Translate this page into:
Efficacy and Safety Comparison of Basic Fibroblast Growth Factor-Related Decapeptide 0.1% Solution (bFGFrP) Plus Oral PUVA Combination Therapy with Oral PUVA Monotherapy in the Treatment of Vitiligo
Address for correspondence: Dr. Priti Sarma, Medical Advisor- Dermatology, Medical Affairs, Alkem Laboratories Ltd., Devashish, Alkem House, Senapati Bapat Marg, Lower Parel, Mumbai -400013, Maharashtra. E-mail: supriya.ambedkar@alkem.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Phototherapy in its different forms, is mainstay of vitiligo management. Combining treatment modalities like topical calcipotriol (for quicker, more intense repigmentation), Low dose azathioprine with PUVA have proven to be beneficial in management of vitiligo due to different mechanisms of repigmentation and their synergistic effects. Topical bFGF-related decapeptide (bFGFrP) application followed by sun exposure/ UVA phototherapy yields effective repigmentation. bFGFrP has shown to aid the targeted phototherapy in smaller lesions and its combinations with other treatment modalities have been very promising. However, there is paucity of studies on combination treatments; especially oral PUVA along with bFGFrP. This study was aimed at evaluating safety and efficacy of combination of bFGFrP with Oral PUVA in vitiligo (larger body surface area 20% or more).
Materials and Methods:
Phase IV, randomized, multicentre study (N = 120) in adult patients with stable vitiligo of 6 months treatment period with monthly follow up visits. Psoralen (Tab. Melanocyl) dosage 0.6 mg/kg orally 2 h before exposure to UVA phototherapy. Oral PUVA therapy, initially, at an irradiation dose 4 J/cm2 (PUVA group), followed by increments 0.5 J/cm2 every four sittings if tolerated for twice weekly. Primary end point was improvement in extent of repigmentation (EOR) in target lesion (at least 2 cm × 2 cm in greatest dimension, without leukotrichia), while secondary endpoints were improvement in patient global assessment (PGA) and safety at end of 6 months of treatment period in bFGFrP + oral PUVA combination group and Oral PUVA monotherapy group.
Results:
End of 6 months, significantly greater EOR >50%) was achieved in 61.8% (34 patients, n = 55) from combination group while 30.2% (16 patients, n = 53) from the oral PUVA monotherapy group (n = 53). Regarding Grade of repigmentation (GOR), complete repigmentation was observed 5.5% (3 patients, n = 55) in combination group whereas no patient showed complete repigmentation in monotherapy group (p ≤ 0.05), PGA showed significant overall improvement in combination group (p ≤ 0.05); 6 patients (10.9%) from combination group Vs one (1.9%) showed complete improvement. During treatment period, there were no reported adverse events.
Conclusions:
Addition of bFGFrP to oral PUVA therapy resulted in intense and faster induction of repigmentation than oral PUVA monotherapy with favorable safety profile.
Keywords
Basic fibroblast related decapeptide
PUVA
repigmentation
response rate
stable vitiligo
INTRODUCTION AND BACKGROUND
Vitiligo is a common acquired hypo-pigmentatory disorder (affects 1-2% of global population) with progressive patchy loss of pigmentation from the skin, overlying hair and underlying mucosal membranes as well (oral mucosa). Clinically, characterized by well-defined depigmented macules or patches thought to occur secondary to loss of melanocytes from involved areas or melanocytes dysfunction.[1] Highest recorded incidence is noted in Indian subcontinent; ranging between 0.5 to 4% with positive family history patients (6% to 40%)[2] In India, Gujarat and Rajasthan states have reported highest prevalence around 8.8%.[3] Onset of Vitiligo can be at any age and disease has grave psychological impact mainly due to social stigma attached to this condition. It is a disorder with multifaceted etio-pathogenesis with diverse pathogenic mechanisms of pigment loss.
The complex interplay of genetic and non-genetic factors and several mechanisms leading to progressive melanocytes destruction; like autoimmune processes, impaired oxidants-antioxidants balance, biochemical, cytotoxic as well as viral and neural processes is involved in pathogenesis of vitiligo. Yet it remains not completely understood posing a challenge about selection of treatment modalities; many of which are being cumbersome, tedious, expensive and are associated with various adverse effects. Thus, treatments fail after long periods of hopeful trying, thus adding to the psychological burden of the patient.[4] As there are multiple mechanisms, operating at the pathogenesis of vitiligo, to improve the repigmentation success, the combination of two or more treatment modalities with different mechanisms of action/targeting different stages of depigmentation have been proven more effective than the individual monotherapies.
Topical corticosteroids, or topical calcineurin inhibitors are the most valuable treatments for localized vitiligo[5] while for generalized vitiligo, phototherapy in its different forms, including Psoralen-UV-A (PUVA) and Narrowband UV-B (NBUVB), excimer laser therapy is mainstay of vitiligo management. Majority of patients retain PUVA-induced repigmentation for many years, especially when treated until stagnation of repigmentation occurred. Available medical therapies, high repigmentation percentages mostly on facial and neck lesions are achieved, they are less effective on trunk and limbs and poor on the acral parts of the extremities. Additionally, shortcomings like slow and variable repigmentation of available medical treatment modalities has prompted the use of Phototherapies for management of vitiligo.
PUVA (oral psoralens and UVA) is one of the preferred treatments for generalized vitiligo affecting more than 10–20% of the cutaneous surface; however, phototherapy demands frequent clinic visits, requires long treatment durations for several months to years. Also, sometimes results in disappointing outcomes especially the monotherapy. Thus, patient’s adherence and clinician’s confidence in challenging vitiligo management; are crucial for successful phototherapy[6]
Existing Oral PUVA partially addresses the need incorporation of distinct approaches for strategy to achieve successful stable repigmentation in stable generalized vitiligo. PUVA therapy is a modality with level of evidence B or C, is better tolerated among Indians; with better response when combined with other topical modalities like topical steroids, Oral MiniPulse steroid regimens. Newer topical modalities like bFGF (a melanocyte mitogen) related decapeptide has provided promising therapeutic benefits by repigmentation of vitiligo lesions. The bFGFrP has been shown to act synergistically to give better results in terms of better repigmentation and faster response rates when combined with other treatment modalities for vitiligo.[7]
As per the concurrent clinical use of bFGFrP with PUVA phototherapy, improved patient outcome and better repigmentation can be expected by virtue of distinct mechanisms of repigmentation in vitiligo. However, there is no published clinical data available to substantiate benefits of boosting action of bFGFrP in combination. Hence, the current phase IV study was conducted to evaluate and to compare the efficacy and safety of the combination of bFGFrP and PUVA therapy as well as to compare extent and grade of repigmentation; response rates of combination after 6 months of therapy in patients with clinically diagnosed stable vitiligo (no progression for 6 months and more).
MATERIALS AND METHODS
A phase IV, open label, randomized; prospective, comparative, multicenteric study (6 sites across India) was conducted in compliance with Good Clinical Practice guidelines after ethical clearance was obtained for all the sites. Informed consent was taken from the participating patients. The sites for the study included, major contributors to sample size like TNMC, Nair Ch. Hospital and Sir JJ group of Hospitals, Mumbai; School of Tropical Medicine, Kolkata, D.Y. Patil Medical College, Navi Mumbai as well as Oyster & Pearl Hospital, Pune and PSG IMSR, Coimbatore,
A total of 120 adult patients (age range 18–65 years) with generalized (affected body surface area 20%–40 %) and clinically diagnosed stable vitiligo (without any new lesions and no progression of existing lesions for ≥ 6 months) with no treatment taken for past 3 months were included in the study. After the routine investigations as per the individual investigators discretion, the patients randomized 1:1 ratio, 60 patients in combination and monotherapy group. The simple randomization was performed through software system and the statistician generated schedule using SAS® (SAS Institute Inc., USA).
A target lesion (vitiligo patch) selected was of 2 × 2 cm in greatest dimension with no leukotrichia. Treatment period was of 6 months for both the groups with monthly follow up visits. In combination group, patients topically applied bFGFrP solution 0.1% (Melbild marketed by Alkem Laboratories Ltd.) at bedtime on target lesions, which was followed by exposure to morning sunlight for 5-10 minutes between 11 am-3pm daily. The PUVA [psoralen (P) and Ultraviolet A (UVA) therapy) regimen consisted of Oral Psorlen (Melanocyl tablet) in dosage of 0.6 mg/kg, was given 2 h before exposure to UVA phototherapy. Oral PUVA was administered initially, an irradiation dose 4 J/cm2 (PUVA group), followed by increments 0.5 J/cm2 every four sitting if tolerated for twice weekly. The Oral PUVA therapy in monotherapy group consisted of the same oral PUVA dosage regimen.
The Primary end point was improvement in Extent of repigmentation (EOR) while secondary end points were grade of repigmentation (GOR) at the end of the treatment period. Safety assessment with incidences of adverse events and serious adverse events was also noted throughout the study.
Data is presented in mean ± SD for qualitative data and as number (% of total) for categorical data. Comparison of PGA scores (categories), EOR and GOR were analyzed by Friedman test (non-parametric test) and overall trend comparison was done across all visits. Chi square test was used to compare percentages of all efficacy variables between two groups at the end of 6 months. Fisher’s exact probability test was applied to compare percentages between two groups for two by two data. Level of Significance was taken as P≤ 0.05 [S = Significant, NS= Non-significant, DF=, degrees of freedom].
RESULTS
Out of 120 randomized patients, 55 (n = 60) from combination and 53 (n = 60) from monotherapy group completed the study and were included for final statistical analysis. The demographic variables were comparable and well distributed in both groups at baseline. The demographic data analysis findings are mentioned in [Table 1]
| Parameters | bFGFrP + Oral PUVA | Oral PUVA |
|---|---|---|
| Enrolled no. | 60 | 60 |
| Follow up loss | 5 | 7 |
| No of patients completed | 55 | 53 |
| Males | 29 (52.7%; n = 55) | 27 (52.9%, n = 51) |
| Females | 26 (47.3%; n = 55) | 24 (47.1%, n = 51) |
| Mean Age (years) | 39.47 ± 11.12 (n = 53) | 40.51 ± 11.97 (n = 51) |
At the end of 6 months (at Visit 8), significantly better improvement was noted for the primary endpoint EOR in combination than monotherapy group, from 3 months of treatment period onwards. More than 50% EOR was achieved in 61.8 % (34 patients, n = 55) from combination while in 30.2% (16 patients, n = 53) from oral PUVA monotherapy group. [Graphs 1 and 2].
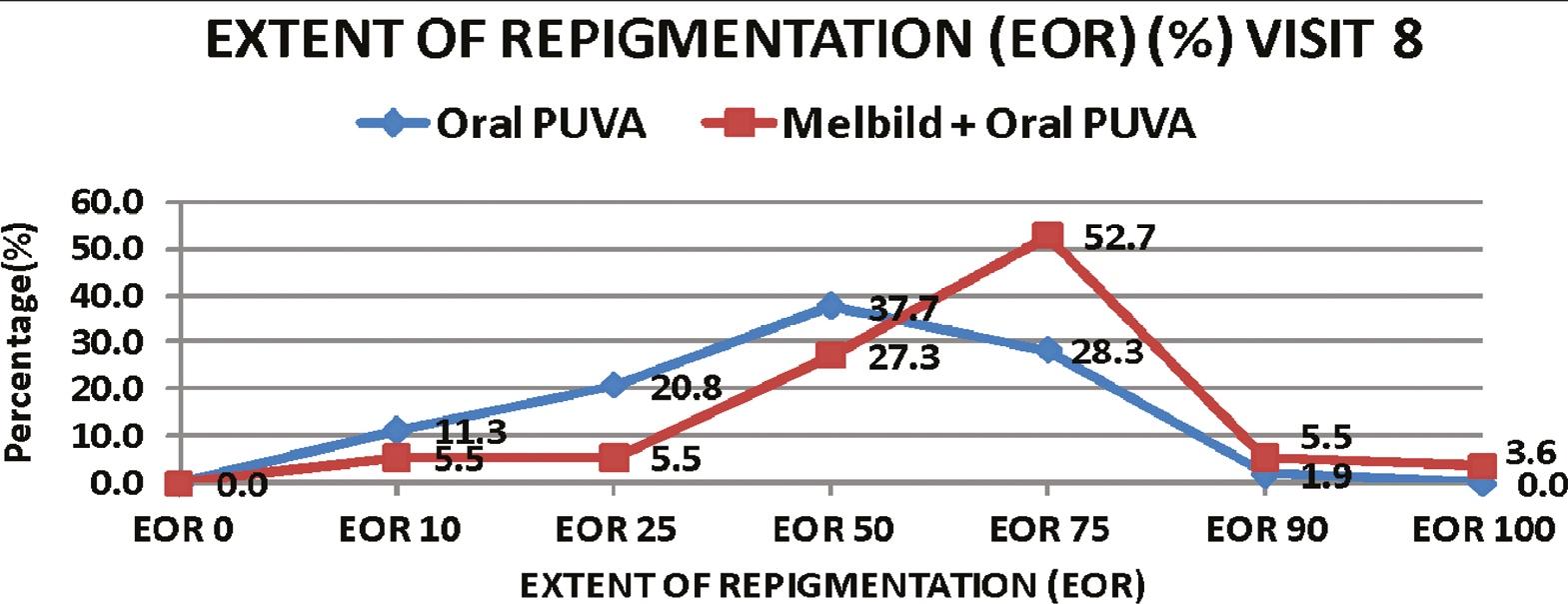
- Comparison of extent of repigmentation (%) (EOR) at end of 6 months between 2 groups
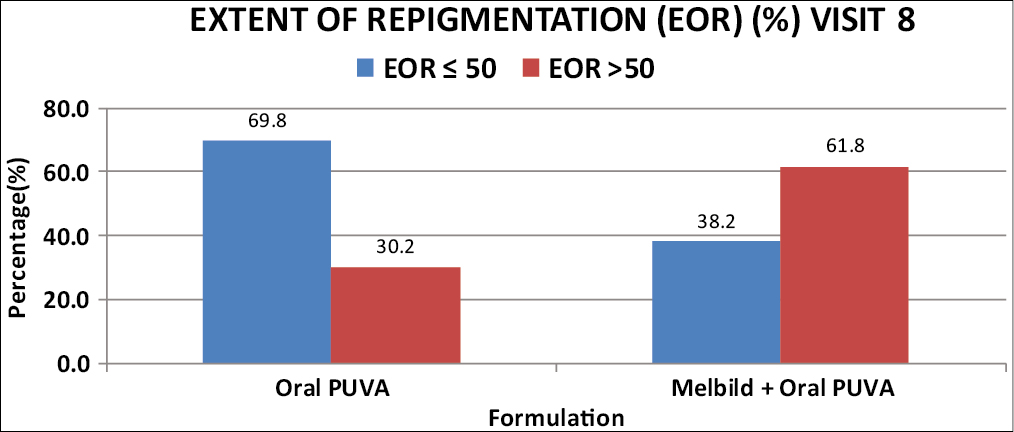
- Comparison of extent of repigmentation (%) (EOR) at visit 8 between 2 groups
Significant improvement was also noted for secondary endpoints like GOR and overall patient outcome in terms of improved PGA. Complete repigmentation at the end of 6 months, was achieved in 3 patients (5.5 %, n = 55) from combination group as opposed to none from monotherapy group. [Graph 3] As per patients global assessment complete repigmentation was noted in 6 patients (10.9%, n = 55) from combination group while only 1 patient (1.9%, n = 53) reported complete repigmentation.
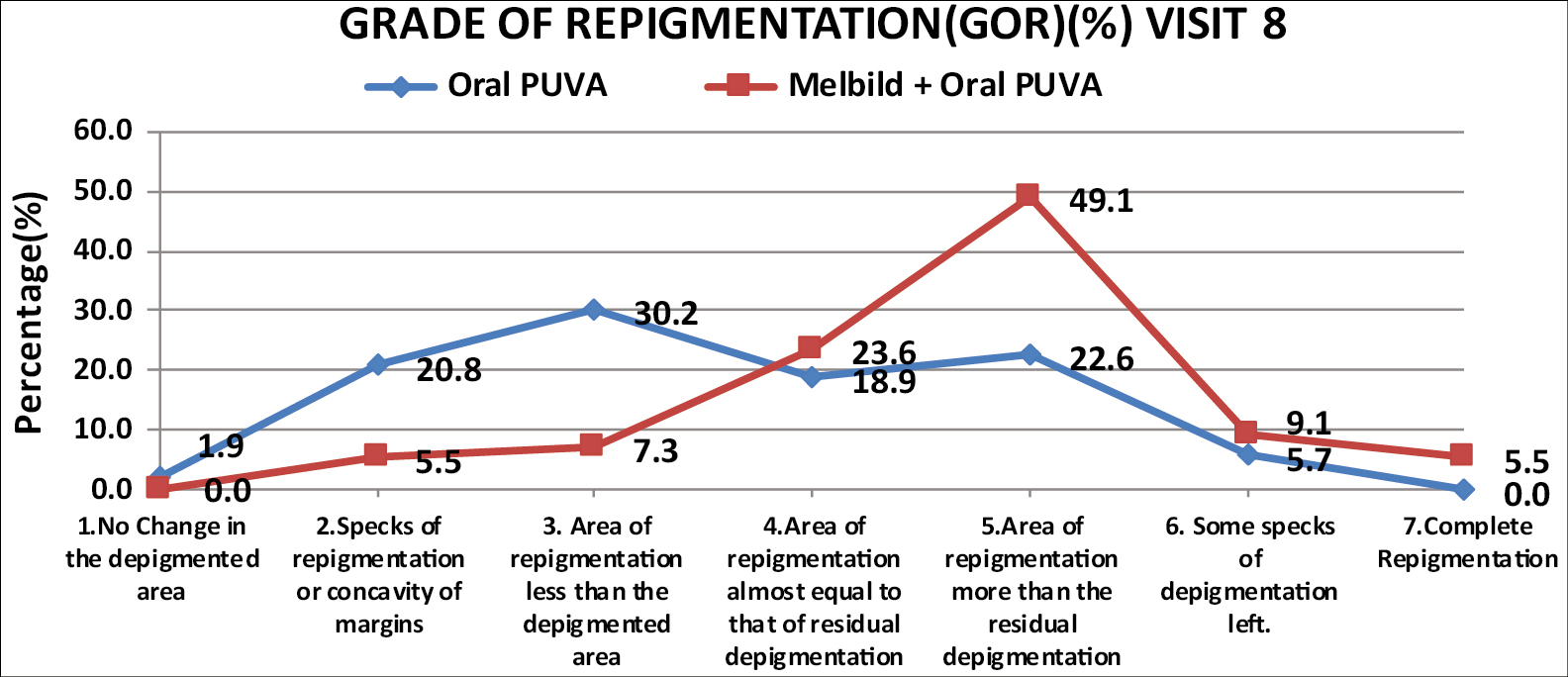
- Comparison of grade of repigmentation (%) between two groups
Significantly, higher mean response rates were seen in combination group from 3 months onwards. At the end of study (6 months) higher mean response rate was observed for the combination therapy group, 63.64 ± 21.25 (n = 55) compared to 48.11 ± 22.90 (n = 53) in PUVA monotherapy group. (p<0.001) [Graph 4]. No significant serious adverse events were noted in both the groups throughout the study period.
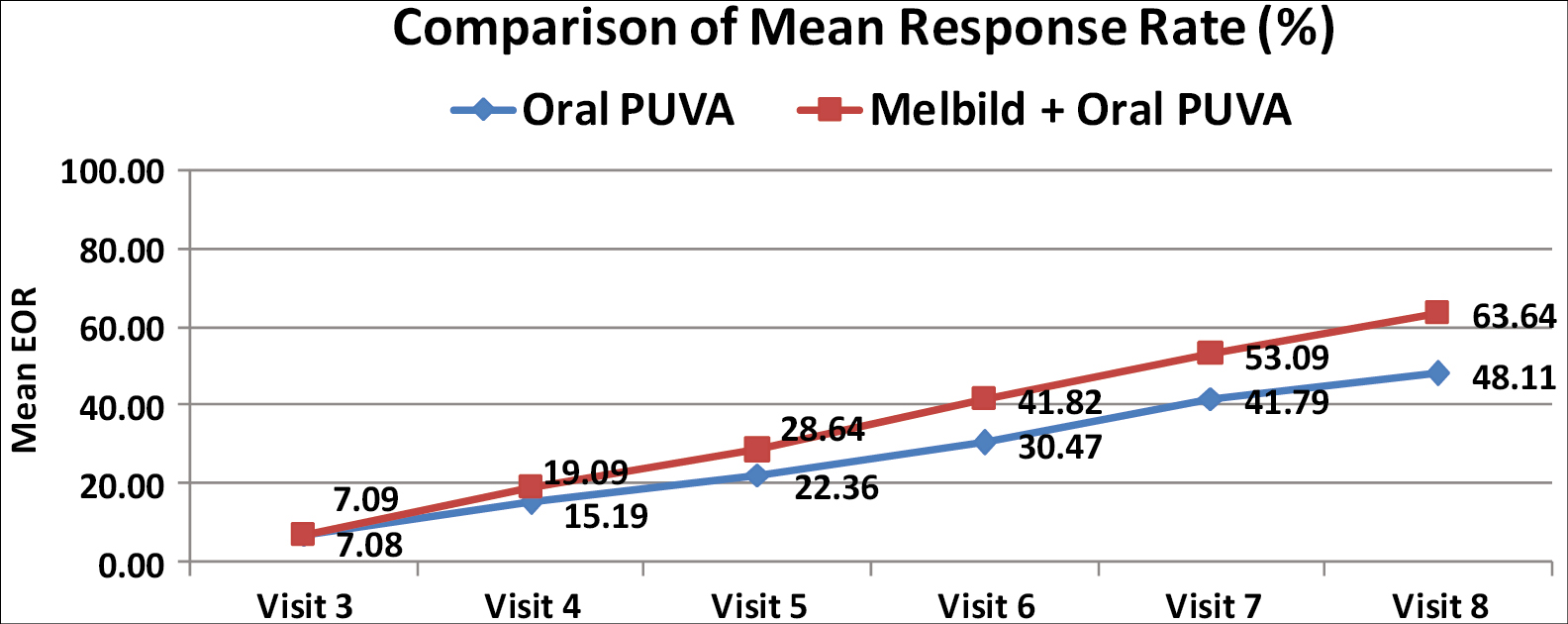
- Comparison of mean response rates in % between the groups
DISCUSSION
Achieving disease stability by halting underlying destructive processes is the treatment goal for vitiligo (multifactorial disease with complex etio-pathogenesis), however, once the stability of the lesions is obtained; complete, faster as well as stable repigmentation is the management goal. For successful, faster and stable repigmentation, combining various treatment modalities supersedes the results obtained with individual medical and phototherapies.
PUVA phototherapy has shown to have promising treatment response rate 23.5% with more than 50% repigmentation at the end of 6 months and has “A” level of evidence (highest level of evidence, systematic review and meta-analysis). PUVA therapy is often preferred under special conditions, like unstable/ spreading vitiligo due to deeper penetration of UV-A.[8] Majority of patients retain PUVA-induced repigmentation for many years, especially when treated until stagnation of repigmentation occurred.[9] The mechanism by which oral psoralen with UV-A (PUVA) stimulates melanocyte proliferation in vitiligo via release of growth factors into the circulation which can stimulate a proliferation of melanocytes and of other cells. This could account for the repigmentation of vitiligo by PUVA treatment.[10]
However, PUVA therapy has several concerns, like photosensitization, higher cumulative dose that significantly limits its higher doses. Narrow band UV-B (NBUVB) which is preferred practically as a gold standard phototherapy isn’t completely devoid of side effects. Combined treatments with phototherapies have been found to be superior to monotherapies regarding efficacy, early response and safety, especially in difficult to treat areas and refractory cases. The phototherapies have been used in combinations with lasers, although effective; intolerance to side effects limit their wide use of these combinations. The widely preferred topical corticosteroids in combination with other treatment modalities also have long term safety concerns in the form of skin atrophic changes as well as with the risks associated with the systemic side effects etc.
Studies like Nada & Rashed et al.[11] have documented the role of bFGF in vitiligo; as a mitogen stimulating the growth as well as migration of melanocytes. Rationale for bFGFrP (derived from bFGF) in treatment of vitiligo is based on the melanocyte growth factor deprivation theory and the clinical evidence generated in the Indian clinical trials[412] support bFGFrP as a treatment modality for repigmentation in the management of vitiligo.
Mechanisms of repigmentation induced by photo-biomodulation therapy in vitiligo revealed He-Ne laser irradiation significantly increase in basic fibroblast growth factor (bFGF) release from both keratinocytes and fibroblasts; a putative melanocyte growth factor, which stimulates melanocyte migration. Melanocyte migration is an important event in re-pigmentation of vitiligo. It was demonstrated that narrow-band ultraviolet B (UVB) irradiation stimulated cultured keratinocytes to release a significant amount of basic fibroblast growth factor (bFGF). Furthermore, narrow-band UVB enhanced migration of melanocytes via increased expression of phosphorylated focal adhesion kinase (p125FAK) on melanocytes. Thus, the effect of recombinant human bFGF (rHbFGF) on melanocyte migration was studied.[13]
A 16 weeks study comparing topical active bFGF 0.1% solution with topical corticosteroid, Betamethasone valerate 0.1% ointment among 62 patients, showed that bFGF active fragment is more efficacious than the topical betamethasone with considerable safety and can be an efficacious alternative to topical corticosteroids.[14] There is accumulation of evidence from various clinical studies about addition of different growth factors such as bFGF leading to better and faster results of re-pigmentation. A double blind randomized study by Ramaiah A. et al, evaluated the efficacy of bFGF related decapeptide in combination with NBUVB among 62 patients for 12 weeks of treatment. The study demonstrated that combination therapy leads to faster repigmentation of vitiligo macules due to synergistic action.[4] The synergistic action must be due to the boosting effect of the bFGF peptide on the melanogenesis stimulated by Psoralens.
The present study suggested that patients with vitiligo showed better repigmentation outcomes as well as better response rates than patients who were given oral PUVA as monotherapy. The combination leads to more greater repigmentation as compared to Oral PUVA monotherapy by taking care at different mechanisms. [Figure 1]
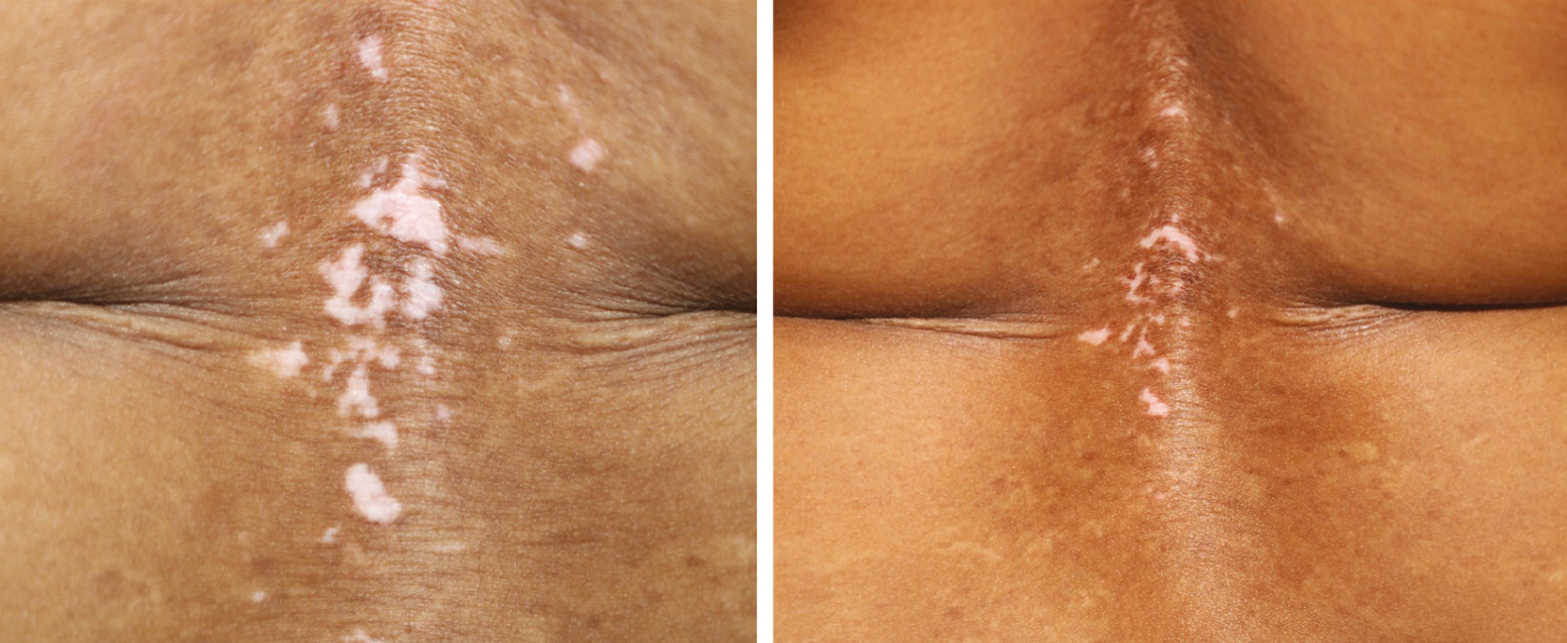
- Repigmentation of Vitiligo patch treated with bFGFrP and oral PUVA after 6 months of treatment
Limitations
Although the study was randomized, it was an open label. A double-blinded study with large sample size would have been of value addition for evidence generation.
Although PUVA phototherapy is commonly practiced, in certain tertiary care centers, NBUVB Is preferred over PUVA due to lesser side effects. A more rigorous larger sample size would be more enlightening to the day-to-day preferences and certainly would support the booster effect role of bFGFrP, to facilitate the faster repigmentation with higher response rates. An analysis including the active dynamic lesions patients should have been done since PUVA therapy is also widely prescribed for the spreading generalized unstable vitiligo. Patient follow up for 1 year with Relapse rates analysis would have been beneficial for clinical correlation.
CONCLUSION
The study shows that after 6 months of treatment, the combination of Oral PUVA therapy with bFGF related peptide leads to significantly better and rapid repigmentation as well as higher response rates in stable vitiligo patients with considerable safety. The combination group shown to have significantly better repigmentation, observed as early as 3 months as well as complete repigmentation as compared to the oral PUVA monotherapies group. The combination therapy with bFGFrP achieved better and faster successful patient outcomes than monotherapy highlight the booster effect role of bFGFrP, with higher response rates.
Financial support and sponsorship
The study was sponsored by Alkem Labs Ltd.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Autoimmunity - pathogenesis, clinical aspects and therapy of specific autoimmune diseases. Croatia: IntechOpen; 2015.
- [Google Scholar]
- Clinico-epidemiological profile of patients with vitiligo: A retrospective study from a tertiary care center of North India. Indian Dermatol Online J. 2019;10:38-44.
- [Google Scholar]
- Acetylcholine esterase levels in different clinical types of vitiligo in Baroda, Gujarat. Indian J Dermatol. 2006;51:289-91.
- [Google Scholar]
- Double blind randomized phase IV clinical trial of basic fibroblast growth factor related deca peptide in vitiligo. Pigmentary Disorders. 2015;S3:S3-004.
- [Google Scholar]
- Update on skin repigmentation therapies in vitiligo. Pigment Cell Melanoma Res. 2009;22:42-65.
- [Google Scholar]
- Photochemotherapy (PUVA) in psoriasis and vitiligo. Indian J Dermatol Venereol Leprol. 2014;80:497-504.
- [Google Scholar]
- Efficacy and safety of basic fibroblast growth factor (bFGF) related decapeptide solution plus Tacrolimus 0.1% ointment versus Tacrolimus 0.1% ointment in the treatment of stable vitiligo. Dermatol Ther. 2019;32:e13109.
- [Google Scholar]
- Phototherapy for vitiligo: A systematic review and meta-analysis. JAMA Dermatol. 2017;153:666-74.
- [Google Scholar]
- Vitiligo: A comprehensive overview Part II: Treatment options and approach to treatment. J Am Acad Dermatol. 2011;65:493-514.
- [Google Scholar]
- Oral psoralen with UV-A therapy releases circulating growth factor(s) that stimulates cell proliferation. Arch Dermatol. 1997;133:1530-3.
- [Google Scholar]
- Basic fibroblast growth factor & vitiligo. Journal of the Egyptian Women’s Dermatologic Society. 2012;9:22-5.
- [Google Scholar]
- A double blind randomized clinical trial on basic fibroblast growth factor related deca-peptide to reduce wrinkles on skin and to treat non sun exposed vitiligo macules. Cosmetol & Oro Facial Surg. 2017;3:112.
- [Google Scholar]
- Basic fibroblast growth factor promotes melanocyte migration via increased expression of p125FAK on melanocytes. Acta Derm Venereol. 2006;86:498-502.
- [Google Scholar]
- Comparative study of efficacy and safety of Topical active fragment of basic fibroblast growth factor (bFGF) 0.15 solution Vs Betamethasone Valerate 0.1% Ointment in the treatment of vitiligo patients. Journal of Dental and Medical Sciences (IOSR-JDMS). 2015;14:41-7.
- [Google Scholar]






