Translate this page into:
Deoxycholate (ATX-101) Mixed with Lidocaine to Minimize Pain/Discomfort in Nonsurgical Treatment of Submental Fullness Appearance
Address for correspondence: Prof. Raffaele Rauso, Department of Maxillo-Facial Surgery, University of Campania “Luigi Vanvitelli”, Piazza Miraglia, 80138 Naples, Italy. E-mail: raffaele.rauso@unicampania.it
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the present study pain/discomfort reduction in submental fullness treatment with the injections of a DC based drug (ATX-101, Allergan, Dublin, Ireland) premixed with lidocaine 2% on a sample of 12 patients retrospectively evaluated has been performed All patients indicated improvement in skin tightening from the 2nd month postinjection. Three patients had minor ecchymoses at the injection sites, which resolved spontaneously within 10 days posttreatment. One patient experienced dysesthesia of the treated area, which lasted approximately 40 days and resolved spontaneously. No other complications—such as nerve paresis or alopecia—were recorded. No patient required analgesic drugs postinjection.
Keywords
Deoxycholate
discomfort
fat reduction
injection adipocytolysis
INTRODUCTION
ATX-101 (Allergan, Dublin, Ireland), an injectable drug containing deoxycholate (DC), was recently introduced into the US, Canadian, and European markets for noninvasive treatment of submental fullness (SMF). Submental fat accumulates in a distinct compartment within the preplatysmal fat[1]; it is considered aesthetically unappealing and has a negative psychological impact on patients.[2] In a recent study, Jacono and Malone[3] determined that the preplatysmal fat pad is enlarged in the elderly patients.
In recent years, several nonsurgical technologies have been proposed to treat SMF, which include high-intensity focused ultrasound and cryolipolysis. A major advantage of injectable drugs for submental fat reduction is that the practitioner can obtain the drug on demand. “I have 1 patient to treat; I can buy only the vials of ATX-101 I need to treat him!”
ATX-101 was shown to be safe and effective for noninvasive reduction of SMF in two randomized controlled trials[45]; however, patients have raised concerns about a burning sensation felt immediately after injection. To mitigate this discomfort, the manufacturer of the drug suggests placement of ice packs or induction of local anesthesia before injection. In this case series, the author describes his experience in treating SMF by injection of a premixed solution of ATX-101 and 2% lidocaine. This approach allowed for pain relief on drug delivery.
MATERIALS AND METHODS
From May 2017 to September 2017, 12 patients (10 women, 2 men) who presented with SMF were treated by injection with ATX-101 (10mg/mL; trade name, Belkyra) that had been premixed with lidocaine hydrochloride (20mg/mL; Bioindustria L.I.M., Novi Ligure, Italy).
Injection procedure
Before treatment, the preplatysmal fat was identified, and the potential change in neck anatomy associated with managing the SMF was assessed. The area to be treated was marked with a surgical pen, and a grid (with points spaced at 1-cm intervals) was applied to mark the injection sites, as suggested by the manufacturer [Figure 1].[6] Preparations of ATX-101 (10mg/mL; 2mL per vial) were premixed with 0.5mL of lidocaine at a concentration of 20mg/mL. The solution then was injected as 0.25-mL aliquots by means of a 12-mm, 32-gauge needle. The injections were made at 1-cm intervals at an approximate depth of 7mm in the central area of the grid and at an approximate depth of 3–4mm in lateral areas of the grid [Figure 2].
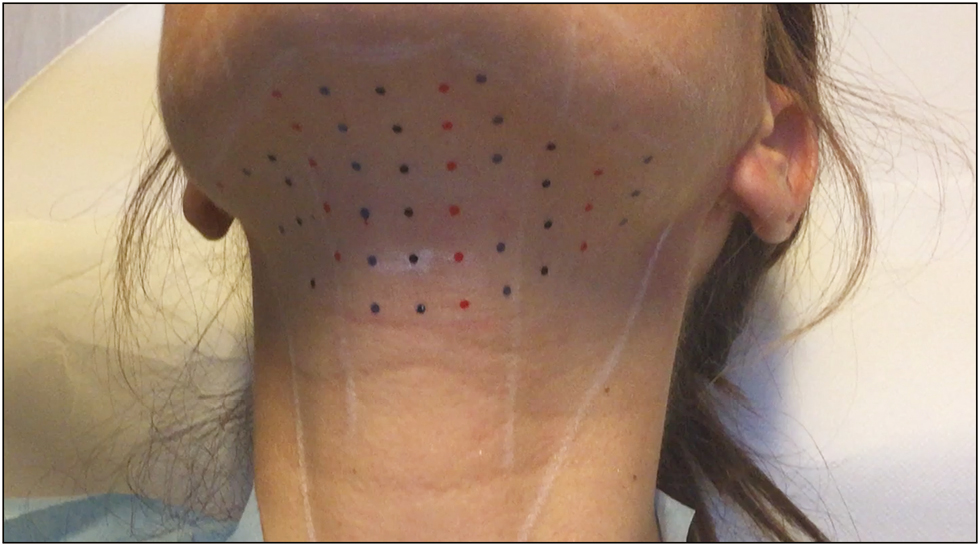
- Pretreatment markings on the 41-year-old woman who presented for reduction in SMF by means of ATX-101 injection. The sites for injection were marked with a grid supplied by the manufacturer
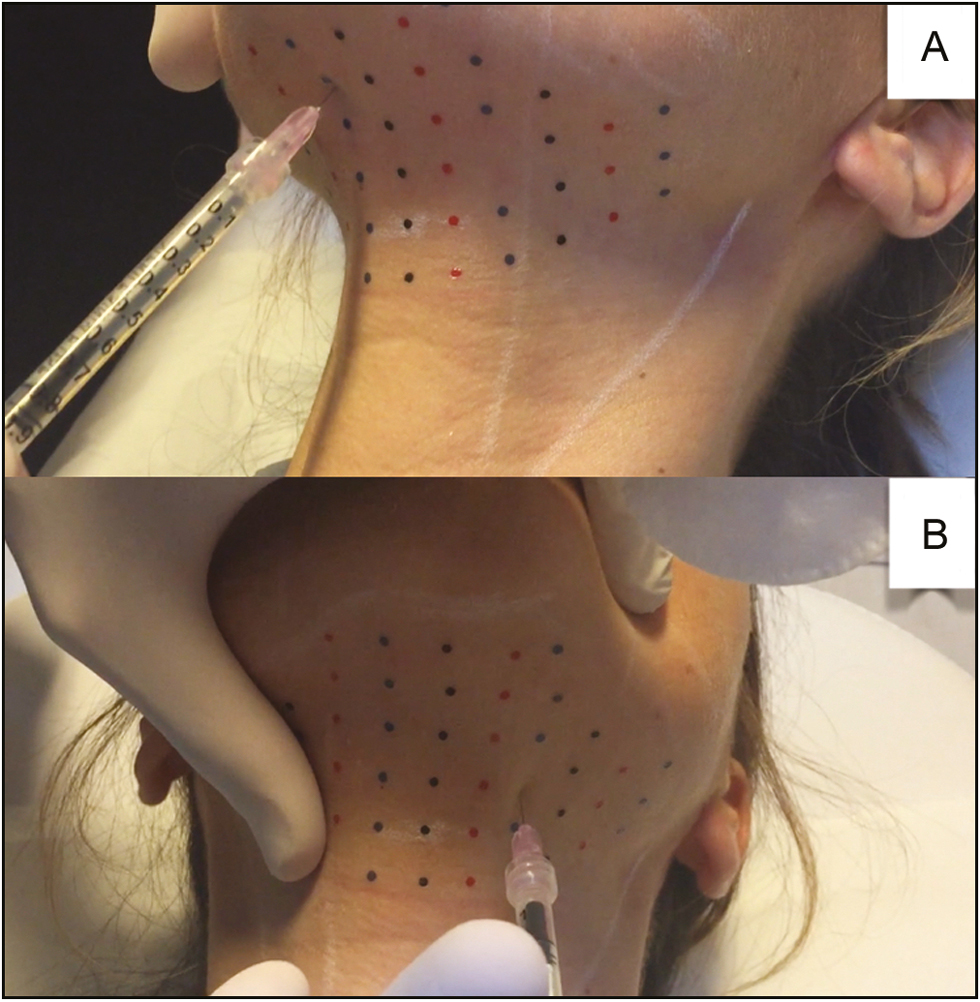
- (A) Injections in the central area of the grid were made at a depth of approximately 7mm. (B) Injections in the lateral areas of the grid were made at a depth of approximately 3–4 mm
Assessment of treatment efficacy and pain severity
Patients were photographed pretreatment and 2–3 months posttreatment. At the follow-up visit, the degree of skin tightening over the preplatysmal fat was assessed and pre- and posttreatment photographs were compared.
After the treatment session, the patients were asked to indicate whether the injection was painful. Specifically, the patients were asked to compare the level of pain or discomfort to that of a previous aesthetic treatment involving the face, such as placement of filler or botulinum toxin type A. Of the 12 patients included in the study, only 1 had not undergone a facial aesthetic treatment previously.
RESULTS
The age range of the patients was 22–56 years. Each patient received multiple injections in a single session, with one to four 2-mL vials of ATX-101 delivered per treatment (mean, 2.16 vials; total, 26 vials; Table 1).
| Patient (gender) | Number of vials injected (each vial contains 2 mL) |
|---|---|
| Patient 1 (F) | 2 |
| Patient 2 (F) | 1 |
| Patient 3 (F) | 3 |
| Patient 4 (F) | 2 |
| Patient 5 (F) | 2 |
| Patient 6 (M) | 1 |
| Patient 7 (F) | 2 |
| Patient 8 (F) | 2 |
| Patient 9 (F) | 3 |
| Patient 10 (F) | 2 |
| Patient 11 (F) | 2 |
| Patient 12 (M) | 4 |
F = female, M = male
All patients experienced postinjection swelling that persisted for an average of 15 days (range, 10–28 days). All patients indicated improvement in skin tightening from the 2nd month postinjection. Three patients had minor ecchymoses at the injection sites, which resolved spontaneously within 10 days posttreatment. One patient experienced dysesthesia of the treated area, which lasted approximately 40 days and resolved spontaneously. No other complications—such as nerve paresis or alopecia—were recorded. No patient required analgesic drugs postinjection. In Table 2, patients’ perceptions of pain and discomfort associated with this procedure are summarized relative to previous facial aesthetic medical treatments that that patients had undergone.
| Patient (gender) | More painful/discomfortable | Same pain/discomfort | Less painful/discomfortable |
|---|---|---|---|
| Patient 1 (F) | X | ||
| Patient 2 (F) | X | ||
| Patient 3 (F) | X | ||
| Patient 4 (F) | X | ||
| Patient 5 (F) | X | ||
| Patient 6 (M) | X | ||
| Patient 7 (F) | X | ||
| Patient 8 (F) | X | ||
| Patient 9 (F) | X | ||
| Patient 10 (F) | X | ||
| Patient 11 (F) | X | ||
| Patient 12 (M) | N/A | N/A | N/A |
F = female, M = male, N/A = not applicable
DISCUSSION
The effectiveness of DC injection in nonsurgical fat reduction was first described by Rotunda et al.[7] in 2004; since then, this procedure has been gaining in popularity. Duncan and Rotunda[8] noted that DC injection primarily is indicated for the treatment of localized adiposities, such as submental fat, back rolls (“bra-strap fat” in women), and lipomas. A drug containing DC (ATX-101) was introduced into the US and Canadian markets in 2015 and into the European market in 2017. The sole purpose of this drug is to reduce unwanted SMF. A key innovation of this drug is its specific injection protocol. A grid is applied to the treatment area and 0.2-mL aliquots of ATX-101 are injected into prespecified sites at 1-cm intervals.[569]
To ease the pain of ATX-101 delivery, the manufacturer suggests preparing the area to be injected with ice packs. However, the efficacy of ice packs for pain relief after DC injections is debatable. In a recent practical and theoretical course, Sherman[10] noted that the patient discomfort could be reduced by prefilling 1-mL syringes with the amount of drug solution needed and performing the procedure as quickly as possible. The manufacturer also has suggested local anesthesia for pain mitigation before ATX-101 injection. However, the induction of local anesthesia before ATX-101 injection has anatomic consequences because the anesthetic leads to swelling. Therefore, it is necessary to wait for approximately 15–20min between delivery of the anesthetic and injection of ATX-101.
In this study, the author mixed each 2-mL vial of ATX-101 with 0.5mL of lidocaine and injected 0.25mL of the mixed solution into each 1-cm2 area of the grid. No patient in this case series indicated that pain was felt during the injections. The results summarized in Table 2 support the hypothesis that premixing with lidocaine allowed for drug delivery with little discomfort compared with other aesthetic medical treatments involving the face. Eleven of the twelve patients in this series had previously undergone a facial aesthetic medical treatment. Five of these eleven stated that they felt the same amount of pain and discomfort that they had felt during other aesthetic facial procedures; six patients indicated that the injection of the ATX-101 mixture was less painful. We attribute this result to premixing with lidocaine.
The volume of 2% lidocaine added to each vial was chosen because it is a sufficient amount required for pain reduction and it allows for simple calculation and delivery. The manufacturer recommends a dose of 0.2mL of ATX-101 per site, and each vial contains 2mL of product. Therefore, from each vial, two 1-mL syringes can be filled for injection at 10 sites. After premixing each vial with 0.5mL of lidocaine, the practitioner can fill two and a half 1-mL syringes and inject 0.25mL of solution at 10 sites.
The amount of lidocaine used in this study is much less than the amount of lidocaine used by other authors to induce local anesthesia before ATX-101 injection.[11] The premixed solution of ATX-101 and lidocaine was found to be effective for managing SMF as shown by posttreatment photographs that depict improvements in preplatysmal fat reduction and skin tightening [Figures 3]. The anhydrous disodium phosphate buffer in ATX-101 is robust to pH changes on addition of another drug; therefore, we did not expect to see a decline in effectiveness with lidocaine premixing. Importantly, no precipitate was observed in the mixture.
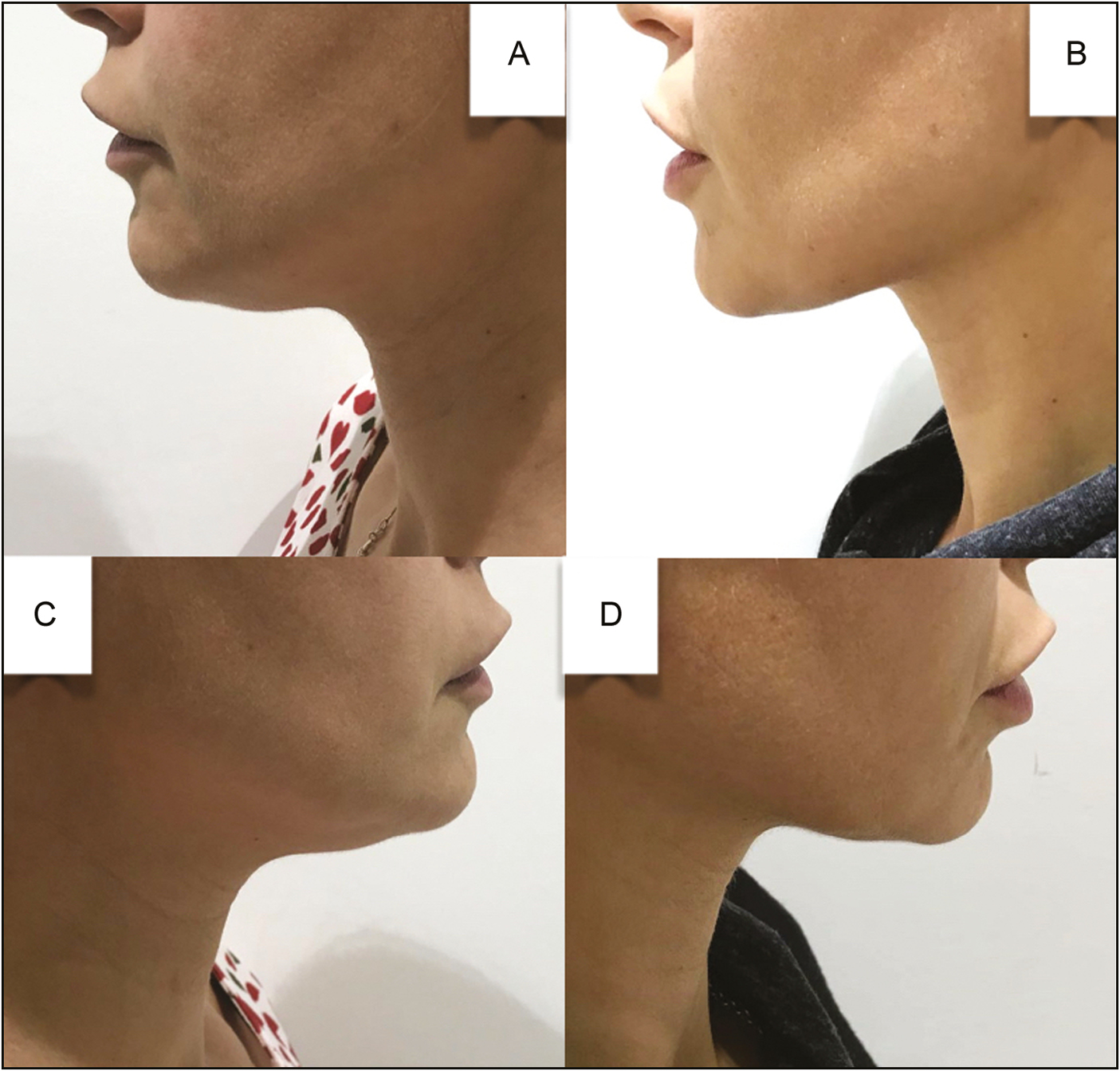
- (A, C) Pretreatment and (B, D) 3-month-posttreatment lateral views of the 41-year-old woman who presented with SMF and underwent injection of a total of 4mL (2 vials) of ATX-101 that had been premixed with lidocaine
Other investigators have noted several adverse outcomes of ATX-101 injection.[11] These include edema, site-injection ecchymosis, nausea, vomiting, headache, alopecia, and paresis. Patients in this case series experienced only edema and site-injection ecchymosis following treatment with ATX-101 plus lidocaine.
CONCLUSION
ATX-101 is a relatively new drug that contains DC and is indicated for noninvasive treatment of SMF. To address a common patient concern of pain associated with injection of ATX-101, we performed injections of a premixed solution of ATX-101 and lidocaine (0.5mL of lidocaine per 2-mL vial of ATX-101). In a series of 12 patients, we determined that pain was well controlled and aesthetic improvements in SMF were achieved with few side effects.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Validated composite assessment scales for the global face. Dermatol Surg. 2012;38:294-308.
- [Google Scholar]
- Characterization of the cervical retaining ligaments during subplatysmal facelift dissection and its implications. Aesthet Surg J. 2017;37:495-501.
- [Google Scholar]
- Reduction of unwanted submental fat with ATX-101 (deoxycholic acid), an adipocytolytic injectable treatment: results from a phase III, randomized, placebo-controlled study. Br J Dermatol. 2014;170:445-53.
- [Google Scholar]
- Efficacy, patient-reported outcomes and safety profile of ATX-101 (deoxycholic acid), an injectable drug for the reduction of unwanted submental fat: results from a phase III, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2014;28:1707-15.
- [Google Scholar]
- Belkyra [package insert]. Markham, Ontario: Allergan; 2016.
- Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatol Surg. 2004;30:1001-8.
- [Google Scholar]
- Injectable therapies for localized fat loss: state of the art. Clin Plast Surg. 2011;38:489-501, vii.
- [Google Scholar]
- Results from a pooled analysis of two European, randomized, placebo-controlled, phase 3 studies of ATX-101 for the pharmacologic reduction of excess submental fat. Aesthet Plast Surg. 2014;38:849-60.
- [Google Scholar]
- Early experience in 100 consecutive patients with injection adipocytolysis for neck contouring with ATX-101 (deoxycholic acid) Dermatol Surg. 2017;43:950-8.
- [Google Scholar]






