Translate this page into:
Comparison of efficacy of intralesional vitamin D3 versus intralesional triamcinolone acetonide in keloid – A randomized double-blinded non-inferiority study
*Corresponding author: Nibedita Dixit, Department of Dermatology, Institute of Medical Sciences and Sum Hospital, Bhubaneswar, Odisha, India. nibeditadixit@soa.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Mohapatra L, Kar BR, Singh S, Singh BS, Dixit N. Comparison of efficacy of intralesional vitamin D3 versus intralesional triamcinolone acetonide in keloid – A randomized double-blinded non-inferiority study. J Cutan Aesthet Surg. doi: 10.25259/jcas_56_24
Abstract
Objectives:
Keloids are often difficult to treat and have a high chance of recurrence. Multiple modalities of therapy have been tried with variable success rates. Intralesional triamcinolone acetonide (TA) remains the most common modality of treatment of keloids. We have conducted a randomized controlled trial comparing the efficacy of intralesional injection TA versus intralesional vitamin D3 (VD3) in keloids.
Material and Methods:
Group TA (n = 30) received an intralesional TA 40 mg/mL, and group vitamin D (VD) (n = 30) received intralesional VD3 (cholecalciferol) 60000 IU every 4 weekly till 12 weeks and all the patients were followed up for another 4 weeks. At each session, the scar size was assessed by the Vancouver Scar Scale (VSS), and the Visual Analog Scale assessed the pain.
Results:
The mean score of VSS was significantly decreased in both group TA (7.91 ± 1.5–4.9 ± 1.6, P < 0.001) and group VD (7.84 ± 0.8–5.0 ± 1.6, P < 0.001). The pain was severe in group VD compared to group TA. There was fluid discharge with severe itching and pain in one keloid site in the VD group. The keloids reduced faster in size in the TA group compared to the VD group. There was no significant difference in response to TA versus VD.
Conclusion:
Both intralesional triamcinolone and VD3 were found to be efficacious with triamcinolone achieving a faster effect. The pain was a limiting factor in the intralesional VD group.
Keywords
Keloid
Intralesional vitamin D3
Intralesional triamcinolone acetonide
INTRODUCTION
Keloid is an abnormal, benign, well-demarcated growth caused by the deposition of collagen by dermal fibroblasts that extend beyond the original site of the skin injury (e.g., ear piercing, surgical injury, burns, or any type of inflammation due to trauma wounds, insect bites, acne, chickenpox, and herpes zoster infection).1-3 Keloid frequently occurs in high-tension body surfaces, such as the chest and upper back.4 It clinically appears as raised amorphous growth and is frequently associated with contractures, pruritus, and pain. Larger keloids may cause physical/functional disability, cosmetic deformation, and psychological distress and hamper the quality of life. The prevalence of keloid is not gender or skin-type dependent but may occur more in dark individuals, with an incidence rate of 16% in Hispanic, African American, or black cohorts.2,3 Many researchers described a significant association between keloid scars, obesity, and hypertension.5,6
The exact patho-mechanism behind the keloid formation is still unclear.7 There is a strong association between dysregulation of transforming growth factor beta (TGF-β) isoform levels (TGF-β1, -β2, and -β3), cytokines (interleukin [IL]-4, IL-5, IL-10, and IL-13), elastin, and fibrillin-1, mediating the tissue repair and regeneration processes.8-10
Interestingly, various treatments were used to treat keloids with varying degrees of success. However, the recurrence of the disease is the most challenging thing to manage. Keloids can be treated with medical and surgical interventions and combination therapies. Topical treatments (mitomycin C, imiquimod, and topical silicone), intralesional injections (triamcinolone acetonide [TA], verapamil, 5-fluorouracil [5-FU], etc.), radiations, laser-based therapy, and combination therapies have been tried for keloids.7,11,12 Numerous clinical trials have reported the effectiveness of various treatments on keloid scarring. Yet, most treatment for keloid remains empirical and unsatisfactory.13 The gold standard of treatment, which is the need of the hour, should be minimally invasive, cost-effective, with very few side effects, and a low recurrence. Among all of these, intralesional TA is the most widely used treatment in keloid.7,14,15 It is considered to be the gold standard in the non-surgical management of hypertrophic and keloid scars. It suppresses the collagen and glycosaminoglycan synthesis level and decreases fibroblast development and the collagen degradation process.7,14,15 Previously, vitamin D3 (VD3) has been documented as having anti-inflammatory, anti-proliferative, and anti-cancerous properties. A limited number of studies have found VD3 to be effective in the treatment of keloid.16 Therefore, this study was undertaken to compare the efficacy of intralesional injection of VD3 and intralesional TA.
Aims and objective
Primary
To compare the efficacy of 3 weekly intralesional TA injections versus 3 weekly intralesional VD3 injections in keloids at the end of 12 weeks.
Secondary
To compare the side effect profile and recurrence during follow-up of either group.
MATERIAL AND METHODS
The study was a randomized, non-inferiority trial. This study was approved by the Institutional Ethical Committee and registered with the Clinical Trials Registry-India bearing number REF/2022/09/058153. Patients with clinically diagnosed keloid attending the dermatology outpatient department in a tertiary healthcare center in the eastern part of India were assessed for eligibility. The sample size was determined to be 60 based on the percentage of success in the control group being 73.1%, and 54.68% in the experimental group, with a non-inferiority limit of 40 with a significance level of 0.05 and power of 80%. A total of 15 patients with 76 numbers of clinically confirmed keloids were enrolled of which 9 patients with 60 numbers of keloids satisfying the eligibility criteria were included in this study after obtaining consent. Six patients were excluded. The clinicodemographic data, such as age, gender, disease duration, family history, triggering factor, and affected sites, were recorded in a case report form. A total of sixty (n = 60) keloids were included in the study, randomly divided into two groups: Group TA (n = 30) keloids were treated with intralesional TA and group vitamin D (VD) (n = 30) received intralesional VD3. A computer-generated software was used to generate a random allocation sequence. Allocation concealment was done using a similar-looking syringe for both medications. Both the patient and assessor were blinded. A flowchart of the treatment assigned to both groups is shown in Figure 1.
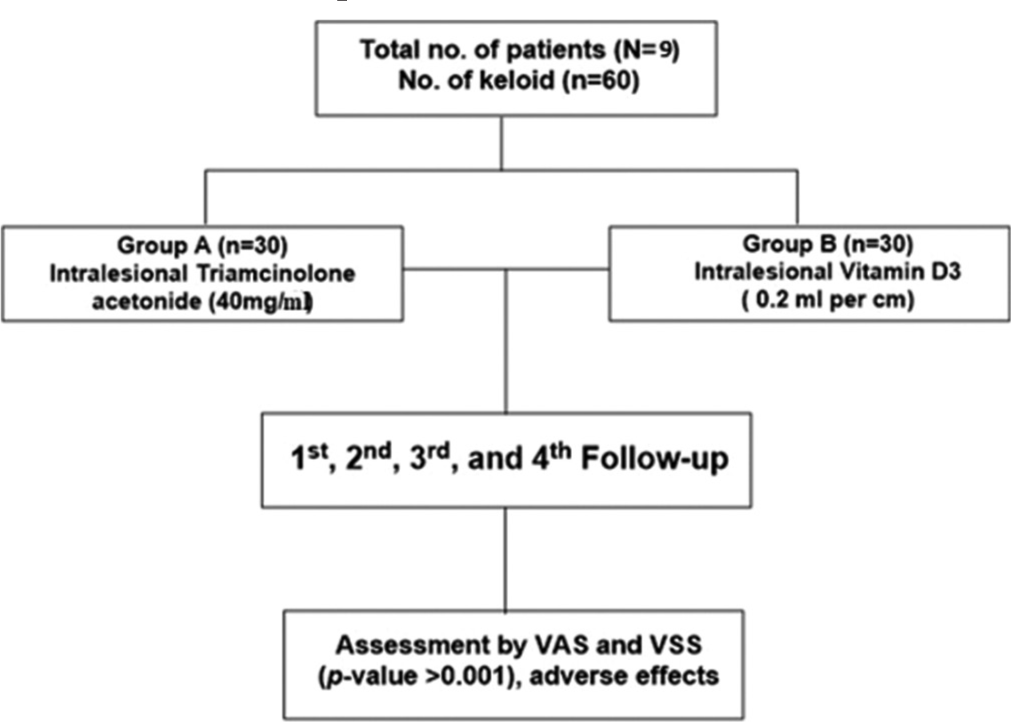
- Treatment allocation flow chart. VSS: Vancouver scar scale, VAS: Visual analog scale.
Inclusion criteria
All patients with keloids above 18 years of age
Patients having a minimum of two keloids not more than 5 cm
Keloids without any treatment in the past 6 months.
Exclusion criteria
Pregnancy and lactation
Diabetes mellitus
Systemic disorder, immune-compromised status
Retinoid use in previous 6 months.
Group TA received intralesional TA 40 mg/mL till blanching was seen with a maximum total dose of 80 mg for sizes up to 5 cm, using 1 mL U-100 insulin syringe every 4 weekly and group VD received intralesional injection VD3 (cholecalciferol) 60000 IU at a dose of 0.2 mL/cm up to a maximum of 1 mL. Treatment was continued for three 4 weekly sessions. Patients were followed for 1 month after the last injection.
This study used two scoring systems to assess the pre and post-treatment results. The Vancouver Scar Scale (VSS) score [Figure 2] was evaluated for vascularity, pigmentation, pliability, and height.17,18 The pain score was determined through self-assessment using the Visual Analog Scale (VAS), a 0–10 scale ranging from no to extremely unbearable pain.17,19 The comparison of VSS scores among the two groups is depicted in Figure 3.
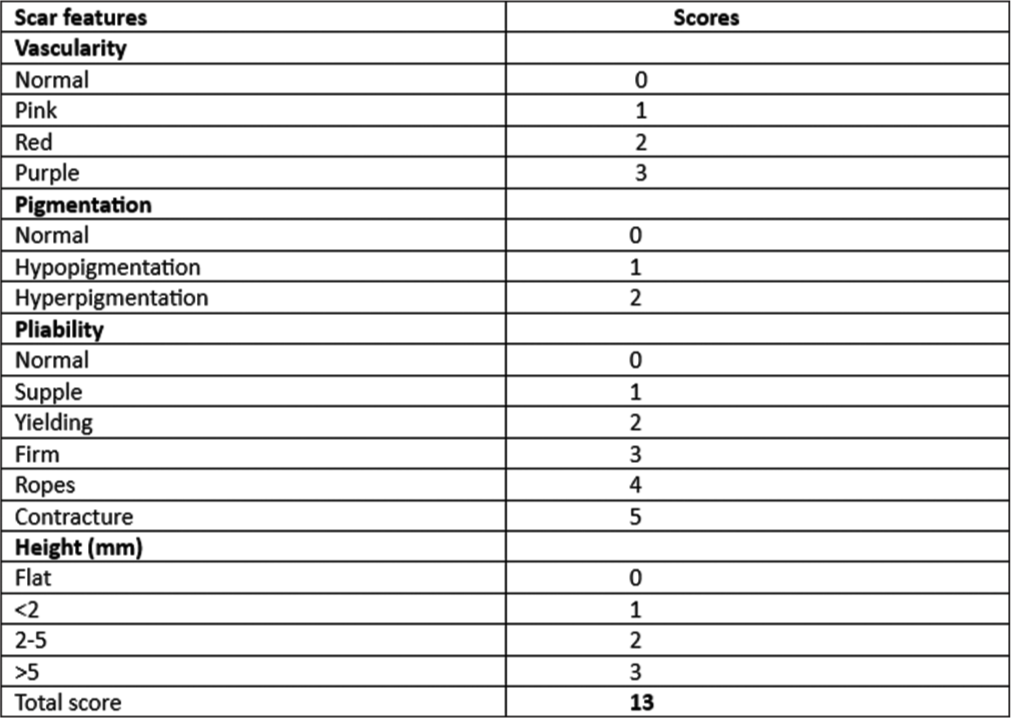
- Vancouver skin scar scale.
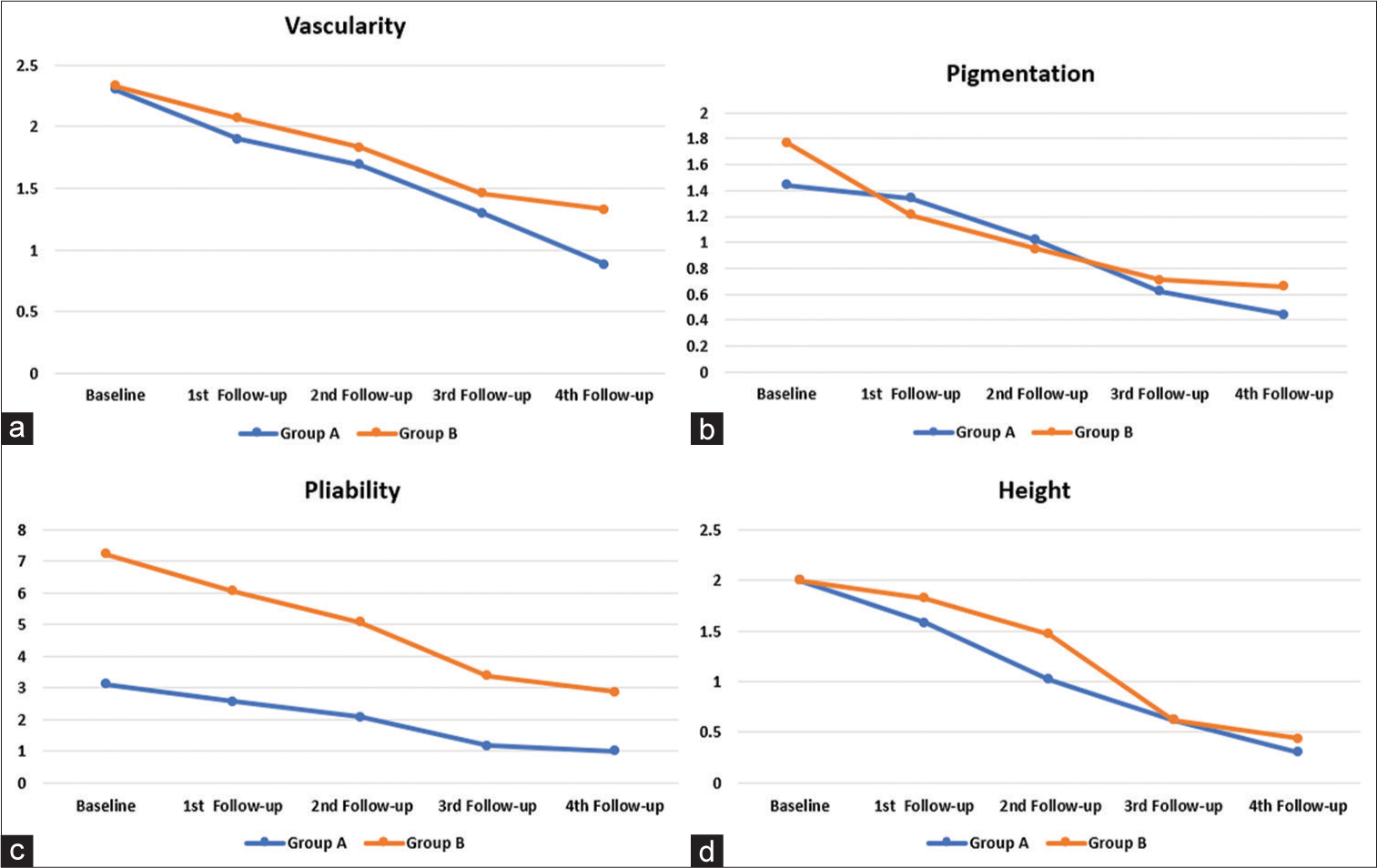
- Comparison of individual parameters of Vancouver Scar Scale score among the two groups. (a) Line graph comparing vascularity. (b) Line graph comparing pigmentation. (c) Line graph comparing pliability. (d) Line graph comparing height.
All the baseline data were presented as percentages (%) for categorical variables and continuous variables by mean ± Standard deviation. The chi-square test was used to compare data between groups before and during every session of follow-up periods. All the statistical analyses were performed using IBM Statistical Package for the Social Sciences software, version 26.0. A P < 0.05 was considered significant. The primary investigator did the group allocation, and the second investigator assessed the effectiveness and safety parameters at baseline and during the follow-up procedure.
RESULTS
Five males and four females with a total of sixty (n = 60) keloids were included in the study and were randomly divided into two groups: Group A (n = 30) keloids were treated with intralesional TA and Group B (n = 30) received intralesional VD3. A family history of keloid was found in two cases (28.57%). The majority (63.33%) of keloids were distributed either on the chest or around the waist [Table 1]. The VSS score for group TA was 7.91 ± 1.5 and 4.9 ± 1.6 at baseline and fourth follow-up, respectively [Table 2]. The baseline VSS score of the VD group reduced from 7.84 ± 0.8 to 5.0 ± 1.6 at the end of the fourth follow-up visit [Table 3]. The difference was found to be statistically significant (P < 0.001) with both modalities of treatment [Table 4]. VAS score was used to analyze injection pain. Patients in Group VD experienced a more severe form of pain than the group TA. 5 out of 9 patients in group VD had a score of “Hurts even more or score value 3.” Injection pain and mild itching were the commonly reported adverse effects in the VD group. However, 3 patients developed atrophy in group TA after the second visit, and 5 patients developed depigmentation after the third visit. One case in group VD experienced sterile fluid discharge from the keloid after the third injection. The discharge was sent for culture sensitivity, which showed no growth. Clinical images comparing the response are shown in Figure 4a and b.
| Characteristics | n | % |
|---|---|---|
| Gender | ||
| Male | 5 | 71.42 |
| Female | 4 | 28.57 |
| Duration of disease | ||
| 1–5 years | 5 | 60 |
| 5–10 years | 4 | 10 |
| Family history | ||
| Mother | 1 | 14.28 |
| Father | 1 | 14.28 |
| None | 7 | 71.42 |
| Trigger | ||
| Spontaneous | 16 | 25 |
| Trauma | 41 | 68.33 |
| Post acne | 4 | 5 |
| Immunization/None | 1 | 1.66 |
| Sites affected | ||
| Chest | 25 | 38.33 |
| Waist | 18 | 25 |
| Bilateral arms | 13 | 20 |
| Upper back | 10 | 16.66 |
| VSS | Baseline versus 1st follow-up | Baseline versus 2nd follow-up | Baseline versus 3rd follow-up | Baseline versus 4th follow-up |
|---|---|---|---|---|
| Vascularity | 0.320 | 0.001 | <0.001 | <0.001 |
| Pigmentation | 0.603 | 0.012 | 0.001 | <0.001 |
| Pliability | <0.001 | <0.001 | <0.001 | <0.001 |
| Height | 0.401 | 0.023 | 0.01 | <0.001 |
VSS: Vancouver scar scale
| VSS | Baseline versus 1st follow-up | Baseline versus 2nd follow-up | Baseline versus 3rd follow-up | Baseline versus 4th follow-up |
|---|---|---|---|---|
| Vascularity | 1.008 | 0.086 | 0.012 | <0.001 |
| Pigmentation | 0.033 | <0.001 | <0.001 | <0.001 |
| Pliability | 0.083 | 0.002 | <0.001 | <0.001 |
| Height | 0.950 | 0.102 | 0.003 | <0.001 |
VSS: Vancouver scar scale
| VSS assessment | Baseline | 4th follow-up | ||
|---|---|---|---|---|
| Mean±SD | P-value | Mean±SD | P-value | |
| TA | 7.91±1.5 | <0.001 | 4.9±1.6 | <0.001 |
| VD3 | 7.84±0.8 | 5.0±1.6 | ||
VSS: Vancouver scar scale, TA: Triamcinolone acetonide, VD3: Vitamin D3, SD: Standard deviation
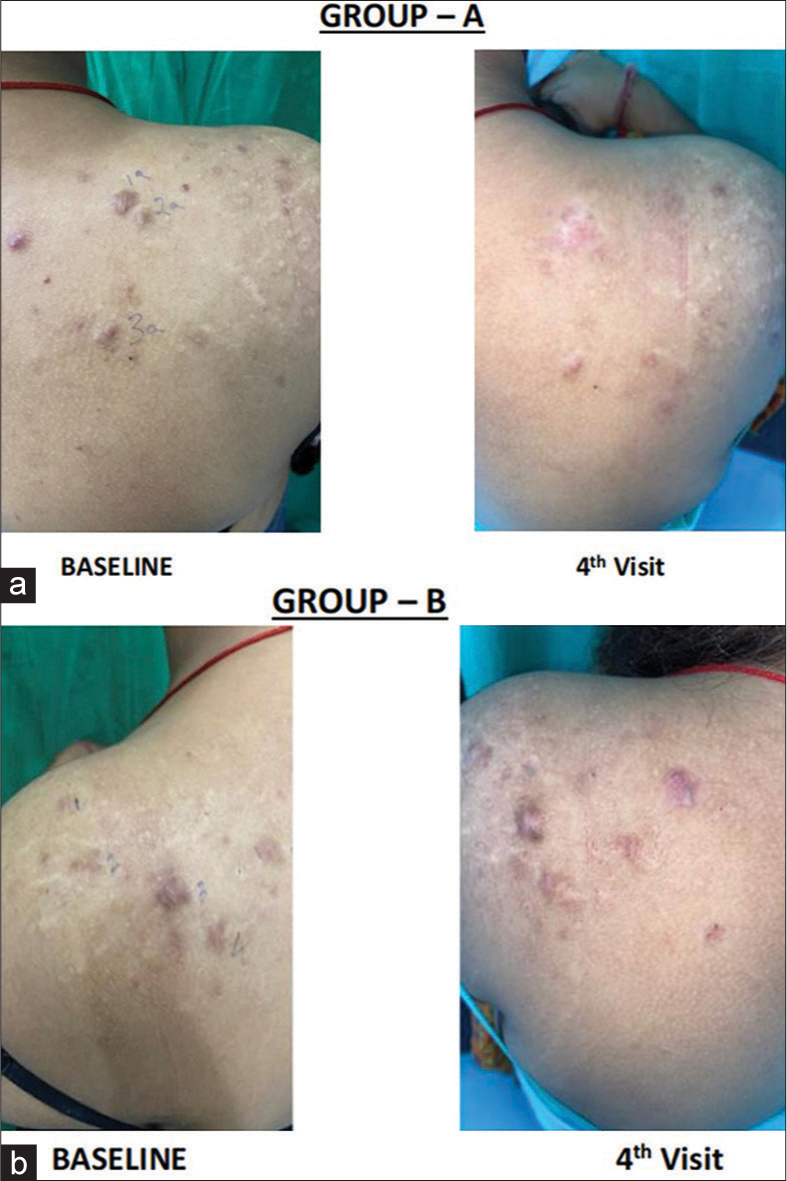
- (a) Clinical images showing the response of each visit in the triamcinolone acetonide group. (b) Clinical images showing the response of each visit in the vitamin D group.
The pliability of the keloids was the first to improve in the TA group as early as 1st week, and the last parameter to improve significantly was the height of keloids at the fourth follow-up visit. Contrarily, vascularity was the last facet of keloids to improve in the VD group, and pigmentation and pliability showed significant improvement by the second follow-up. By the third follow-up in the VD group, there was a significant decrease in the height of keloids.
DISCUSSION
Keloids are stubborn scars that are difficult to treat and manage. Corticosteroids remain the gold standard among the myriad treatment modalities.20 At the end of this study, we found a noticeable improvement in the keloids, as demonstrated by the significant reduction in VSS scores in both groups. There was a faster improvement in the intralesional TA group by the 1st month itself; however, subsequently, the change was gradual. In the VD group, the decline in VSS was slow in onset and gradual in nature. Pain was the most common side effect in the VD group in our study compared to Mamdouh et al., where redness, swelling, and tenderness were reported.21 We presume that the oily nature of the VD3 injection may be the cause of the pain. VD plays an important role in cell proliferation and differentiation, has an anti-fibrotic effect, and inhibits collagen synthesis in dermis fibrosis. The African American population has an increased incidence of keloids and hypertrophic scars. Interestingly, the same ethnic black population has VD3 deficiency due to decreased synthesis of VD3 in the skin and genetic defects in VD3 metabolism.22 This hypothesis can be extrapolated to the fact that VD3 prevents the formation of scars. Yu et al. compared serum 1,25-dihydroxyvitamin D level and VD3 gene polymorphism among 261 patients with keloids to a control group of 261 healthy people without keloids. Patients with keloids had significantly lower levels of circulating serum 1,25-dihydroxyvitamin D than the healthy controls.2 A cross-sectional study by Cho et al. reported higher pigmentation and low scar elasticity in hypertrophic burn scar cases having VD3 deficiency.23 The severity of keloid has been found to be higher in patients with a deficiency of VD3.24-26
Intralesional TA has long been the mainstay in the management of keloid, alone or in combination with other therapeutic or non-therapeutic procedures.27,28 However, corticosteroids come with their own set of adverse effects, limiting their prolonged usage. Various newer treatment modalities are being tried to match the efficacy and response rate of intralesional TA. Botulinum toxin type A, corticosteroids (including diprospan and TA), verapamil, and 5-FU are the main drugs for local injection in the treatment of pathological scars.7 TA was associated with a significant improvement in vascularity over the medium-term and long-term follow-up. There was no significant difference in pliability across TA and verapamil in the medium-term and long-term follow-up.29 In our study also, there was a significant increase in pliability as early as 4 weeks in TA and 8 weeks in the VD group. In our study, both the groups showed a statistically significant reduction in all the parameters of the VSS score; however, the pliability component responded as early as 4 weeks in the steroid group. In terms of patient safety, studies have shown that TA often causes adverse effects like skin atrophy and telangiectasia in up to 63% of patients. TA was more effective in improving scars than silicone gel sheets, verapamil, and cryotherapy. In addition, combining 5-FU with TA showed a significant improvement in keloid in comparison with TA alone. Although TA treatment could lead to complications, including skin atrophy and telangiectasia, the difference was not statistically significant compared to 5-FU or verapamil.30 A systematic review has concluded that multiple injections of TA and doses of more than 40 mg per session are known to cause hypothalamic-pituitary-adrenal axis suppression and subsequent development of Cushing syndrome.31 This may be attributed to the slow release of triamcinolone from the injection site. Patients receiving 75–100 mg (40 mg/mL) intralesional TA can have adrenal suppression for as long as 5 days.32 Patients receiving successive injections can have adrenal suppression for up to 8 months. This study supports the potential role of VD as an alternative, practical, and safe method in the treatment of keloid. There is delay in response with intralesional vitamin D3 as compared to intralesional steroids. Our results were comparable with the findings of the study by Mamdouh et al. However, in their study, the treatment interval was weekly for 4 weeks, in contrast to the 4 weekly gap in our study.21
Limitation
Shorter follow-up time.
CONCLUSION
We did not find any significant difference in the efficacy of intralesional TA and VD in the treatment of keloids. Intralesional TA as opposed to intralesional VD3 has a faster effect; however, VD3 is a safe and effective option. The side effects were atrophy and depigmentation in the intralesional steroid group, whereas pain was the major adverse effect in the VD3 group. However, the pain associated with the injection was transient and subsided without any medication. A large-scale study with a more extended follow-up period is recommended to assess the efficacy of VD3 as a keloid treatment and its role in preventing relapses in keloids.
Authors’ contributions
Liza Mohapatra: Concepts, design, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing and review. Bikash Ranjan Kar: Concepts, design, definition of intellectual content, clinical studies, experimental studies, and manuscript review. Surabhi Singh: Concepts, design, definition of intellectual content, clinical studies, experimental studies, data analysis, statistical analysis, manuscript editing and review. Bhabani STP Singh: Concepts, design, definition of intellectual content, data acquisition, and data analysis. Nibedita Dixit: Statistical analysis, Manuscript editing and manuscript review.
Ethics approval
The research/study was approved by the Institutional Review Board at IMS AND SUM HOSPITAL, Reg. No. DRI/IMS.SH/SOA/2021/176, dated 21/7/21.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The role of the renin-angiotensin system and vitamin D in keloid disorder-a review. Front Surg. 2019;6:67.
- [CrossRef] [PubMed] [Google Scholar]
- The TaqI gene polymorphisms of VDR and the circulating 1,25-dihydroxyvitamin D levels confer the risk for the keloid scarring in Chinese cohorts. Cell Physiol Biochem. 2013;32:39-45.
- [CrossRef] [PubMed] [Google Scholar]
- High-mobility group box protein-1, matrix metalloproteinases, and vitamin D in keloids and hypertrophic scars. Plast Reconstr Surg Glob Open. 2015;3:e425.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical forces in skin disorders. J Dermatol Sci. 2018;90:232-40.
- [CrossRef] [PubMed] [Google Scholar]
- Association of keloids with systemic medical conditions: A retrospective analysis. Int J Dermatol. 2016;55:e38-40.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of patient registries in skin of color. J Investig Dermatol Symp Proc. 2017;18:S31-3.
- [CrossRef] [PubMed] [Google Scholar]
- Keloids: A review of therapeutic management. Int J Dermatol. 2021;60:661-71.
- [CrossRef] [PubMed] [Google Scholar]
- Insights into the pathophysiology of hypertrophic scars and keloids: How do they differ? Adv Skin Wound Care. 2018;31:582-95.
- [CrossRef] [PubMed] [Google Scholar]
- The role of TGFβ in wound healing pathologies. Mech Ageing Dev. 2018;172:51-8.
- [CrossRef] [PubMed] [Google Scholar]
- The role of elastic fibers in scar formation and treatment. Dermatol Surg. 2017;43(Suppl 1):S19-24.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of 2 representative topical agents to prevent keloid recurrence after surgical excision. J Oral Maxillofac Surg. 2017;75:401.e1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of keloid scars using light-, laser-and energy-based devices: A contemporary review of the literature. Lasers Med Sci. 2017;32:2145-54.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in scar management: Prevention and management of hypertrophic scars and keloids. Curr Opin Otolaryngol Head Neck Surg. 2016;24:322-9.
- [CrossRef] [PubMed] [Google Scholar]
- The keloid disorder: Heterogeneity, histopathology, mechanisms and models. Front Cell Dev Biol. 2020;8:360.
- [CrossRef] [PubMed] [Google Scholar]
- Keloids: The paradigm of skin fibrosis-pathomechanisms and treatment. Matrix Biol. 2016;51:37-46.
- [CrossRef] [PubMed] [Google Scholar]
- A role for vitamin D and the vitamin D receptor in keloid disorder. Wound Repair Regen. 2023;31:563-75.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: A randomized clinical trial. Int Wound J. 2022;19:1729-35.
- [CrossRef] [PubMed] [Google Scholar]
- Rating the resolving hypertrophic scar: Comparison of the Vancouver Scar Scale and scar volume. J Burn Care Rehabil. 2000;21:205-12.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical applications of visual analogue scales: A critical review. Psychol Med. 1988;18:1007-19.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of intralesional triamcinolone acetonide (20mg/mL) in the treatment of keloid. Int Surg J. 2018;5:868-72.
- [CrossRef] [Google Scholar]
- Role of vitamin D in treatment of keloid. J Cosmet Dermatol. 2022;21:331-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of hypertrophic scars among African Americans linked to vitamin D-3 metabolism? J Natl Med Assoc. 2005;97:1004-9.
- [Google Scholar]
- The association between Postburn vitamin D deficiency and the biomechanical properties of hypertrophic scars. J Burn Care Res. 2019;40:274-80.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between serum 25-hydroxyvitamin D levels with keloid severity. Open Access Maced J Med Sci. 2019;7:65-7.
- [CrossRef] [PubMed] [Google Scholar]
- Does vitamin D deficiency predispose to keloids via dysregulation of koebnerisin (S100A15)? A case-control study. Wound Repair Regen. 2021;29:425-31.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidities of keloid and hypertrophic scars among participants in UK biobank. JAMA Dermatol. 2023;159:172-81.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of intralesional triamcinolone acetonide, 5-fluorouracil, and their combination in treatment of keloids. World J Plast Surg. 2018;7:212-9.
- [Google Scholar]
- Comparison of fractional CO2 laser, verapamil, and triamcinolone for the treatment of keloid. Adv Wound Care (New Rochelle). 2019;8:7-13.
- [CrossRef] [PubMed] [Google Scholar]
- The safety and efficacy of intralesional triamcinolone acetonide for keloids and hypertrophic scars: A systematic review and meta-analysis. Burns. 2021;47:987-98.
- [CrossRef] [PubMed] [Google Scholar]
- The Efficacy of triamcinolone acetonide in keloid treatment: A systematic review and meta-analysis. Front Med (Lausanne). 2016;3:71.
- [CrossRef] [PubMed] [Google Scholar]
- Cushing's syndrome after intralesional triamcinolone acetonide: A systematic review of the literature and multinational survey. Burns. 2013;39:549-57.
- [CrossRef] [PubMed] [Google Scholar]
- Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1966;40:271-327.
- [Google Scholar]







