Translate this page into:
Correction of nasal skin necrosis after filler injection with micro and nanofat grafting
*Corresponding author: Navamon Plasen, Department of Otolaryngology, Thammasat University, Pathum Thani, Thailand. navamonp@tu.ac.th
-
Received: ,
Accepted: ,
How to cite this article: Plasen N, Soontornwesn T, Bunaprasert T. Correction of nasal skin necrosis after filler injection with micro and nanofat grafting. J Cutan Aesthet Surg. doi: 10.25259/JCAS_43_2025
Abstract
Vascular compromise leading to skin necrosis is the most serious complication of filler injection. This report presents two cases of nasal skin necrosis treated with microfat and nanofat grafting. Microfat was harvested from the thigh, rinsed with saline, and filtered through sterile gauze. Nanofat was produced by mechanically emulsifying the microfat. Microfat was injected into the pre perichondrium and subcutaneous layers, while nanofat was delivered into the subdermal and intradermal layers. Both patients showed satisfactory outcomes without complications. The nasal tips healed with soft, natural-looking skin indistinguishable from the surrounding tissue. This technique offers a cost-effective alternative for repairing skin necrosis, avoiding the need for debridement or more invasive procedures such as skin grafts or flaps, which may result in scarring and asymmetry.
Keywords
Adipose-derived stem cells
Filler complication
Microfat grafting
Nanofat grafting
Skin necrosis
INTRODUCTION
The most serious complications following hyaluronic acid (HA) filler injections are vascular complications1 which can result in persistent skin necrosis, unilateral or bilateral vision loss, and stroke. In the cases of skin necrosis, standard treatments are surgical debridement, wound care with different materials that promote wound healing and defect coverage with skin grafts or local flaps that might give final results of noticeable scarring and asymmetry.
Adipose-derived stem cell (ASC) therapy was suggested as early as possible in acute phase after ischemia to achieve wound healing in a short period of time.2 The ASCs were derived from stromalvascular fraction (SVF) which was obtained from enzymatic dissociation (collagenase type II) of lipoaspirate followed by centrifugation to separate mature adipocytes from stromal cells. However, the use of enzymes is associated with high costs and presents some conflicts with regulatory agencies.
Instead of using ASCs, we decided to use microfat and nanofat grafting to conduct wound healing in two cases who have nasal skin necrosis after receiving filler injection at their noses. Both of them were treated successfully with the healed skin quality that appears very similar to normal skin.
CASE SERIES
Case 1
A 34-year-old woman received filler injection on her nasal dorsum and tip by a medical professional at a private clinic. She reported erythema and painful swelling developed from nasal tip to nasal dorsum on the day after injection which then turned black without pus. She came to our department 14 days after filler injection and presented with regional necrosis (dry gangrene/eschar) 1.5 × 2 cm on nasal tip [Figure 1a]. Hyaluronidase (3000 IU diluted with 6 mL of NSS) was injected at the affected area on the day she visited the hospital. The wound was taken care using oxytetracycline with Polymyxin B sulfate (terramycin) ointment and the patient received oral amoxicillin/clavulanic acid twice daily without wound debridement [Figure 1b].
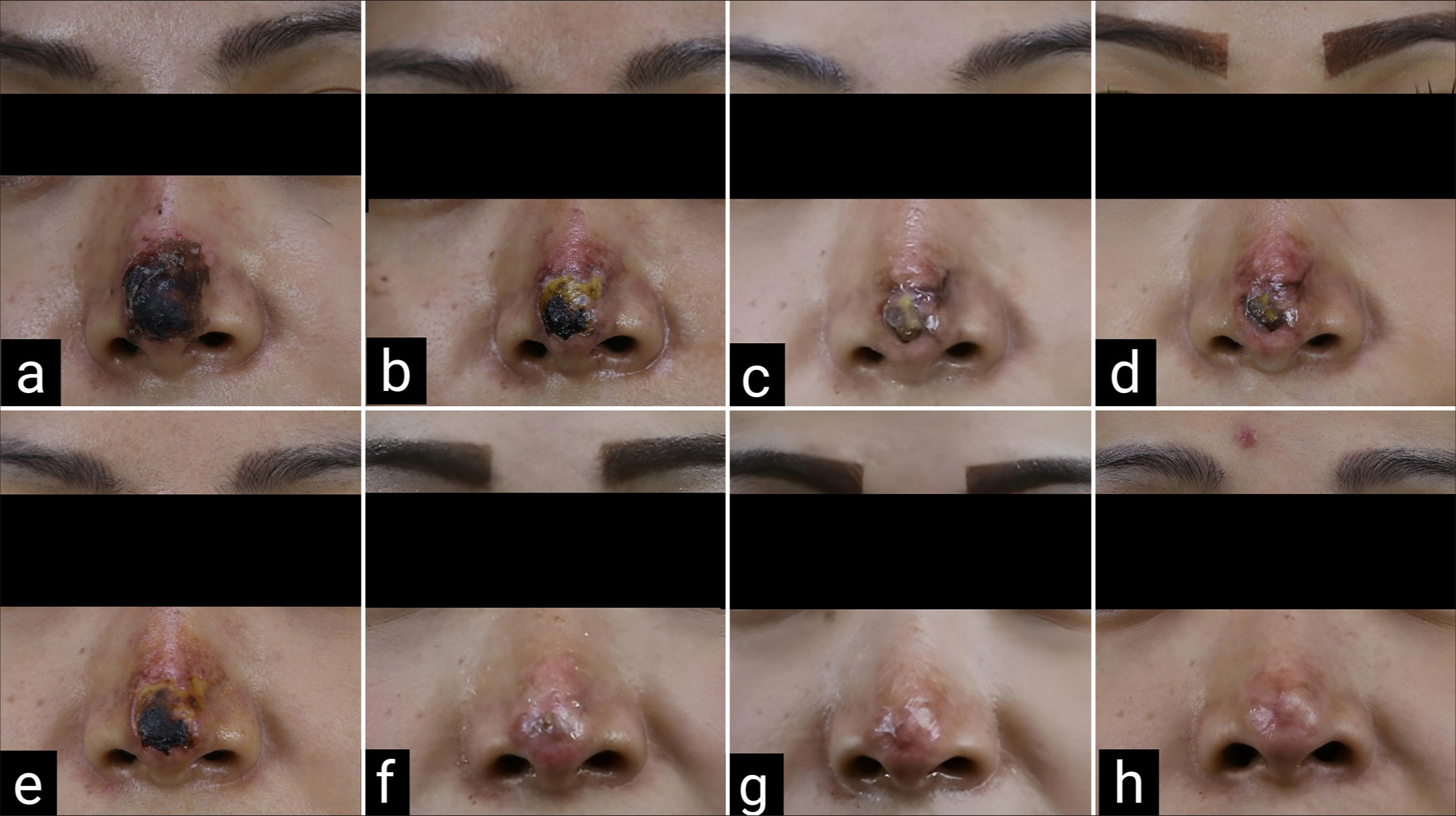
- Case 1 dry gangrene on nasal tip (a) 14-day post-filler injection; hyaluronidase was injected (b) 17-day post-filler injection (c) 22-day post-filler injection; 1st lipofilling was done (d) 1-week post 1st lipofilling (e) 2-week post 1st lipofilling (f) 10-week post 1st lipofilling; 2nd lipofilling was done (g) 6-week post 3rd lipofilling (3rd lipofilling was done 4 weeks after 2nd operation); 4th lipofilling was done (h) 4 months after 4th lipofilling.
Twenty-two days after the filler injection [Figure 1c], she was scheduled for the operation of lipofilling at the lesion using micro and nanofat graft. After the injection, the wound was dressed twice daily with oxytetracycline with Polymyxin B sulfate (Terramycin) ointment to prevent infection and monitor for signs of scar contracture. After the first treatment, the area of necrotic skin gradually diminished [Figure 1d and e]. At 10-week post-operative follow-up, no area of skin necrosis was observed [Figure 1f]. After the first lipofilling, treated areas resembled normal skin, with minor discoloration and slight volume depression. The lipofilling was done repeatedly when we found that the wound edge started to contract and was going to be a depressed scar at 3, 4, and 6 months [Figure 1g] after the first operation.
After four sessions of treatments, the wound was completely re-epithelialized and nasal contour was similar to her previous one [Figure 1h]. Four months after the final treatment, the patient was referred to a dermatologist for laser resurfacing of a slightly noticeable scar.
Case 2
A 37-year-old woman underwent filler injection on her nasal dorsum and tip at a private clinic. She developed erythema and painful swelling in the injected area on the day after injection. Three days after filler injection, her nose color became darker with a small ulcer and pus discharge. She received oral antibiotics (augmentin and clarithromycin) and IV antibiotics (ceftriaxone) but symptoms were getting worse. She came to our hospital 7 days after injection with skin discoloration (purplish color) at supratip area and dermal loss at tip with yellowish necrotic tissue [Figure 2a]. Hyaluronidase, 3000 IU diluted with 6 mL of NSS, was injected to all layers around the area which seemed to have vascular compromise on day 8th after filler injection [Figure 2b and c]. She was scheduled for the operation lipofilling 21 days after the filler injection [Figure 2d]. The wound was completely re-epithelialized and the patient achieved skin quality resembling normal skin at 6 weeks after a single session of lipofilling without complications [Figure 2e]. There is minimal scar remaining and she was sent to a dermatologist for laser resurfacing.
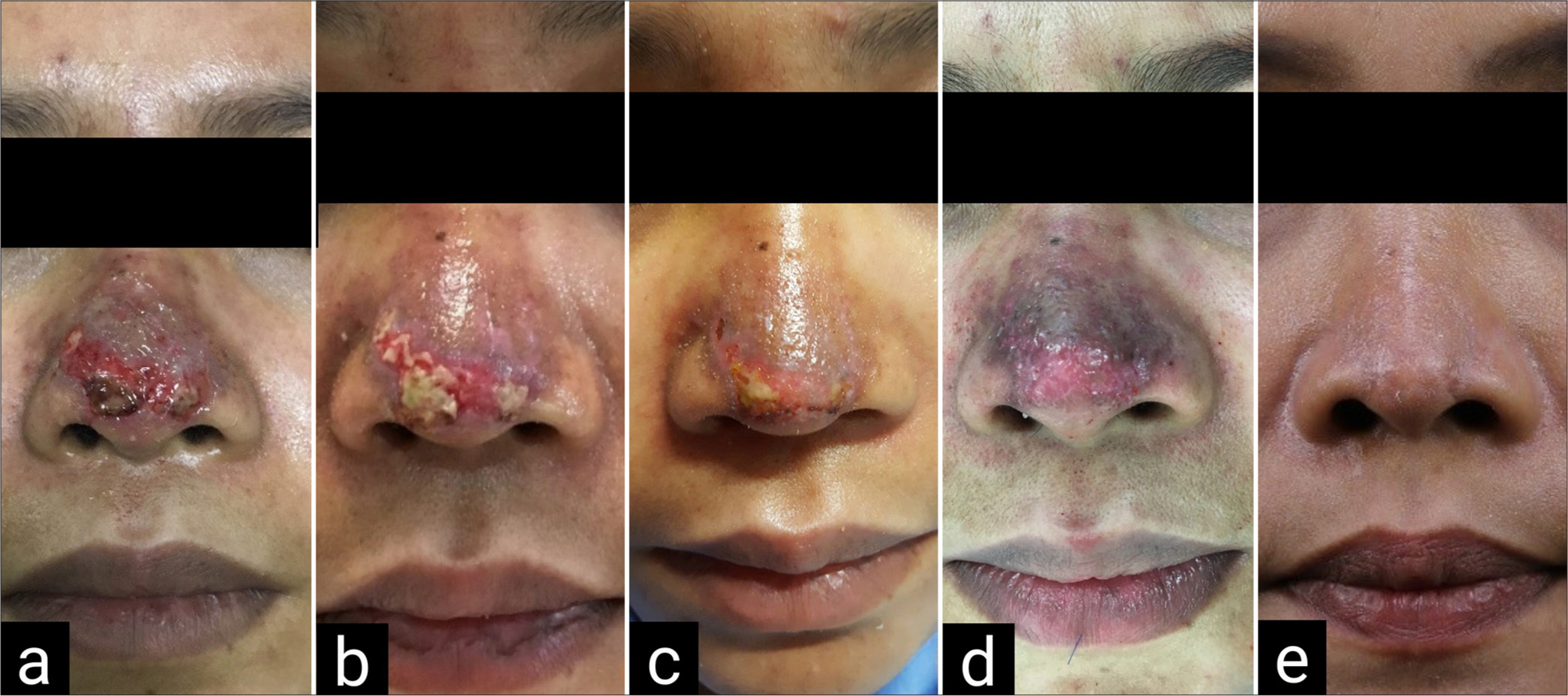
- Case 2 dermal loss at tip with the area of yellowish necrotic tissue (a) 7-day post-filler injection (b) 8-day post-filler injection; hyaluronidase was injected (c) 11-day post-filler injection (3 days after hyaluronidase injection) (d) 21-day post-filler injection; lipofilling was done (e) 6 weeks after lipofilling.
Treatment procedures
Preparation of microfat
Fat was harvested from the inner thigh after infiltration with a modified Klein solution (NSS 100 mL; 1% xylocaine, 10 mL; adrenalin (1:1000), 2 microdrops; 7.5% NaHCO3, 2 mL) and waiting for 15–20 mins. Multi-port 3 mm cannula with sharp side holes of 1 mm in diameter obtained from Tulip Medical products was used. The cannula was connected to 20 mL syringe and liposuction was done using negative pressure. The harvested fat was rinsed with NSS and filtered through a sterile gauze with 0.5 mm pore size without further processing. This effluent is called “Microfat” [Figure 3a].
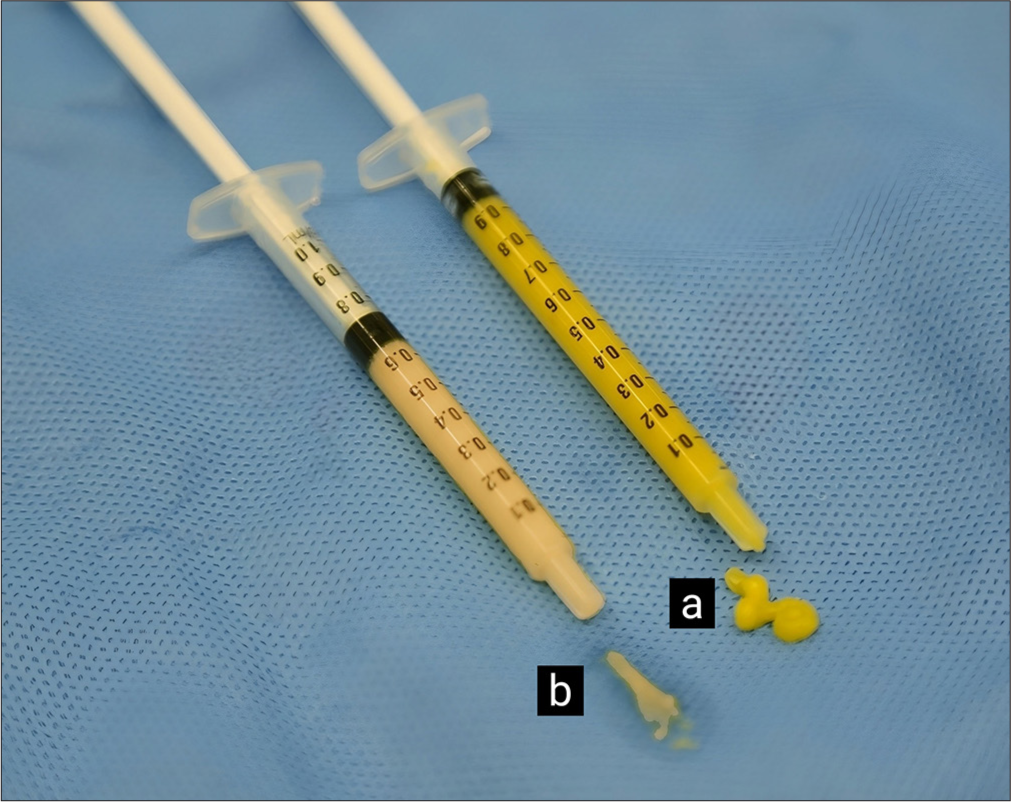
- (a) Microfat. (b) Nanofat: More liquid and whitish appearance.
Preparation of nanofat
The microfat was further mechanically emulsified by shifting it between 1-mL syringe connected to each other using Stopcock, 3-way, 2-Female lock connector. After 30 passes, the fat transformed into a liquid and appeared as a whitish emulsion. This effluent is called “Nanofat” [Figure 3b].
Injection of microfat and nanofat
The procedure involved performing subcision using an 18-gauge needle beneath the necrotic area, targeting the supraperichondrial plane. Microfat was then injected as a bolus with a blunt cannula into the subcision pocket. Next, microfat was injected in diffuse small parcels into the middle layer of the necrotic area, superficial to the created pocket (the subcutaneous layer of the nose), to restore volume. This was followed by nanofat injection into the most superficial layer of the necrotic area (the dermis of the nose). The injection was performed using a retrograde technique with multiple radiating passages to distribute fat in all directions.
DISCUSSION
When vascular compromise happens, appropriate and immediate treatments should be performed. Injection should be stopped. Immediate intralesional hyaluronidase injection, massage, and warm compression are advised to dissolve crosslinked HA, reduce vessel pressure, and restore perfusion.3,4 The ideal time window to use hyaluronidase to prevent further ischemic tissue injury is generally 48 h after filler injection.5
These two cases presented to the hospital late, at 14 and 7 days, respectively, following filler injections. As a result, they were in the late phase of tissue ischemia, characterized by skin necrosis and eschar formation. Intralesional hyaluronidase was administered in both cases and we ensured that the affected area is exposed to adequate amounts of the enzyme by injection in every layer of the ischemic tissue with the highest concentrations within the most severely injured area. This treatment cannot revitalize necrotic tissue but is effective in preventing further tissue damage.3
Mechanically disaggregated nanofat was obtained by sequential passes through different Luer-lock sizes where the lipoaspirate is exposed to shear forces, resulting in mature adipocyte rupture and sizing down of stromal tissue fragments. Sesé et al.6 found that nanofat yields more cells than enzymatic dissociation (SVF) using 10 times less fat, with no difference in cell viability. In addition to isolated SVF cells, nanofat contains a stromal cell population organized as cell aggregates, which retain their vasculature while remaining attached to their natural matrix niche, which has been shown to enhance cell yield performance by promoting cell viability, proliferation, and differentiation.7,8 Thus, we decided to use nanofat instead of ASCs (from SVF) due to comparable regenerative effects, cost-effectiveness, and simplicity in processing and harvesting. Tonnard et al.9 also reported that microfat preserved adipocytes and tissue structure, while nanofat lacked both but was rich in ASCs.
Microfat grafting was applied in two methods which are diffuse small parcels and bolus in created pocket by subcision. A goal of small parcel injection is to improve survival so it can restore volume or function as a filler in depressed scar. In the meantime, we also injected bolus microfat grafting in the pocket created by subcision. This fat behaves as a scar softener and scar expander to maintain expansion. Most of the fat injected in bolus will be necrosed and activate a release of matrix metalloproteinase (MMP). This MMP helps in the degradation of collagen fiber which contributes to scar softening. For the nanofat grafting, we injected intradermal and sub-dermal area. According to Spiekman et al.,10 ASCs prevent scar contracture by inhibiting transforming growth factor-β1 induced myofibroblast differentiation and stimulating gene expression of MMP enzymes, MMP-1 and MMP-2. Thus, the aims of nanofat grafting enriched with ASCs are to improve skin quality without volume change due to its regenerative effect and scar contracture inhibition.
To summarize, micro and nanofat grafting was applied to restore volume in depressed scar, inhibit scar contracture, soften healing tissue, and enhance wound repair from ASCs potentiality. In the first case, the nasal tip was injured from subcutaneous layer to epidermis, while the injury was limited to deep dermal layer in the second case. Hence, the duration of treatment is longer in the first case because lipofilling was done to reconstruct the whole destroyed subcutaneous and skin layer. All procedures were done repeatedly when we observed that the wound edge began to contract to prevent scar contracture.
CONCLUSION
Micro and nanofat grafting are effective for treating skin necrosis after filler injections. They offer a cost-effective, easy-to-process solution with fewer regulatory hurdles, aligning with stem cell legislation. This technique avoids aggressive debridement and the need for skin grafts or flaps, reducing donor site morbidity and scarring at the affected area.
Author’s contributions:
Navamon Plasen: Validation, investigation, resources, writing - original draft, writing - review & editing, project administration. Thitinij Soontornwesn: Investigation, resources. Tanom Bunaprasert: Conceptualization, methodology, supervision.
Ethical approval:
The research/study was approved by the Institutional Review Board at Chulalongkorn University, number IRB No. 289/59, dated March 03, 2016.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Vascular complications after facial filler injection: A literature review and meta-analysis. J Clin Aesthet Dermatol. 2019;12:E65-72.
- [Google Scholar]
- Early intervention with highly condensed adipose-derived stem cells for complicated wounds following filler injections. Aesthetic Plast Surg. 2016;40:428-34.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline for the management of hyaluronic acid filler-induced vascular occlusion. J Clin Aesthet Dermatol. 2021;14:E61-9.
- [Google Scholar]
- Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol. 2011;10:1007-12.
- [Google Scholar]
- Hyaluronic acid filler-induced vascular occlusion-three case reports and overview of prevention and treatment. J Cosmet Dermatol. 2024;23:1217-23.
- [CrossRef] [PubMed] [Google Scholar]
- Nanofat cell aggregates: A nearly constitutive stromal cell inoculum for regenerative site-specific therapies. Plast Reconstr Surg. 2019;144:1079-88.
- [CrossRef] [PubMed] [Google Scholar]
- Extracellular matrix aggregates from differentiating embryoid bodies as a scaffold to support ESC proliferation and differentiation. PLoS One. 2013;8:e61856.
- [CrossRef] [PubMed] [Google Scholar]
- Aggregation promotes cell viability, proliferation, and differentiation in an in vitro model of injection cell therapy. J Tissue Eng Regen Med. 2012;6:e61-73.
- [CrossRef] [PubMed] [Google Scholar]
- Nanofat grafting: Basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017-26.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose tissue-derived stromal cells inhibit TGF-β1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg. 2014;134:699-712.
- [CrossRef] [PubMed] [Google Scholar]







