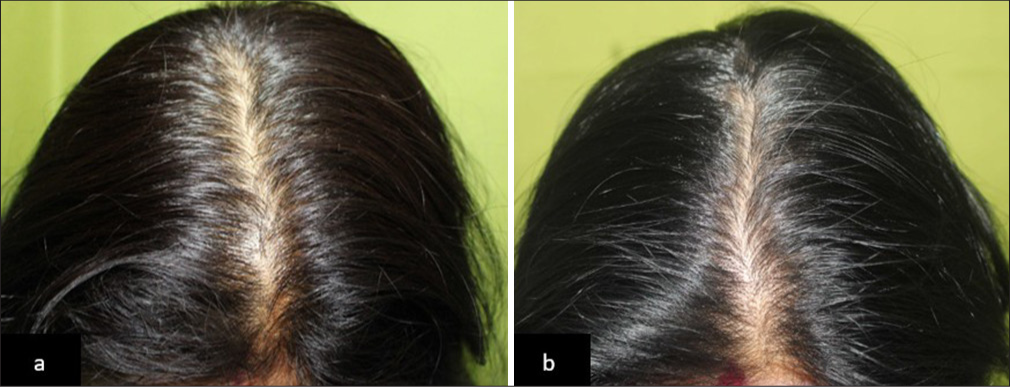
- (a) Global photographs of a patient with Telogen effluvium at baseline and (b) after 90 days of hair serum treatment in Group 1.
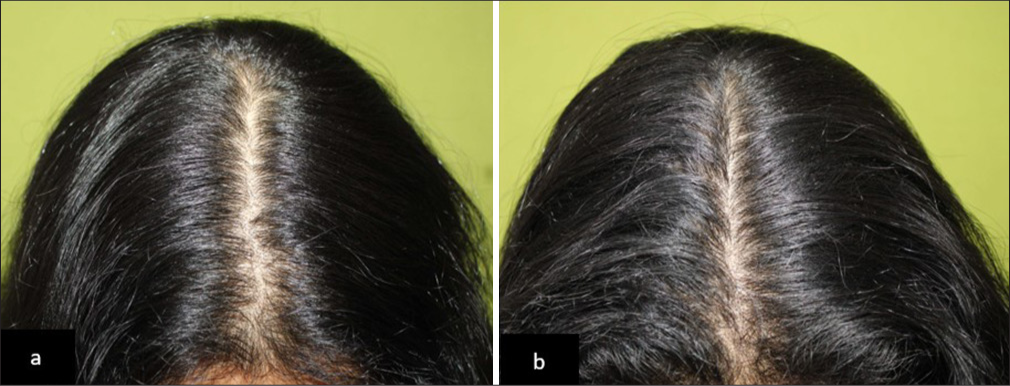
- (a) Baseline global photography of a patient with Telogen effluvium and (b) noticeable improvement in hair density after 90 days of hair serum treatment in Group 2.
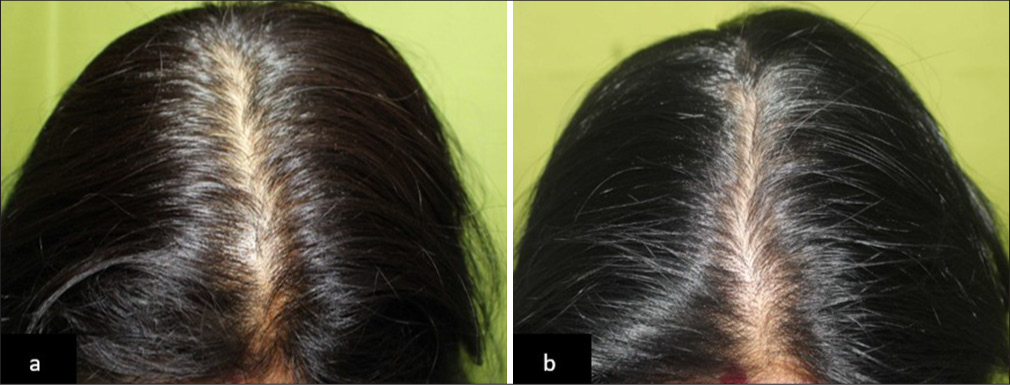
- (a) Global photographs of a patient with Telogen effluvium at baseline and (b) after 90 days of hair serum treatment, showing improved hair density in Group 3.
Assessment of subject’s satisfaction
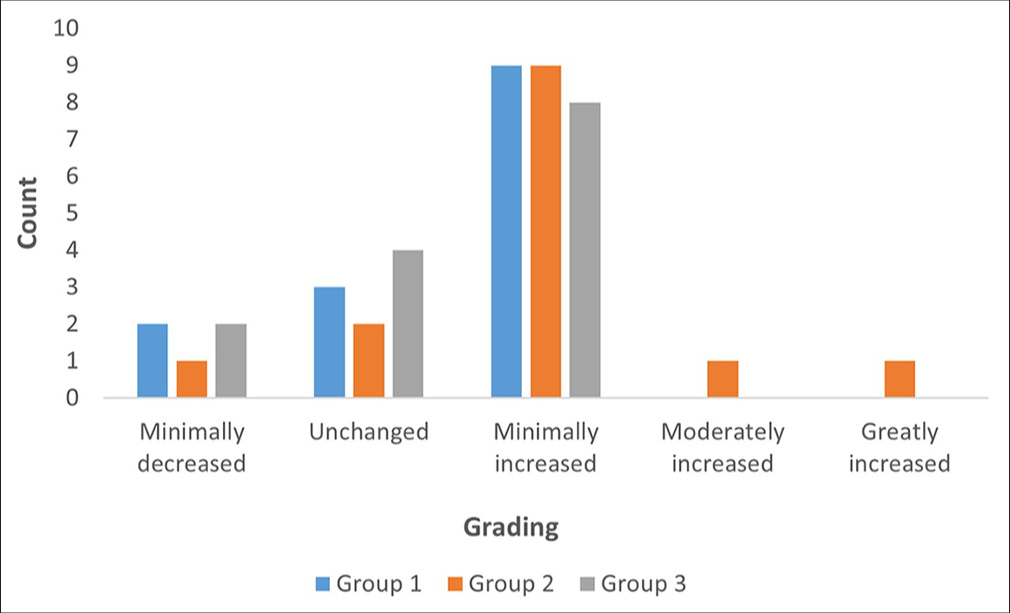
A minimal decrease was noted in two subjects from Groups 1 and 3, and one subject from Group 2. Most subjects in all groups reported a minimally increased satisfaction, with nine subjects each in Groups 1 and 2, and eight in Group 3. A moderately increased satisfaction was observed in one subject from Group 2, while a greatly increased satisfaction was only reported by one subject from Group 2 [Figure 6].
- Subject satisfaction grading across the groups.
Both physician assessment and patient satisfaction scores did not show statistically significant differences across the groups, as their P-values exceeded the 0.05 threshold.
DISCUSSION
TE is primarily driven by disruptions in the hair growth cycle, where hair follicles prematurely enter the telogen (resting) phase, often triggered by factors such as inflammation, stress, vitamin deficiencies, or hormonal shifts, which can act as potential confounders.2 The present study assessed the effectiveness of three different hair serums, each with unique formulations, in reducing hair fall and promoting hair regrowth in TE patients. Improvements in parameters such as hair density, count, and anagen phase duration across all groups suggest that the formulations effectively counteracted TE-related hair loss.
Group 1: This serum contained peptides, Capilia longa®, and stem cell-derived nutrients and showed a moderate reduction in hair shedding (23.9%) and significant improvement in trichoscopic parameters. Capilia longa® known to promote anagen phase initiation and follicular health, played a central role.14 Peptides help in enhancing follicular activity, while biotin, glycerin, and lecithin support hair structure and scalp hydration, which is key for mitigating TE-related dryness. The combination of niacinamide and caffeine further stimulated follicular activity, promoting circulation and contributing to overall scalp health. The result is an effective reduction in hair shedding and increased flexibility and resilience in hair strands.15,16
Group 2: This serum showed the greatest reduction in hair shedding (54.6%) and significant improvements in anagen percentage. Its formulation contained anti-inflammatory milk-based cytokines such as IGF-1, BFGF, PDGF, and KGF, with IGF-1 which has a potential role in promoting linear hair growth, extending the anagen phase, and increasing the expression of platelet-derived growth factors PDGF-A and PDGF-B.17 The cytokine penetration could be through skin appendage pathways, such as hair follicles and sweat glands, providing natural routes for larger molecules to enter the deeper layers of the scalp. In addition, chemical enhancers present in the formulation, such as alcohol, helped in disrupting the lipid matrix of the stratum corneum, further facilitating cytokine diffusion and enhancing their bioavailability and therapeutic effects on hair follicle regeneration.18,19 Acetyl tetrapeptide-3 promotes the synthesis of collagen III in hair follicles. This elastic, fibrous protein, produced by the dermal papilla during the hair cycle, is vital for the growth and stability of hair follicles.20 Ethyl panthenol and inositol further support hair shaft strength and follicular hydration, while extracts such as fennel and hops provide antioxidant benefits.21 The formulation’s combination of ingredients likely promoted both anti-inflammatory and regenerative effects on the hair follicles and enhanced hair strength. Given the consistent efficacy observed across multiple visits, the Group 2 formulation appears particularly effective in addressing the inflammatory and immune response aspects associated with TE.
Group 3: The hair serum containing redensyl and aminexil also demonstrated effectiveness in reducing hair fall (26.3%) and supporting hair regrowth. Redensyl, known to reactivate hair follicle stem cells, likely encouraged a shift from the telogen phase back to the anagen phase, essential for managing TE-related hair shedding. Aminexil promotes hair growth by preventing follicle shrinkage through anti-fibrotic action, prolonging the growth phase, enhancing scalp blood flow, and reducing hair shedding, creating a supportive environment for stronger, denser hair.22 The addition of biotin, D-calcium pantothenate, and promois WJ, along with antioxidants such as dihydroquercetin glucoside, supported scalp health, providing a conducive environment for hair regeneration.23-26
The findings of this study align with previous research by Merja et al.27 which highlighted the potential of formulations containing redensyl and Capilia longa® to stimulate hair regrowth with marked efficacy. Karaca and Akpolat28 demonstrated the combined effectiveness of redensyl, capixyl (combination of acetyl tetrapeptide-3 and red clover extract), and procapil (combination of apigenin, vitaminated peptide, and Oleanolic Acid), showing superior results compared to minoxidil. Similarly, in the present study, Group 2’s superior results in hair fall reduction, density, and anagen phase increase emphasize the role of cytokines and peptides in enhancing scalp health and follicular resilience.
Both physician global evaluations and patient satisfaction scores did not show statistically significant differences across groups, possibly due to a high baseline efficacy among all formulations. However, Group 2 formulation, by consistently demonstrating the greatest improvements in key hair metrics, appears particularly beneficial for patients with TE, likely due to its focused action on inflammation, dihydrotestosterone inhibition, and follicular repair. Further studies could explore the long-term effects of these serums on sustained hair regrowth and compare their efficacy with conventional treatments like minoxidil in a larger and more diverse population.
Limitations and future directions
The study limitations include small sample size, short duration, lack of vitamin level measurements, and lack of male participants, affecting the generalizability of the results. Future research should focus on longer treatment periods, diverse demographics, and active ingredient-specific mechanistic studies, particularly on cytokines, redensyl, aminexil, acetyl tetrapeptide-3, and C. longa stem cell derivatives, to delineate their precise roles in follicular modulation and growth phase induction.
CONCLUSION
The study showed that all three formulations provided significant improvements in hair growth parameters, with Q-sera (group 2) hair serum exhibiting quick, consistent, and substantial results. These findings suggest that a combination of cytokines, peptides, plant extracts, and amino acids offers an effective approach to managing hair fall and promoting hair density in individuals experiencing hair loss.
Authors’ contributions
Dr B S Chandrashekar: Contributed to the concepts, study design, manuscript editing and review, and served as the guarantor for the study. Dr Roopa M S: Involved in the concepts, study design, defining the intellectual content and conducting the literature search, and manuscript preparation. Dr Kusuma M R: Involved in clinical trial, data analysis and result interpretation Dr Madura C: Involved in clinical trial, and result interpretation. Dr Chaithra Shenoy: Involved in clinical trial, and result interpretation. Mr. Lakshmi Narayana N: Conducted the literature search, assisted in clinical trial and contributed to data acquisition. Ms. Pallabi Ghoshal: Contributed to study design, literature search and assisted in manuscript review.
Ethical approval
The research/study approved by the Institutional Review Board at CUTIS Institutional Ethics Committee, number ECR/930/Inst/KA/2017/RR-20, dated June 05, 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Telogen effluvium In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430848 [Last accessed on 2024 May 01]
- [Google Scholar]
- Pathogenesis, diagnosis, and management of telogen. J Ilmiah Kesehatan Media Husada. 2022;11:44-55.
- [CrossRef] [Google Scholar]
- Recent modalities in treatment of telogen effluvium: Comparative study. Dermatol Ther. 2022;35:e15720.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of telogen effluvium using a dietary supplement containing Boswellia Serrata, Curcuma longa, and Vitis vinifera: Results of an observational study. Dermatol Ther. 2019;32:e12842.
- [CrossRef] [Google Scholar]
- Clinical study to evaluate the efficacy and safety of a hair serum product in healthy adult male and female volunteers with hair fall. Clin Cosmet Investig Dermatol. 2020;13:691-700.
- [CrossRef] [PubMed] [Google Scholar]
- Revolutionizing cosmetic ingredients: Harnessing the power of antioxidants, probiotics, plant extracts, and peptides in personal and skin care products. Cosmetics. 2024;11:157.
- [CrossRef] [Google Scholar]
- Topical and nutricosmetic products for healthy hair and dermal antiaging using “dual-acting” (2 for 1) plant-based peptides, hormones, and cannabinoids. FASEB Bioadv. 2021;3:601-10.
- [CrossRef] [PubMed] [Google Scholar]
- The role of vitamins and minerals in hair loss: A review. Dermatol Ther (Heidelb). 2019;9:51-70.
- [CrossRef] [PubMed] [Google Scholar]
- Hair shedding evaluation for alopecia: A refined wash test. Clin Cosmet Investig Dermatol. 2022;15:117-26.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: A randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2014;2014:549721.
- [CrossRef] [PubMed] [Google Scholar]
- First generation of phytopeptides from curcuma longa plus a blend of nutrients associated with carboxytherapy for the treatment of male androgenetic alopecia. Braz J Nat Sci. 2019;2:161.
- [CrossRef] [Google Scholar]
- Niacinamide down-regulates the expression of DKK-1 and protects cells from oxidative stress in cultured human dermal papilla cells. Clin Cosmet Investig Dermatol. 2021;14:1519-28.
- [CrossRef] [PubMed] [Google Scholar]
- Role of caffeine in the management of androgenetic alopecia. Int J Trichology. 2012;4:185-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of IGF-I on hair growth is related to the anti-apoptotic effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Ann Dermatol. 2012;24:26-31.
- [CrossRef] [PubMed] [Google Scholar]
- Improved topical drug delivery: Role of permeation enhancers and advanced approaches. Pharmaceutics. 2022;14:2818.
- [CrossRef] [PubMed] [Google Scholar]
- Description of the intrafollicular delivery of large molecular weight molecules to follicles of human scalp skin in vitro. J Pharm Sci. 1997;86:1022-9.
- [CrossRef] [PubMed] [Google Scholar]
- Co-delivery of bioactive peptides by nanoliposomes for promotion of hair growth. J Drug Deliv Sci Technol. 2022;72:103381.
- [CrossRef] [Google Scholar]
- An optimal combination of inositol and phytic acid effectively promotes hair growth. Biomed J Sci Tech Res. 2024;55:46771-8.
- [CrossRef] [Google Scholar]
- A full factorial design to optimize aminexil nano lipid formulation to improve skin permeation and efficacy against alopecia. AAPS PharmSciTech. 2023;24:40.
- [CrossRef] [PubMed] [Google Scholar]
- A review of the use of biotin for hair loss. Skin Appendage Disord. 2017;3:166-9.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing the effects of zinc sulfate, calcium pantothenate, their combination and minoxidil solution regimens on controlling hair loss in women: A randomized controlled trial. J Res Pharm Pract. 2017;6:89-93.
- [CrossRef] [PubMed] [Google Scholar]
- A study on the effectiveness and safety of herbal extract combination compared to 3% minoxidil solution for the treatment of androgenetic alopecia: A randomized, double-blind, controlled trial. Open Dermatol J. 2024;18:1-8.
- [CrossRef] [Google Scholar]
- Transparent silicone-free shampoo containing proteins with strong hair conditioning properties. Int J Cosmet Sci. 2024;46:106-18.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of REGENDIL™ infused hair growth promoting product in adult human subject having hair fall complaints (alopecia) J Cosmet Dermatol. 2024;23:938-48.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study between topical 5% minoxidil and topical “redensyl, capixyl, and procapil” combination in men with androgenetic alopecia. J Cosmetol Trichol. 2019;5:1000140.
- [Google Scholar]






