Translate this page into:
Efficacy of 15% trichloroacetic acid peel versus 35% glycolic acid peel in acanthosis Nigricans: A randomized open-label study
Address for correspondence: Dr. Rashmi Sarkar, Department of Dermatology, Lady Hardinge Medical College, New Delhi, India. E-mail: rashmisarkar@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction:
Acanthosis Nigricans (AN) is an acquired disorder of keratinization. It presents as hyperpigmentation, velvety texture of skin that can involve any part of the body including the face. Different topical, systemic therapies, or physical therapies including laser have been explored. However, there are not many randomized controlled studies for the majority of therapy alternatives besides lifestyle modifications and weight reduction.
Objectives:
The aim of this study was to compare the effectiveness of 15% trichloroacetic acid (TCA) and 35% glycolic acid (GA) peel for AN.
Materials and Methods:
Forty participants were included and randomized into two groups. In groups A and B, peeling with 15% TCA and 35% GA was done, respectively. The effectiveness of each peel was assessed using changes in the Acanthosis Nigricans Area and Severity Index Score (ANASI) and Physician Assessment Score. Statistical analysis included Wilcoxon–Mann–Whitney test, Friedman test, and generalized estimating equations.
Results:
The overall change in ANASI over time was compared in the two groups using the generalized estimating equations method. A significant difference was observed in the trend of ANASI over time between the two groups (P < 0.001). TCA peel group showed more change in ANASI as compared with GA peel group.
Conclusion:
In our research, 15% TCA has a better efficacy when compared with 35% GA peel after three sessions of chemical peeling. We therefore recommend the use of 15% TCA peel in AN as a safe and effective treatment option. However, more comprehensive randomized control studies are required for supporting data.
Keywords
Acanthosis Nigricans
glycolic acid peel
trichloroacetic acid peel
INTRODUCTION
Acanthosis Nigricans (AN) is an acquired disorder of keratinization. It presents as hyperpigmentation, a velvety texture of skin that can involve any part of the body including the face. AN can be benign or associated with underlying systemic problems such as insulin resistance (IR), metabolic abnormalities, or malignancies.[1] The treatment of AN has been and remains a therapeutic challenge for the physician.
Various treatment modalities including topical agents, oral agents, and interventional modalities have been tried in the treatment of AN. Only a small number of the studies are randomized controlled trials; the majority of the studies are case-control or pilot studies. Topical retinoids are regarded as the first-line treatment for AN.[23] Topical vitamin D analogs, depigmenting agents, and keratolytics have also been tried in AN.[45] Oral insulin sensitizers have been tested as a treatment for AN because, as we know, IR plays a significant part in the etiology of the disease.[6] In the generalized form of AN, oral retinoids are also used. Very superficial and superficial chemical peels such as glycolic acid (GA), trichloroacetic acid (TCA), mandelic acid, and lactic acid have been tried in AN.[7] To the best of our knowledge, there have not yet been any randomized control trials that compare the effectiveness of different peels in AN. Long-pulsed alexandrite lasers, fractional erbium fiber lasers, and carbon dioxide lasers have also been tried.[89]
MATERIALS AND METHODS
This was an open-label randomized controlled trial conducted at the Department of Dermatology and STD of our hospital from January 2021 to June 2022. The Institutional Ethics Committee granted ethical approval for the study. All patients provided informed consent in writing. The clinical trial is registered with the clinical trial registry, India (Ctri/2021/02/030952).
Patient selection
All adults 18 years and older with a clinical diagnosis of AN affecting the face, neck, or elbow, or any combination of these three, who presented to the dermatology outpatient department of our hospital were enrolled in the study. Patients with any of the following conditions were excluded from the study:
Pregnant and lactating females.
Hypersensitivity to the peeling agent.
Skin infection at the site of AN (herpes, pyoderma, and folliculitis).
Individuals prone to keloid formation.
History of any topical application in the last 4 weeks.
Twenty adults in two groups who met the inclusion and exclusion criteria were randomized in the study following the distribution of a patient information sheet and the receipt of written consent. All of the cases were thoroughly reviewed, and their demographic information, medical history, and clinical examinations were recorded.
At baseline, weight, height, waist circumference (WC), and blood pressure were measured. The body mass index (BMI) was determined (weight in kilos divided by height in meters squared). Dermatoscopy was also performed at baseline. Each patient was tested for fasting blood sugar, serum insulin, and lipid profile.
A 20-min post-auricular test peel was performed to evaluate any hypersensitivity to the peeling agent. Patients were instructed to apply tretinoin 0.05% cream at night for two weeks before peeling sessions as part of a pre-peel priming program.
After degreasing the area with acetone, the peel was applied with a cotton tip applicator. We used 15% TCA in one group and 35% GA peel (pH 2) in the other group. The endpoint of TCA peel was frosting and of GA peel was the appearance of erythema or burning sensation. The neutralization was accomplished using cold water. Three peeling sessions were performed at 2-week intervals. The patients were followed up for another 2 weeks to check for any improvement or worsening of results. Throughout this duration, patients were instructed to apply broad-spectrum sunscreen. Counseling on lifestyle modifications was also done.
Efficacy assessment
Assessment of improvement of AN was done as follows:
Acanthosis Nigricans Area and Severity Index Score (ANASI) score at baseline and at each peeling session.[10]
Photograph of the lesion at baseline and then at each session. The photographs were compared and evaluated independently by an observer after three sessions using a 5-point scale, that is, Physician Assessment Score (PAS): 0 = no improvement, 1 = 1%–25% improvement, 2 = 26%–50% improvement, 3= 51%–75% improvement, and 4 = 76%–100% improvement
Patients were asked to record their satisfaction score, that is, Patient Satisfaction Score (PSS): worsening (–1), no improvement (0), mild improvement (1), moderate improvement (2), and excellent improvement (3).
Safety assessment
After each session, the patients were interviewed and examined for any adverse effects like erythema, peeling of skin, post-inflammatory hyperpigmentation (PIH), or any burning sensation related to peeling.
Statistical analysis
Non-Parametric tests were used to make statistical inferences as data were not normally distributed. Wilcoxon–Mann–Whitney test was used to compare the two groups in terms of ANASI at each of the timepoints. Friedman test was used to explore the change in ANASI over time within each group. Generalized estimating equations method was used to explore the difference in change in ANASI between the two groups over time.
RESULTS
A total of 40 patients were enrolled in the study. The mean age of patients in the two groups was 30.73 ± 10.39 years. Out of 40 patients, 19 (47.5%) were males and 21 (52.5%) participants were females. Thirty-two (80%) of the patients were obese, the rest 7 (17.5%) were overweight and one had a normal BMI. Among all the participants, 18 (45%) participants had a history of weight gain. It was seen that participants with no weight gain had a larger proportion of static disease, while participants in the group with a history of weight gain had a larger proportion of progressive disease (χ2 = 14.207, P < 0.001). The rest other demographic data and baseline clinical information of participants are presented in Table 1.
| Parameters | Type of peel | P Value | |
|---|---|---|---|
| GA peel (n = 20) | TCA peel (n = 20) | ||
| Age (years) | 29.05 ± 9.12 | 32.40 ± 11.52 | 0.4481 |
| Gender | 0.7523 | ||
| Male | 9 (45.0%) | 10 (50.0%) | |
| Female | 11 (55.0%) | 10 (50.0%) | |
| Sites: neck (yes) | 20 (100.0%) | 20 (100.0%) | 1.0003 |
| Sites: face (yes) | 3 (15.0%) | 3 (15.0%) | 1.0002 |
| Sites: elbow (yes) | 5 (25.0%) | 5 (25.0%) | 1.0003 |
| Sites: others (axilla/groin) (yes) | 8 (40.0%) | 14 (70.0%) | 0.0573 |
| Age of onset | 26.65 ± 9.05 | 28.85 ± 11.09 | 0.4964 |
| Duration of the disease (years) | 2.64 ± 2.06 | 3.64 ± 4.50 | 0.8491 |
| Family history of DM/HTN: no history (yes) | 7 (35.0%) | 11 (55.0%) | 0.2043 |
| Family history of DM/HTN: DM (yes) | 12 (60.0%) | 7 (35.0%) | 0.1133 |
| Family history of DM/HTN: HTN (yes) | 3 (15.0%) | 4 (20.0%) | 1.0002 |
| Family history of DM/HTN: others (yes) | 1 (5.0%) | 1 (5.0%) | 1.0002 |
| BMI | 0.2352 | ||
| Underweight | 0 (0.0%) | 0 (0.0%) | |
| Normal | 1 (5.0%) | 0 (0.0%) | |
| Overweight | 5 (25.0%) | 2 (10.0%) | |
| Obese | 14 (70.0%) | 18 (90.0%) | |
| BP | 1.0002 | ||
| Normotensive | 16 (80.0%) | 16 (80.0%) | |
| Hypertensive | 4 (20.0%) | 4 (20.0%) | |
| Waist circumference | 0.4653 | ||
| Normal range | 16 (80.0%) | 14 (70.0%) | |
| More than upper normal limit | 4 (20.0%) | 6 (30.0%) | |
| Site | 1.0002 | ||
| Neck | 16 (80.0%) | 16 (80.0%) | |
| Face | 2 (10.0%) | 2 (10.0%) | |
| Elbow | 2 (10.0%) | 2 (10.0%) | |
Out of the total participants, hypercholesterolemia, hypertriglyceridemia, increase low-density lipid, and decreased high-density lipid were seen in 9 (22.5%), 8 (20%), 8 (20%), and 3 (7.5%) participants, respectively. Hyperinsulinemia and deranged fasting blood sugar were seen in 11 (27.5) and 8 (20%) participants, respectively.
The most common dermatoscopy findings were crista and sulci, though in some cases dots and globules were also seen [Figure 1].
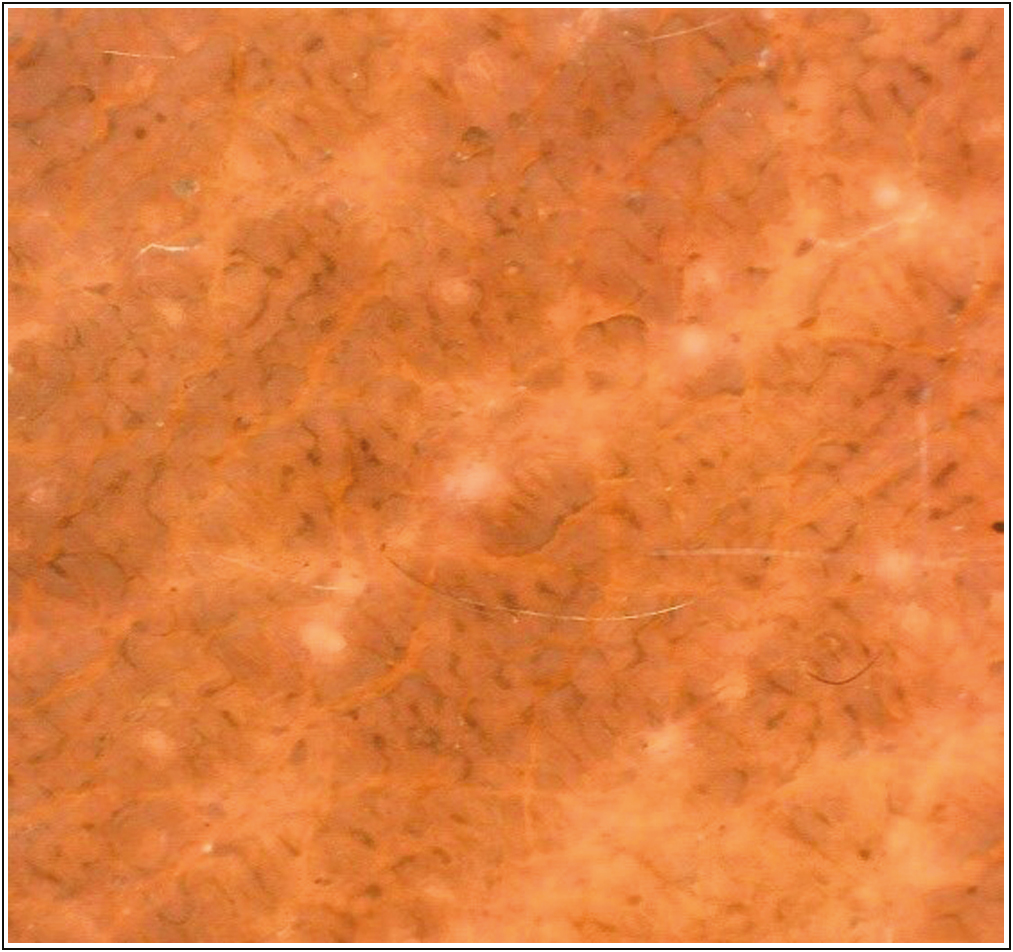
- Dermatoscopy of acanthosis Nigricans showing crista and sulci (Dinolite, polarized, magnification 20×)
Data analysis from 40 participants revealed a statistically significant difference between 15% TCA and 35% GA peel in the treatment of AN [Figures 2A,B, 3A and B]. The two groups did not differ in terms of ANASI at any of the timepoints as shown in Table 2. In the GA peel group (group 1), the mean ANASI decreased from a maximum of 17.07 at the baseline to a minimum of 12.49 at the 8 weeks. This change was statistically significant (P < 0.001). In the TCA peel group (group 2), the mean ANASI decreased from a maximum of 22.33 at the baseline to a minimum of 13.26 at the 8 weeks. This change was statistically significant (P < 0.001). The overall change in ANASI over time was compared in the two groups using the Generalized Estimating Equations method. There was a significant difference in the trend of ANASI over time between the two groups (P < 0.001). TCA peel group (group 2) showed more change in ANASI as compared with GA peel group (group 1). Table 3 summarizes the mean change in ANASI from the baseline to the various follow-up timepoints. It also summarizes the statistical comparison of the two groups in terms of this difference.
| ANASI | Type of peel | P Value for comparison of the two groups at each of the timepoints (Wilcoxon–Mann–Whitney test) | |
|---|---|---|---|
| GA peel | TCA peel | ||
| Mean (SD) | Mean (SD) | ||
| Baseline | 17.07 (9.77) | 22.33 (10.80) | 0.106 |
| 2 weeks | 16.82 (9.48) | 20.18 (9.45) | 0.265 |
| 4 weeks | 16.42 (9.62) | 17.46 (8.97) | 0.673 |
| 6 weeks | 13.49 (8.56) | 14.81 (8.77) | 0.596 |
| 8 weeks | 12.49 (7.84) | 13.26 (8.18) | 0.828 |
| P Value for change in ANASI over time within each group (Friedman test) | <0.001 | <0.001 | |
| Overall P value for comparison of change in ANASI over time between the two groups (generalized estimating equations) | <0.001 | ||
| Timepoint comparison | Change in ANASI from baseline to follow-up timepoints | P Value | |||
|---|---|---|---|---|---|
| Type of peel: GA peel | Type of peel: TCA peel | ||||
| Mean (SD) of absolute change | P Value | Mean (SD) of absolute change | P Value | ||
| 2 weeks baseline | –0.25 (1.12) | 1.000 | –2.15 (4.67) | 0.931 | 0.045 |
| 4 weeks baseline | –0.64 (1.56) | 0.877 | –4.87 (5.06) | 0.009 | <0.001 |
| 6 weeks baseline | –3.58 (2.10) | <0.001 | –7.52 (5.83) | <0.001 | 0.004 |
| 8 weeks baseline | –4.58 (2.81) | <0.001 | –9.07 (5.69) | <0.001 | 0.002 |
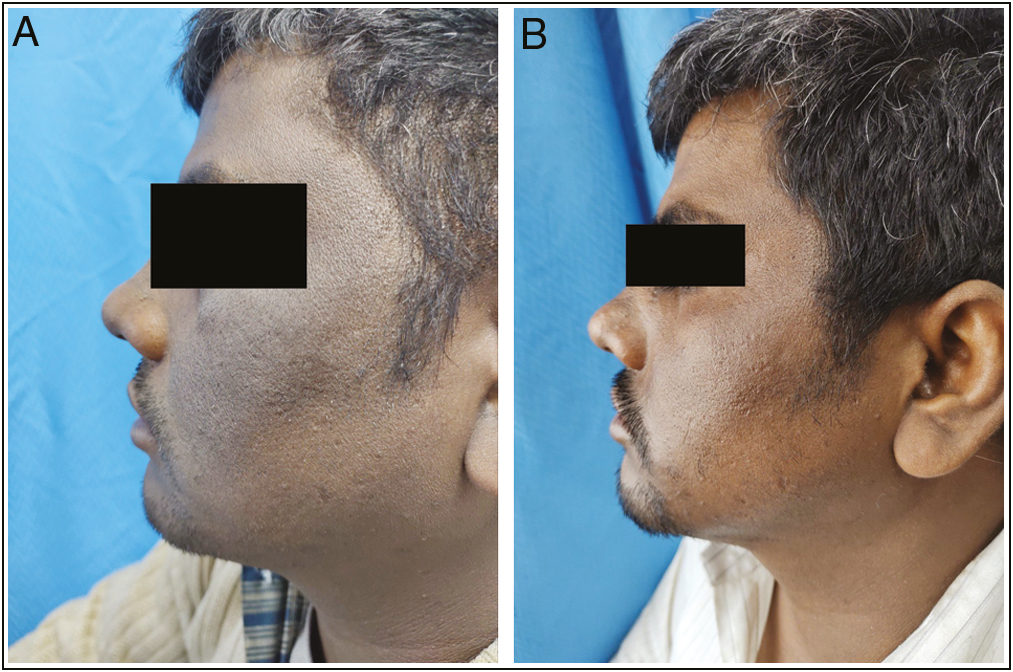
- (A) Global photographs at baseline of patient in TCA peel group. (B) Global photographs at eighth week of patient in TCA peel group.
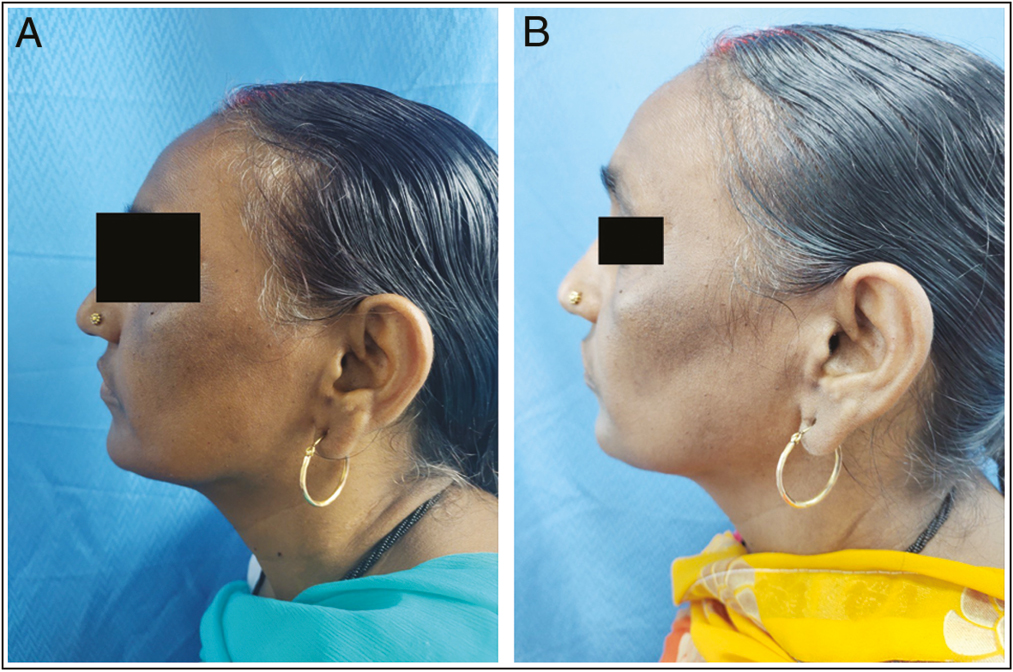
- (A) Global photographs at baseline of patient in GA peel group. (B) Global photographs at 8th week of patient in GA peel group
A significant difference was observed between the two groups in terms of distribution of PAS (χ2 = 15.200, P < 0.001) at 8 weeks. Participants in GA peel group (group 1) had a larger proportion of PAS 1. Participants in TCA peel group (group 2) had a larger proportion of PAS 2. In GA peel and TCA peel groups, 5% and 25% of participants had PAS greater than 2, respectively.
There was no significant difference in ANASI and PAS measured in different sites peeled in patients.
At 8 weeks, there was a significant difference between the two groups in terms of the distribution of PSS (χ2 = 8.300, P = 0.039). In GA peel group (group 1), 35.0% of the participants reported PSS 0 (no improvement), 30.0% reported PSS 1 (mild improvement), 20.0% reported PSS 2 (moderate improvement), 15.0% reported PSS 3 (excellent improvement). In the TCA peel group (group 2), 5% of participants reported PSS 0, 20 % had PSS 1 (mild improvement), 30.0% reported PSS 2 (moderate improvement), and 45.0% reported PSS 3 (excellent improvement). None of the patients in any group reported worsening at 8 weeks
The most common side effect in the GA peel group (group 1) was a burning sensation seen in 10% of the participants at 4 and 6 weeks. In the TCA peel group (group 2), the most common side effect was peeling of skin seen in 70% of the participants at 4 weeks and 6 weeks and in 5% at 8 weeks, followed by a burning sensation reported in 45% at 4 weeks and in 35% at 6 weeks. The other side effects seen were erythema, and PIH [Figure 4A and B].
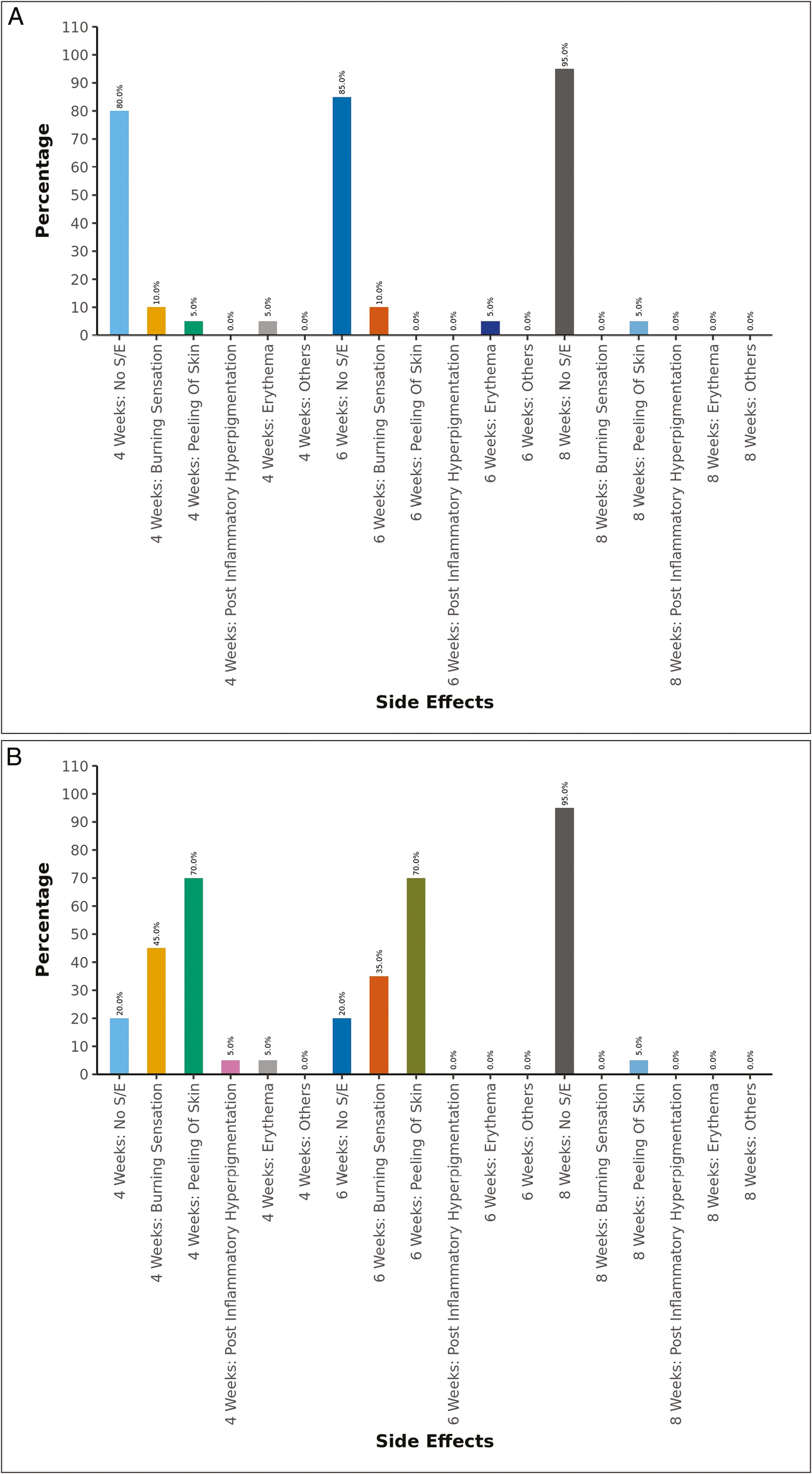
- (A) Side effects in GA peel group at different point of time. (B) Side effects in TCA peel group at different point of time
According to sites, peeling of skin was more frequently seen in patients whose face was peeled, in our study out of four patients with facial AN, three (75%) had reported peeling of skin at some point of time.
DISCUSSION
Treatment for AN has historically been challenging. The numerous therapeutic approaches, including topical, systemic, and interventional modalities, have all been tested. However, the level of evidence is not enough. Consequently, more randomized control studies are necessary. To the best of our knowledge, there are no studies that compare the effectiveness of two chemical peels in the treatment of AN.
In a study conducted by Zaki and Hilal,[10] which was a controlled clinical trial, in which 20 patients with AN over neck were included. It was a split neck comparison study, hence each side of the neck was randomized to either receiving a chemical peel with GA 70% or saline (as a control). Three biweekly sessions were done. There was no statistical difference between the two sides in baseline ANASI. On the control side, there was no statistical significance between pretreatment and posttreatment ANASI values, whereas the post-treatment ANASI values on the treated side were significantly lower than the pre-treatment values. Hence, it was concluded that GA peel is a good treatment option for the management of AN, though in our study TCA peel showed better results in comparison to GA peel in terms of change in ANASI.
In a randomized control study conducted by Rajegowda et al.[2] comparing 15% TCA peel and topical tretinoin, the percentage of patients applying tretinoin had more improvement compared to the 15% TCA peel group. Therefore, it was concluded that topical tretinoin 0.025% is the first-line agent, as it was found to be more efficacious than TCA peel. However, TCA peel being a superficial chemical exfoliating agent with better patient compliance can be considered as a second-line therapy in the management of AN. In another pilot study conducted by Zayed et al., six Egyptian women of AN with 10 lesions were enrolled. Three sessions of 15% TCA peel were done fortnightly, all patients showed improvement in hyperpigmentation, thickness, and overall appearance. The physician assessment was excellent in three lesions, moderate in five, and mild in two. The assessment was based on clinical examination and photographic evaluation.[11] These results support the ones we obtained in the TCA peel group.
Various side effects like burning sensation, peeling of skin, excessive erythema, and PIH of chemical peels have been reported.[12] The side effects profile may vary in different skin types. In our study, it was found that TCA peel has more side effects as compared to GA peel. However, the side effects were of mild severity which neither affected the compliance nor caused anyone to drop out of the study. The burning sensation was short-lived, localized to the peel site, and resolved within minutes of washing with water. The peeling of skin seen in TCA group was marked in the first few days after the peeling session; however, it also resolved on its own. No other studies are available comparing the treatment options as ours. In a pilot study by Zayed et al.,[11] 15% of TCA peel sessions were done fortnightly and the only reported side effect was a burning sensation which was also just after the peeling session and resolved completely after washing with water just like our study.
It was seen that 15% TCA has a better efficacy when compared to 35% GA peel after three sessions of chemical peeling. Due to the modest intensity of the adverse effects, neither compliance nor study dropout was impacted. In order to treat AN safely and effectively, we advise using a 15% TCA peel. However, our study had some limitations, including a small sample size due to time constraints and the lack of long-term patient follow-up. To support our findings, additional randomized trials with larger sample sizes and longer follow-up times are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- To compare the efficacy and safety of trichloroacetic acid peel with topical tretinoin in the treatment of acanthosis Nigricans: A randomized controlled study. J Pak Assoc Dermatol. 2019;29:170-5.
- [Google Scholar]
- Topical tretinoin in acanthosis Nigricans. Indian J Dermatol Venereol Leprol. 1996;62:159-61.
- [Google Scholar]
- The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis Nigricans. J Dermatol Treat. 2021;32:837-42.
- [Google Scholar]
- Current treatment options for acanthosis Nigricans. Clin Cosmet Investig Dermatol. 2018;11:407-13.
- [Google Scholar]
- Comparison of metformin versus rosiglitazone in patients with acanthosis Nigricans: A pilot study. J Drugs Dermatol. 2006;5:884-9.
- [Google Scholar]
- Treatment of acanthosis Nigricans with sequential salicylic acid‐mandelic acid combination peel and maintenance with glycolic acid‐urea combination cream: A retrospective pilot study. J Cosmet Dermatol. 2021;21:3905-09.
- [Google Scholar]
- Treatment of acanthosis Nigricans of the axillae using a long-pulsed (5-msec) alexandrite laser. Dermatol Surg. 2004;30:1158-60.
- [Google Scholar]
- Comparison of the effectiveness of fractional 1550-nm erbium fiber laser and 0.05% tretinoin cream in the treatment of acanthosis Nigricans: A prospective, randomized, controlled trial. Lasers Med Sci. 2020;35:1153-8.
- [Google Scholar]
- Use of a novel quantitative tool for evaluation of pseudo-acanthosis Nigricans: Acanthosis Nigricans area and severity index (ANASI) J Egypt Women Dermatol Soc. 2021;18:119.
- [Google Scholar]
- Using trichloroacetic acid in the treatment of acanthosis Nigricans: A pilot study. J Dermatol Treat. 2014;25:223-5.
- [Google Scholar]
- Standard guidelines of care for chemical peels. Indian J Dermatol Venereol Leprol. 2008;74:S5-12.
- [Google Scholar]






