Translate this page into:
Delayed vascular occlusion following hyaluronic acid-based skin booster – A rare case report
*Corresponding author: Shuba Dharmana, Aesthetic Physician, LeJeune Medspa, Bangalore, India. sdharmana@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dharmana S, Sethi N, Singh S. Delayed vascular occlusion following hyaluronic acid-based skin booster – A rare case report. J Cutan Aesthet Surg. doi: 10.25259/jcas_209_22
Abstract
Hyaluronic acid (HA) based skin boosters used for treating fine lines and for enhancing the quality of skin are quite popular as they help by drawing water under the dermis (hydrofilling effect), helping in prolonged hydration of the skin. They are also considered safe due to the superficial plane of injection and low volumes injected. The patient came to the clinic for skin hydration and rejuvenation treatment. After detailed discussion and informed consent, a non-animal origin HA-based skin booster with a concentration of 20 mg/mL was chosen for facial rejuvenation. As per protocol, 2 mL of the product was injected by micropuncture technique with the automated click technology, delivering 0.01 mL per point in the mid dermis all over the cheeks, chin, and forehead. The patient tolerated the procedure well and presented to us 48 hours post-injection with persisting redness and pain on the central forehead. The redness appeared to be in a triangular pattern, with the apex at the left eyebrow and the base toward the hairline. Diagnosis of vascular compromise was made, and the patient was immediately treated with pulsed doses of hyaluronidase. The entire area healed without any sequelae. We hereby report a rare case of delayed vascular occlusion following skin booster treatment, which was successfully managed by hyaluronidase treatment. Skin boosters are the most sought after treatments for skin hydration and are generally considered safe; this is the first case report with delayed vascular occlusion reported following skin booster injections. Because of the high risk associated with glabella and forehead areas for fillers, one should be mindful in this area even with the skin boosters.
Keywords
Hydration filler
Vascular compromise
Glabella
INTRODUCTION
Skin rejuvenation with hyaluronic acid (HA) based skin boosters has become one of the most sought-after treatments for superficial fine lines and skin rejuvenation.1 Skin boosters are generally safe, with commonly reported side effects such as lumps, bruises, injection site pain, and erythema. However, serious adverse events such as vascular necrosis or blindness have not been reported yet. Although the glabella, along with the nose, is the most commonly reported site for vascular complications when using HA fillers,2 similar complications have not been reported using skin booster treatments for skin hydration and rejuvenation.3
HA-based skin boosters are generally considered safe as they are injected in microdroplets by micropuncture technique and in the intradermal or subdermal planes, but here, we present a case of delayed vascular compromise with such a product injected in the mid-dermis over the glabella and the face for improving fine lines and skin hydration.
CASE REPORT
A 36-year-old Caucasian female presented [Figure 1] with a fair complexion and dynamic frown and forehead lines. She wanted to get treated with HA-based skin boosters for hydration. She had undergone neurotoxin and filler treatments before with no adverse events. She, however, had not undergone any HA-based skin booster treatment in the past. She did not have any relevant medical history of note. Informed written consent was obtained from the patient after she was thoroughly explained the possible associated complications with skin booster injections.
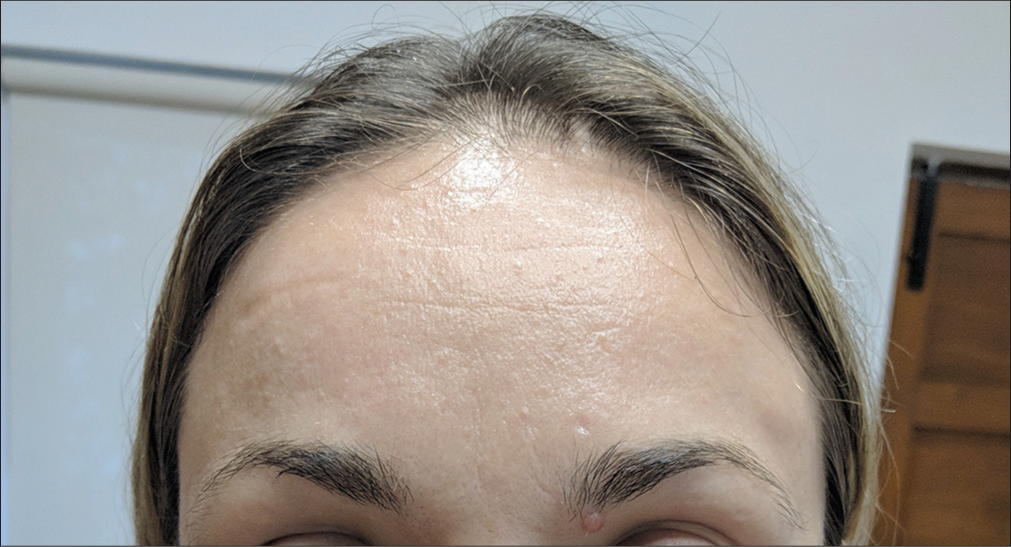
- Pre-procedure.
A non-animal origin HA-based skin booster with a concentration of 20 mg/mL was chosen for facial rejuvenation. As per protocol, 2 mL of the product was injected by micropuncture technique with the automated click technology, delivering 0.01 mL per point in the mid dermis all over the cheeks, chin, and forehead. The procedure was well tolerated, and the patient did not complain of any excessive pain or discomfort after the injections. The patient left the clinic uneventfully. The next day, as a routine, a follow-up call was made, and the patient did not complain about any pain or redness. However, the next day, which was more than 48 hours after the procedure, the patient called up and complained of persisting redness and pain in the forehead. The patient was reviewed at the clinic, and the redness on the central forehead appeared to be in a triangular pattern, with the apex at the left eyebrow and the base toward the hairline. The area was not well demarcated and had mild erythema with localized edema and tenderness. A suspicion of vascular compromise in this triangular area was kept and the patient was advised treatment of the area with hyaluronidase immediately. The patient was very reluctant to use hyaluronidase treatment and sought some time to discuss it with her family. However, within the next 6 h, the erythema further progressed and showed more of a livedo reticularis pattern and also showed some white pustules embedded within [Figure 2]. She agreed to get hyalased immediately, as per our advice.
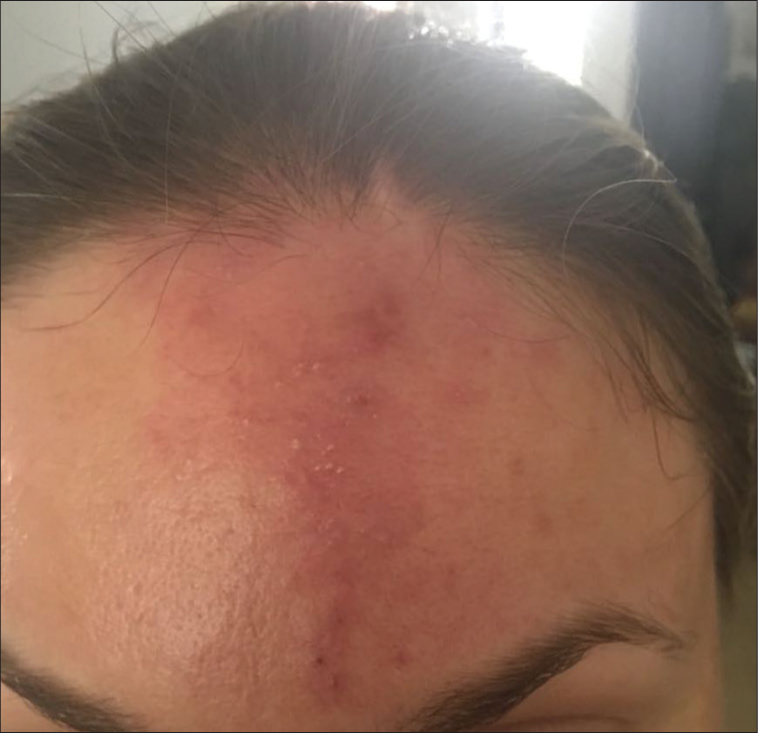
- 2 days post-procedure.
She was injected with hyaluronidase (total 500 U) all over the erythematous area. She was also given 75 mg aspirin stat. The patient was assessed every 10 min and within 30 min, the redness improved considerably with reperfusion and good capillary refill. She was sent home after a further 30-minute observation. She was also followed up later that day [Figure 3], and 1 week later, she recovered from the event without any residual sequelae. She was also reviewed at her follow-up visit 4 months later with no residual sequelae of the vascular event.
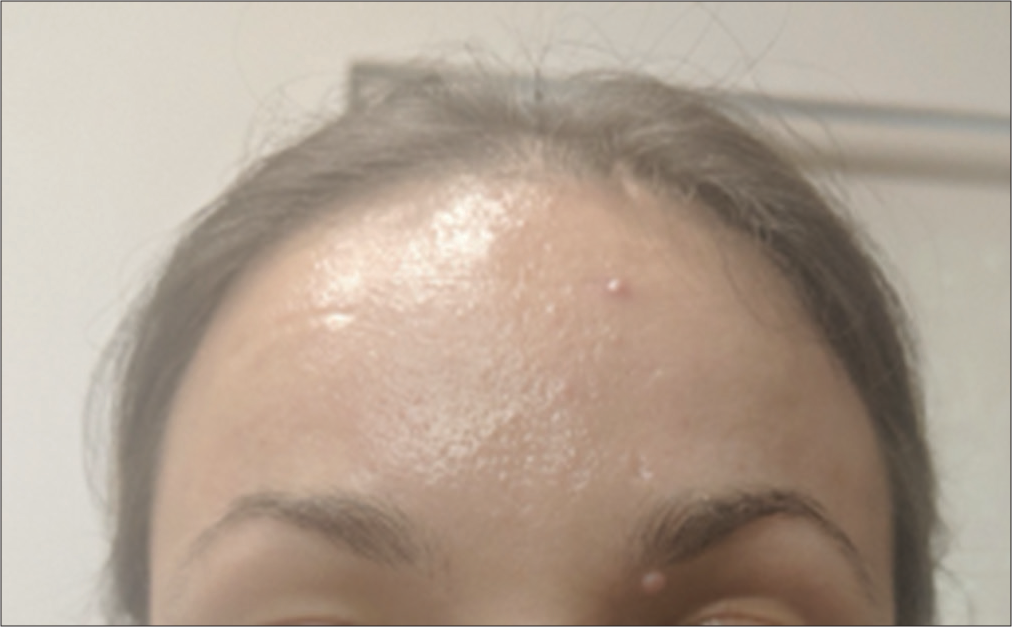
- Post hyalase.
DISCUSSION
A vascular event with fillers is considered a serious side effect, and every effort must be made to avoid such a complication; this includes having a thorough knowledge of the vascular anatomy of the area to be injected and also having a good level of control in relation to depth, with the needle or the cannula which is being used to inject the product. Glabella and the forehead are one of the six filler-related facial unsafe zones.4
Anatomical safe zones and unsafe zones need to be respected by the injectors, even with hydration skin boosters. The superficial and the deep branches of the supratrochlear and supraorbital arteries are in the way during the glabellar injections, and therefore, the chance of intravascular injury is high. The deep branch of the supratrochlear artery passes deep into the corrugators and then penetrates the frontalis, and the superficial branch crosses over the corrugators and then pierces the frontalis. The superficial branch becomes subcutaneous at 13.9 mm distance horizontally from the midline and at 7.6 mm vertically from the supraorbital rim. The deep branch becomes subcutaneous at 25.5 mm horizontally and 30.2 mm vertically from the midline and supraorbital rim, respectively.5
It is recommended, therefore, that injections in the lower forehead area within 2 cm from the supraorbital rim should be as superficial as possible, while injections in the upper forehead should be made on the periosteal plane.6 The consensus recommendations of 2018,7 also recommend injecting in the subdermal plane or the intradermal plane with the needle.
For improving skin quality, low concentration HA-based skin boosters must be injected intradermally for effectiveness. If they are injected deep, they lose their effectiveness in improving skin quality or hydration. Although the skin boosters are considered very safe, we hereby report a case of delayed vascular occlusion following the same and make the injectors wary of such a complication and the need to be extra cautious when injecting in such vascular zones since even the smallest aliquot of 0.01 mL can be dangerous.
CONCLUSION
Although skin boosters are considered absolutely safe, precautions and thorough anatomical knowledge of the vascular territories are of utmost importance. The authors also suggest looking at alternate methods of skin rejuvenation over the glabella and forehead, such as botulinum toxin or high-intensity focused ultrasound. A systematic review of available literature would probably be required to figure out the most effective and safe way of glabellar rejuvenation.
Authors’ contributions
Study conception and design, data collection, analysis and interpretation of results, draft manuscript preparation: Dr.Shuba Dharmana. All authors reviewed the results and approved the final version of the manuscript.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Understanding and using hyaluronic acid. Aesthet Surg J. 2004;24:361-4.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of injectable fillers, Part 2: Vascular complications. Aesthet Surg J. 2014;34:584-600.
- [CrossRef] [PubMed] [Google Scholar]
- Rejuvenating effects of facial hydrofilling using restylane vital. Arch Plast Surg. 2015;42:282-7.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy of the facial danger zones: Maximizing safety during soft-tissue filler injections. Plast Reconstruct Surg. 2017;139:50e-8.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical and ultrasound-based investigation to localize the arteries on the central Forehead region during the Glabellar augmentation procedure. Clin Anat. 2020;33:370-82.
- [CrossRef] [PubMed] [Google Scholar]
- The supratrochlear artery revisited: An anatomic review in favor of modern cosmetic applications in the area study design. Cureus. 2020;12:e7141.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus recommendations for treatment strategies in Indians using botulinum toxin and hyaluronic acid fillers. Plast Reconstruct Surg Glob Open. 2017;5:e1574.
- [CrossRef] [PubMed] [Google Scholar]






