Translate this page into:
Comparison of Safety and Efficacy of Two Brands of Botulinum Toxin A for the Treatment of Lateral Canthal Lines (Crow’s Feet): A Split-Face Study
Address for correspondence: Dr. Byalakere Shivanna Chandrashekar, CUTIS Academy of Cutaneous Science, 5/1, 4th Main MRCR Layout, Vijay Nagar, Bengaluru 560 040, Karnataka, India. E-mail: cutisclinic@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Aim:
To compare the safety and efficacy of Stunnox with the international brand of botulinum toxin A on lateral canthal lines.
Materials and Methods:
This was a nonrandomized, controlled, pilot, split-face study in 47 patients who were given two brands of Botulinum toxin A for the treatment of lateral canthal lines for 12 weeks. Evaluation of lines was assessed with grades of 0 (none), 1 (mild), 2 (moderate), and 3 (severe) at a maximum smile and rest by using ANTERA 3D camera.
Results:
There was a statistical significance in lateral canthal lines wrinkles reduction on the Stunnox sides compared to pretreatment and at weeks 4, 8, and 12, respectively (all P < 0.05). The wrinkle reduction was similar to the effects of the control internationally available brand of botulinum toxin A. The clinical improvement of lateral canthal line wrinkles was greatest at 4 weeks after injection and the improvement lasted until 12 weeks of treatment with no adverse events observed.
Conclusion:
In this split-face study, Stunnox botulinum toxin A showed a moderate but significant wrinkle-soothing effect without obvious side effects on the lateral canthal.
Keywords
ANTERA 3D
botulinum toxin A
lateral canthal lines
spit face
Stunnox
wrinkles
INTRODUCTION
Facial esthetic concerns vary by age group with the younger patients requesting treatment for a smooth contour, while elder groups aged more than 40 years focus on wrinkles, sagging, and improving volume loss.[1] Not all wrinkles are alike. Facial wrinkles are classified as dynamic wrinkles that occur with facial expression and static wrinkles that appear at rest. Static wrinkles are caused by cutaneous sagging or a decrease in the subcutaneous fat layer and atrophy of the dermis. Dynamic wrinkles, caused by repeated muscular contraction, can be improved with botulinum toxin injection. Dynamic lines of the skin occur due to the contraction of muscle in the superficial musculoaponeurotic system layer and results in wrinkles in the skin, perpendicular to the direction of muscle fibers. These wrinkles are fleeting in youth but with facial aging and due to skin atrophy, loss of fat in some superficial areas, and muscular activity, they may become constant.[2] Aging can cause a possible shifting of fat pads, pronounced skin creases, and wrinkles. Static wrinkles, tend to deepen with age with an alteration of dynamic to static facial lines.[3]
Dynamic periorbital wrinkles are a frequent complaint from patients. Lateral orbital wrinkles (“lateral canthal lines”) are horizontal lines in the lateral canthal area or they have a trend toward a superior or inferior position and they appear when smiling. These are predominantly associated with the action of the lateral orbicularis oculi muscle and partially with that of the zygomaticus muscle.[4] These wrinkles are initially seen only with movement with aging and photodamage, these wrinkles seen in activity become static. Age-associated soft-tissue atrophy may also play a role in the development of these lateral orbital lines.[5]
There has been a global rise in the demand for noninvasive facial esthetic treatments to enhance one’s appearance due to the increasing affordability and accessibility of injectable treatments. Since the introduction of Botulinum toxin type-A (Bont - A) more than three decades ago, the indications for use encompass more aesthetic procedures for the aging face and it is the most commonly performed nonsurgical procedure worldwide.[67] Bont-A is used for all wrinkles as a result of persistent muscular contractions. These include forehead lines, glabellar lines, crow’s feet, bunny lines, perioral wrinkles, and platysmal bands. Dynamic wrinkles respond better than static wrinkles.[7]
Treatment of lateral orbital wrinkles
Botulinum toxin is effective in treating lateral canthal lines and is an excellent way to improve lower lid folds and widen the palpebral aperture.[5] In the development of lateral canthal lines, contraction of the underlying muscle can be effectively prevented by using botulinum toxin type A, a neurotoxin produced by the Clostridium botulinum bacterium which blocks the release of acetylcholine in the neuromuscular junction.[4] The mechanism of action of Bont-A is that it prevents the release of acetylcholine, with consequent paralysis of the local muscles, which usually occurs 24 h to 2 weeks following the injection and this effect will extend up to 3–6 months.[8]
The classic application points for treating periorbital wrinkles are recorded. In general, three injection sites are planned for each lateral orbital area. These three injection sites are positioned 1 cm apart and lie 1 cm outside the bony orbital rim (1.5 cm lateral to the lateral canthus). The position of these injection sites should follow the general pattern of the lines, which may be horizontal, superior, or inferior.[8] Appropriate application techniques can produce a reduction in the appearance of lateral orbital wrinkles.
Bont-A injections are a very gratifying product for both doctors and patients alive when done properly. This led us to conduct a nonrandomized, split-face controlled study including 50 patients who were given two brands of Botulinum toxin A for the treatment of lateral canthal lines. Out of the 50 enrolled subjects, 47 patients completed 3 months of evaluation to compare the safety and efficacy of 2 brands of botulinum toxin A for the treatment of lateral canthal lines [Figure 1].
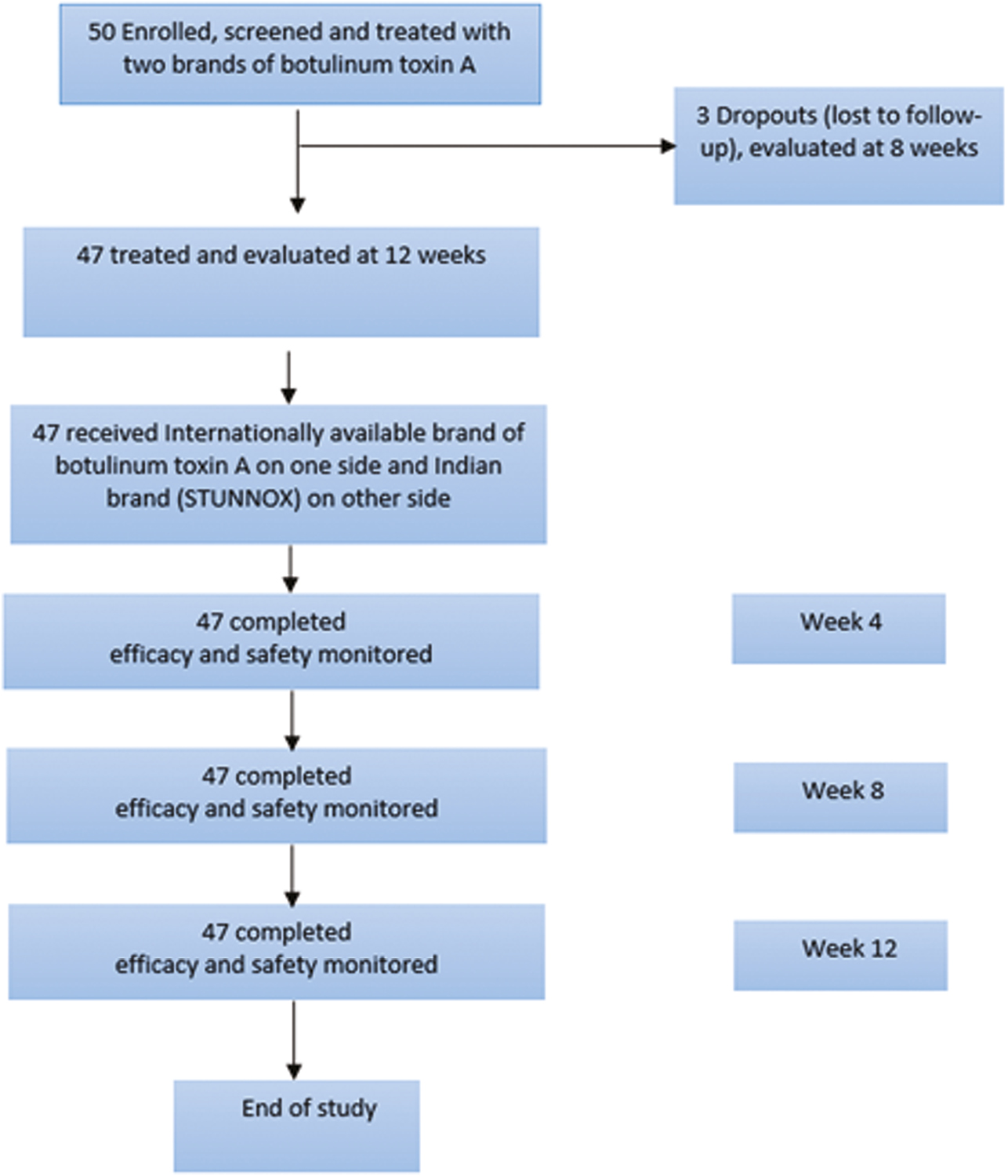
- CONSORT diagram of patient disposition
MATERIALS AND METHODS
Study design
This was a nonrandomized, active controlled, split-face, pilot study. There were 50 patients enrolled who had moderate to severe lateral canthal lines and were treated with two brands of botulinum toxin A (CTRI/2022/09/045312; 1 site in India). The protocol (Protocol number: CACS-GBL-002, Version 1 dated July 26, 2022) and informed consent form were approved by the institutional ethics committee. The trial was undertaken in accordance with current guidelines of the Central Drugs Standard Control Organization, which is the National Regulatory Authority in India,[9] the International Conference on Harmonization Good Clinical Practice, and the Declaration of Helsinki. Written informed consent followed the study explanation to patients before screening procedures or assessments. The objective of the study was to evaluate the safety of Stonnox botulinum toxin A on lateral canthal lines and compare efficacy to the existing brand in the market. The duration of the study was 3 months.
Study population
The study population comprised patients with moderate to severe lateral canthal lines. Key exclusion criteria were known hypersensitivity to botulinum toxin, subjects with neuromuscular disorders, subjects who used medications that interfered with the neuromuscular function, such as aminoglycoside antibiotics and curare-like agents, within 4 weeks before screening, subjects with a previous injection of botulinum toxin type A within 6 months or cosmetic procedures in the periocular area and pregnant or lactating subjects.
Efficacy assessments
There were 50 patients who met the inclusion and exclusion criteria and were selected for the study after informed consent was obtained. These 50 enrolled patients were treated with two brands of botulinum toxin A. Out of the 50 subjects enrolled, 47 subjects completed the study and were evaluated at 3 months. There were three subjects who were lost to follow-up at the second month and completed evaluation 2 months into the study. A detailed history regarding the treatment taken, and procedures done previously was recorded in the predesigned proforma. The severity of lateral canthal lines on both sides was assessed, using a facial wrinkle scale with grades of 0 (none), 1 (mild), 2 (moderate), and 3 (severe) at maximum smile and at rest.
The assessment was done at the first visit, and every month till the completion of the study by using an ANTERA 3D camera.
Study procedure
Botulinum toxin were supplied in single-dose 100 Units per vial. Prior to intramuscular injection, Botulinum toxin was reconstituted with sterile 2.5 mL of 0.9% saline, preservative-free 0.9% sodium chloride. Injections were given with the needle bevel tip up and oriented away from the eye. Four units (0.1 mL) of reconstituted botulinum toxin were injected into three sites per side (six total injection points) in the lateral orbicularis oculi muscle for a total of 24 Units (0.6 mL). On the right side, the internationally available brand of botulinum toxin A was given as control to assess the efficacy, and the brand (Stunnox) was administered on the left side to assess the efficacy and duration of action in comparison to control.
Statistical analysis
Data was summarized descriptively. ANTERA 3D values were analyzed using repeated measures of ANOVA, with P < 0.05 being considered statistically significant.
RESULTS
The study was conducted between September 16, 2022, and January 30, 2023; 50 patients were enrolled in the study and were treated with two brands of botulinum toxin A. Out of the 50 subjects enrolled, 47 subjects completed the study and were evaluated at 12 weeks (N = 47). These 47 patients were included in the efficacy analysis of reduction in lateral canthal lines wrinkles with the treatment of two brands of botulinum toxin A. There were three subjects who were dropouts and were lost to follow up and complet evaluation at 8 weeks in the study.
Baseline demographic characteristics were recorded in Table 1 and stratification by age is described in Figure 2.
| Parameter | Count (N = 47) |
|---|---|
| Age (years) | |
| Mean | 28.67 |
| Standard deviation | 9.19 |
| Minimum | 25.00 |
| Maximum | 45.00 |
| Gender, n (%) | |
| Male | 24 |
| Female | 23 |
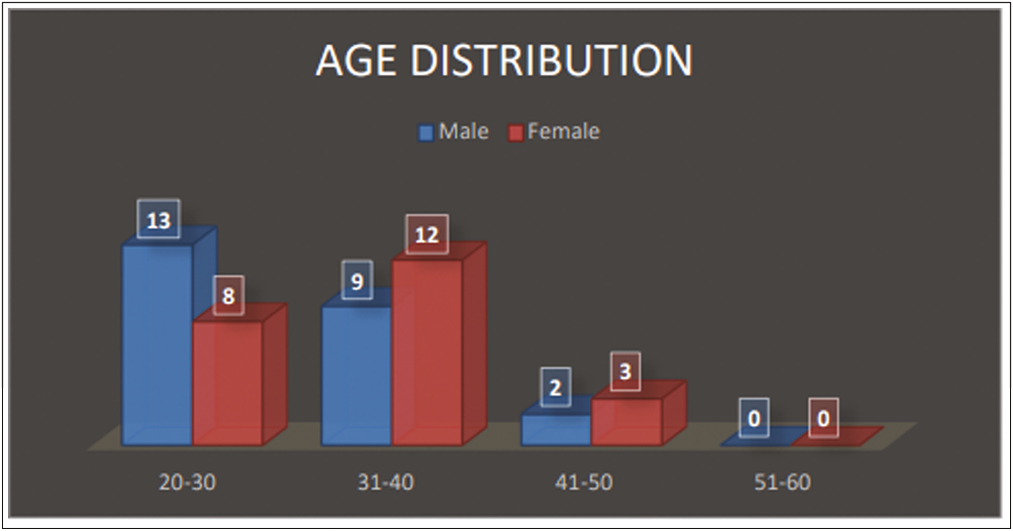
- Age distribution
Total number of 47 subjects were enrolled which included 24 males and 23 females with an age range of 25–45 years [Table 1]. Clinical photographs and 3D images with the ANTERA 3D camera were taken before the procedure and repeated every 4 weeks for 12 weeks and evaluated by a dermatologist. The wrinkles on both Stunnox and control side showed significant improvement on weeks 4, 8, and 12 when compared to the pretreatment on 3D imaging [Table 2,Table 3,Table 4,Table 5 and Figures 3 and 4].
| Wrinkle types | Baseline | Week 4 | Week 8 | Week 12 | P value |
|---|---|---|---|---|---|
| Small wrinkles | |||||
| Size | 20.93617 | 9.614979 | 13.36383 | 18.10638 | 0.04722 |
| Depth | 0.097038 | 0.046702 | 0.074985 | 0.085128 | 0.02359 |
| Width | 0.978872 | 0.461979 | 0.712079 | 0.861149 | 0.00892 |
| Medium wrinkles | |||||
| Size | 33.65957 | 18.77872 | 24.78298 | 30.53617 | 0.04851 |
| Depth | 0.138451 | 0.066211 | 0.092685 | 0.115572 | 0.03150 |
| Width | 1.022489 | 0.479936 | 0.648723 | 0.911723 | 0.00287 |
| Large wrinkles | |||||
| Size | 61.2383 | 29.12191 | 36.97234 | 53.06383 | 0.03248 |
| Depth | 0.225174 | 0.100853 | 0.147955 | 0.196251 | 0.04975 |
| Width | 1.085872 | 0.501102 | 0.813404 | 0.951851 | 0.00125 |
| Wrinkle types | Week 4 (%) | Week 8 (%) | Week 12 (%) |
|---|---|---|---|
| Small wrinkles | |||
| Size | 54.07 | 36.17 | 13.52 |
| Depth | 51.87 | 22.73 | 12.27 |
| Width | 52.81 | 27.26 | 12.03 |
| Medium wrinkles | |||
| Size | 44.21 | 26.37 | 9.28 |
| Depth | 52.18 | 33.06 | 16.52 |
| Width | 53.06 | 36.55 | 10.83 |
| Large wrinkles | |||
| Size | 52.44 | 39.63 | 13.35 |
| Depth | 55.21 | 34.29 | 12.84 |
| Width | 53.85 | 25.09 | 12.34 |
| Wrinkle types | Baseline | Week 4 | Week 8 | Week 12 | P value |
|---|---|---|---|---|---|
| Small wrinkles | |||||
| Size | 21.31489 | 9.48298 | 13.14043 | 17.71702 | 0.04180 |
| Depth | 0.13776 | 0.05910 | 0.09268 | 0.11387 | 0.03316 |
| Width | 1.00200 | 0.45994 | 0.71172 | 0.90557 | 0.01258 |
| Medium wrinkles | |||||
| Size | 35.98936 | 19.45106 | 26.14787 | 32.02340 | 0.03691 |
| Depth | 0.15755 | 0.06603 | 0.10466 | 0.13177 | 0.02597 |
| Width | 1.05670 | 0.42936 | 0.67915 | 0.91255 | 0.00762 |
| Large wrinkles | |||||
| Size | 60.77872 | 22.39362 | 34.61277 | 51.12979 | 0.04897 |
| Depth | 0.251083 | 0.108702 | 0.164872 | 0.21833 | 0.03548 |
| Width | 1.121511 | 0.511638 | 0.811638 | 0.958298 | 0.00249 |
| Wrinkle types | Week 4 (%) | Week 8 (%) | Week 12 (%) |
|---|---|---|---|
| Small wrinkles | |||
| Size | 55.51 | 38.35 | 16.88 |
| Depth | 57.10 | 32.72 | 17.34 |
| Width | 54.10 | 28.97 | 9.62 |
| Medium wrinkles | |||
| Size | 45.95 | 27.35 | 11.02 |
| Depth | 58.09 | 33.57 | 16.36 |
| Width | 59.37 | 35.73 | 13.64 |
| Large wrinkles | |||
| Size | 63.16 | 43.05 | 15.88 |
| Depth | 56.71 | 34.34 | 13.04 |
| Width | 54.38 | 27.63 | 14.55 |
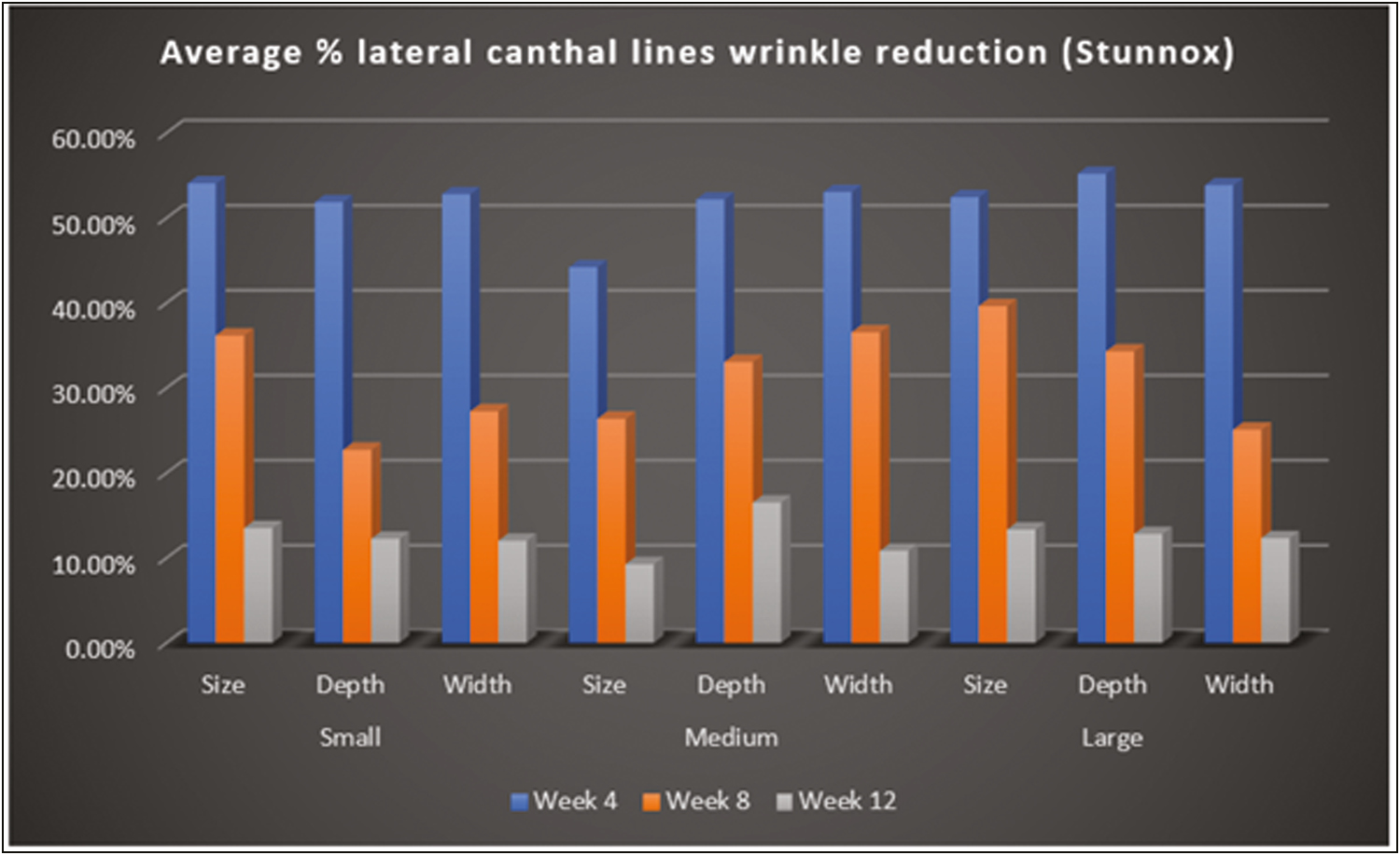
- ANTERA 3D—Average % lateral canthal lines wrinkle reduction (Stunnox)
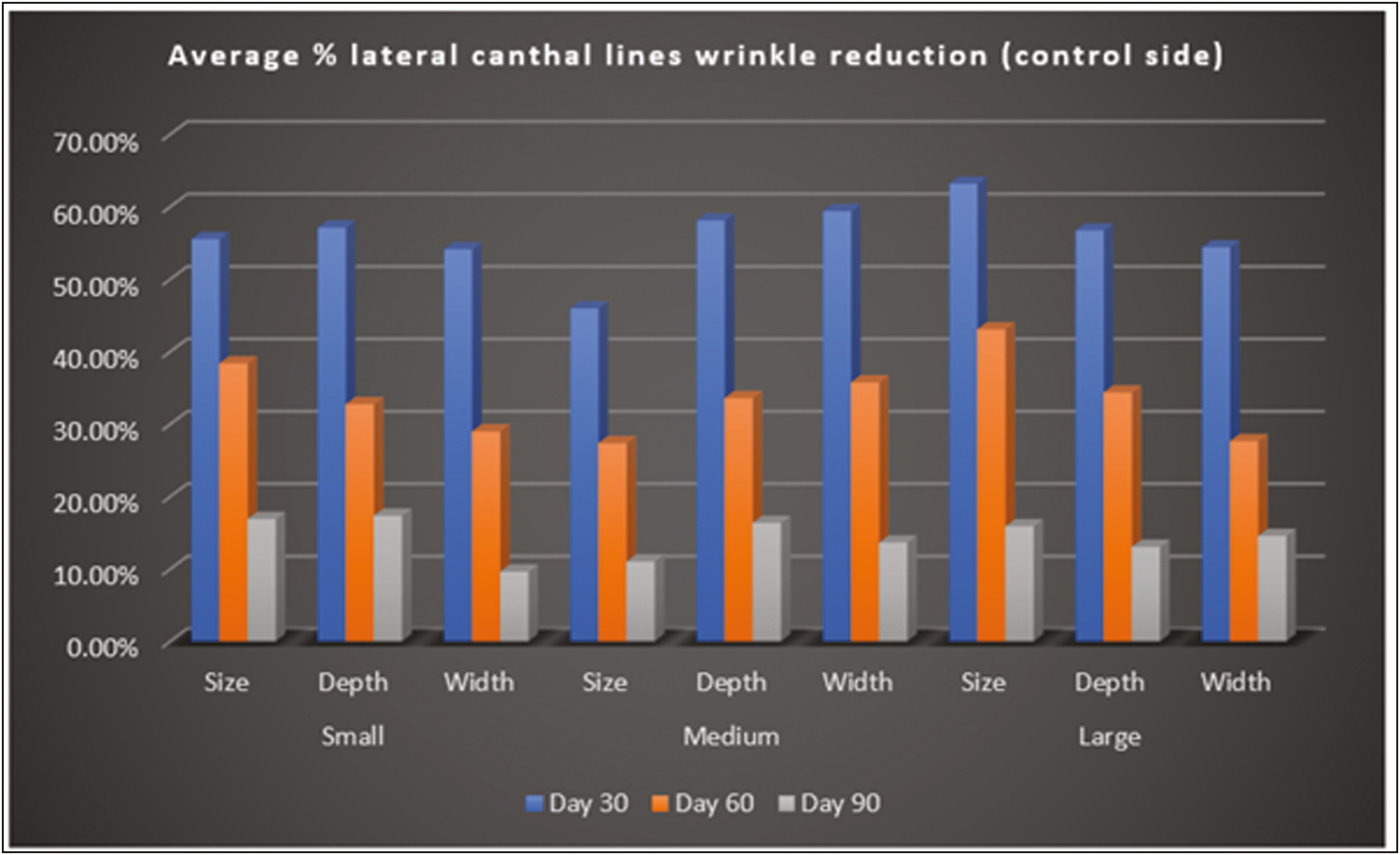
- ANTERA 3D—Average % lateral canthal lines wrinkle reduction (control side)
By photographic documentation using the ANTERA 3D camera, there was a statistical significance in lateral canthal lines wrinkles reduction on the Stunnox sides compared to pretreatment and the wrinkle reduction was similar to the effects of the control, internationally available brand of botulinum toxin A. This effect was noted as early as 1 week after injection and lasted for up to 12 weeks. The clinical improvement of wrinkles was maximum at 4 weeks follow-up after injection and the improvement lasted up to 12 weeks of treatment [Figure 5A–C]. By photographic documentation, there was a significant improvement in the Stunnox sides compared to pretreatment, at weeks 4, 8, and 12, respectively (all P < 0.05). The results were comparable to improvement in reduction of wrinkles on the side treated with the control. Even at week 12, the improvement in wrinkles was maintained on the Stunnox side and control side, compared to the baseline. In our study, the lines had not reached the preinjection state even at 12 weeks.
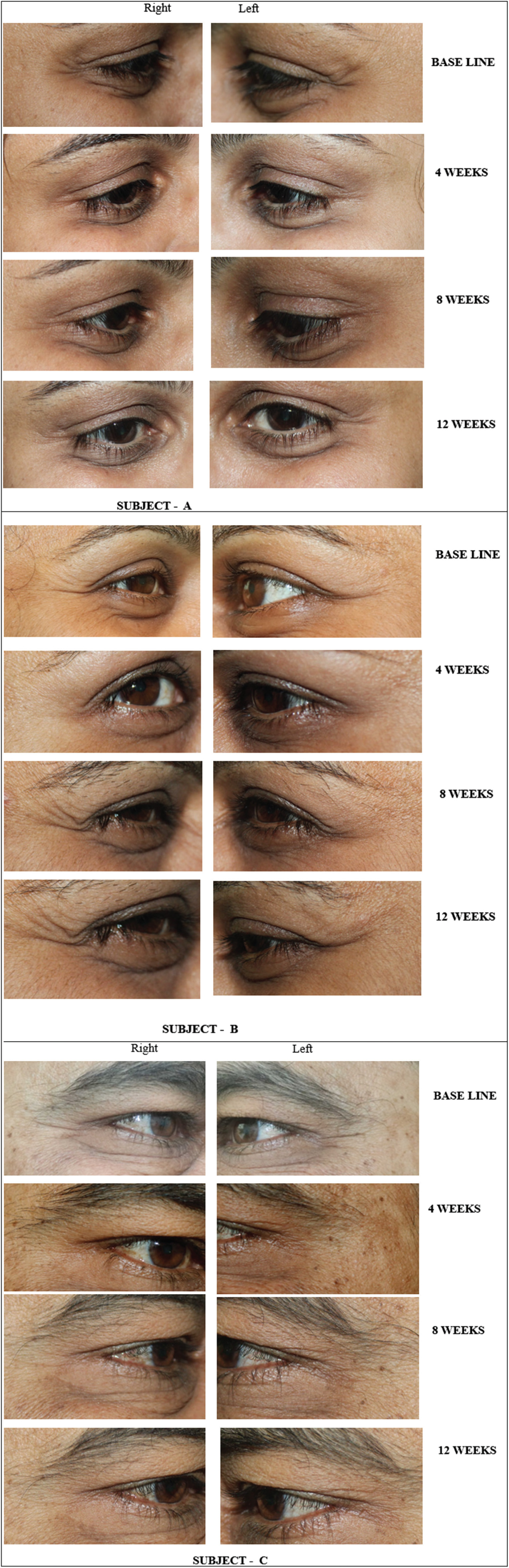
- (A) Photograph showing lateral periorbital wrinkles at baseline, 4, 8, and 12 weeks. (B) Photograph showing lateral periorbital wrinkles at baseline, 4, 8, and 12 weeks. (C) Photograph showing lateral periorbital wrinkles at baseline, 4, 8, and 12 weeks
There were no adverse events observed in the 47 patients evaluated in the study. Treatment of lateral canthal lines wrinkles with Bont-A has been shown to be a safe and effective option up to 12 weeks of treatment.
DISCUSSION
Bont-A is indicated for wrinkles produced due to persistent muscular contractions.[7] It has been established as an excellent agent for improving lateral periorbital wrinkles. The technique is a safe, simple, and effective modality for facial rejuvenation.
In a prospective, longitudinal, analytical study, the effects of Bont-A on the wrinkles of the periorbital region were assessed by means of clinical examination and photographic documentation in 530 patients treated between 2002 and 2007.[4] Thirty percent of patients over 45, and 80% of those under 45, demonstrated complete improvement of the wrinkles after treatment in the classic points. The remaining patients needed treatment in the additional points of the lower eyelid, with the number of units and points varying according to the individual patient.[4]
The results of our pilot study in 47 patients demonstrated that there was statistical significance in lateral canthal lines wrinkles reduction on the Stunnox side compared to pretreatment baseline and the wrinkle reduction was comparable to the control internationally available brand of Bont-A. The wrinkle-smoothing effect was reported by patients on day 7 and objectively recorded by 4 weeks after Bont-A injection and lasted until 12 weeks. The clinical improvement of wrinkles was greatest at 4 weeks after injection.
In this pilot study, Stunnox was similar to the internationally available brand of botulinum toxin A and both treatments showed significant wrinkle-soothing effects compared to pretreatment baseline at weeks 4, 8, and 12. Stunnox caused a less stinging sensation at the site of injection compared to the control. Stunnox is comparable to the internationally available brand of botulinum toxin A when evaluated for safety and efficacy of lateral canthal lines wrinkle reduction in this split-face study. The onset of action of Stunnox is 3–5 days, which is the same as the internationally available brand of botulinum toxin A. The therapeutic effects of Stunnox are similar to of the internationally available brand of botulinum toxin A lasting for 12–16 weeks.
CONCLUSION
Botulinum toxin A injections have become one of the most effective and common aesthetic interventions carried out by physicians for upper face rejuvenation.[10] In this split-face study, Stunnox botulinum toxin A showed moderate but significant wrinkles-soothing effect on lateral orbital wrinkles without obvious side effects, which is similar to the effects of the international brand of botulinum toxin A.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The study was funded by Gufic Biosciences Limited, the manufacturer of botulinum toxin A (Stunnox) in India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Consensus on changing trends, attitudes, and concepts of Asian beauty. Aesthetic Plast Surg. 2016;40:193-201.
- [Google Scholar]
- Treating aging changes of facial anatomical layers with hyaluronic acid fillers. Clin Cosmet Investig Dermatol. 2021;14:1105-18.
- [Google Scholar]
- Classification of periorbital wrinkles and treatment with botulinum toxin type A. Surg Cosmet Dermatol. 2011;3:129-34.
- [Google Scholar]
- ISAPS International Survey On Aesthetic/Cosmetic Procedures performed in 2020. Available from: https://www.isaps.org/media/hprkl132/isaps-global-survey_2020.pdf.
- Guidelines on the use of botulinum toxin type A. Indian J Dermatol Venereol Leprol. 2008;74:S13-22.
- [Google Scholar]
- Regulatory requirements for clinical trials in India: What academicians need to know. Indian J Anaesth. 2017;61:192-9.
- [Google Scholar]
- Regulatory requirements for clinical trials in India: what academicians need to know. Indian J Anaesth. 2017;61:192-9.
- [Google Scholar]
- Upper face rejuvenation using botulinum toxin and hyaluronic acid fillers. Indian J Dermatol Venereol Leprol. 2013;79:32-40.
- [Google Scholar]






