Translate this page into:
Efficacy of Botulinum Toxin in Healing of Posttraumatic Facial Lacerations: A Prospective, Randomized, Comparative Study
Address for correspondence: Dr. Nilesh Kumar, Department of Oral and Maxillofacial Surgery, School of Dental Sciences, Krishna Institute of Medical Sciences Deemed To Be University, Karad 415539, Maharashtra, India. E-mail: drkumarnilesh@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Posttraumatic facial scars are not only unaesthetic but also have a negative sociopsychological impact. Surgeons constantly try to achieve the most aesthetic scar by various suturing methods. The tensile forces acting on the edges of the wound during healing are a major factor in determining the final appearance of a scar.
Aim:
Injecting botulinum toxin type A (BTX-A) locally to produce temporary muscular paralysis to relieve the tensile forces acting on suture line, thus improving the appearance of the scar.
Materials and Methods:
In this study, patients with posttraumatic facial lacerations were locally injected with BTX-A before suturing (with vicryl 4-0 and ethilon 5-0 in layers). BTX-A reformulated with local anesthetic with vasoconstrictive was used in this study for immediate action. Patients were followed for a duration of 6 months with standardized photographs of the scar for evaluation.
Result:
A total of 50 patients with traumatic facial lacerations were included in the study. This study demonstrated significantly less increase in scar width (P = 0.000) and irregularities (P = 0.017), and improved discoloration of the scar (P = 0.000) in patients who received BTX-A injection. The overall results showed significant (P < 0.05) improvement over the period of 6 months. Both visual analog scale scoring and patient self-assessment scale were favorable in the BTX-A group, showing statistically significant improvement (P < 0.05).
Conclusion:
Botulinum toxin-induced immobilization of the wound enhances healing and improves the eventual appearance of the scar.
Keywords
Botulinum toxin
scar
wound healing
INTRODUCTION
“Time heals all wounds—but scars remain.”
Poorly healed facial scars are not only unaesthetic but also have a negative sociopsychological impact.
Wound healing is affected by a variety of intrinsic and extrinsic factors. The healing response differs primarily on the type of tissue involved, the nature of the tissue disruption, and wound closure. Widening of scars resulting in unaesthetic appearance occurs when the suture line is pulled apart due to the opposing forces that are applied to newly formed collagen before it reaches final maturity, which can take several months. These opposing tensile forces are caused by various elements such as muscle pull, elastic forces of adjacent skin, and external pressure. The widening of scars mainly results due to the mechanical influence on the resilient and immature collagen. This concept is justified by the outcome of healing wounds relative to the lines of Langer. Scars aligned with the lines of Langer are subject to reduced tension and heal well, whereas scars oriented against them are subject to repetitive tension, resulting in scar widening.[1] Therefore, to reduce bias, lacerations along the relaxed skin tension lines (RSTL) had been excluded in this study. Variety of guidelines and approaches have been recommended in literature to reduce or avoid wide scars.
Botulinum toxin type A (BTX-A) is produced by the anaerobic bacterium Clostridium botulinum. Botulinum toxin has been extensively used to treat facial rhytids since Carruthers and Carruthers described its cosmetic use in the face.[2] Application of botulinum toxin in the reduction of facial scars was first reported as trial on primates by Gassner et al. in 2002.[3] The cosmetic appearance of scars treated with botulinum toxin was found to be significantly better than that of the control group.
The neurotoxin induces chemodenervation through its action on the presynaptic neuron, preventing release of acetylcholine, which leads to functional denervation of striated muscle for 2–6 months after injection.[4] The result is muscle fiber atrophy and subsequent clinical flaccid paralysis. Temporary paralysis of the facial muscles underlying the wound is expected to give a crucial advantage of providing rest during healing wound until the collagen matures.
BTX-A takes up to 48–72 h to achieve complete flaccid muscle paralysis. Till then the forces of underlying musculature already start acting on the suture line resulting in follow-up visits and reinjections to achieve the desired results. Reformulation of BTX-A before injecting with local anesthetic with vasoconstrictor provides immediate action. The action of the anesthetic agent is to stabilize the neuronal membrane and inhibit the ionic fluxes that are required to initiate and conduct the neuronal impulses. Owing to this mechanism, the efferent fibers get blocked and muscle paralysis is achieved immediately. The action of the vasoconstrictive agent is to achieve its effect through its sympathomimetic properties that act on the receptors, reducing the local diffusion of the anesthetic agent, which would also prevent diffusion of BTX-A, thereby improving its local availability.[5]
The aim of the study was to evaluate the efficacy of botulinum toxin-induced immobilization of facial muscles on wound healing of facial lacerations.
The objective of the study includes evaluation of wound healing of facial lacerations treated with and without botulinum toxin for color, width, elevation, and induration.
MATERIALS AND METHODS
This study was a prospective, randomized, comparative, clinical study conducted from January 2019 to January 2020 on a total of 50 patients randomly divided into two groups (25 patients each) after due approval of the institutional ethical committee.
The inclusion criteria were as follows:
Patients older than 18 years
Patients with traumatic lacerations on face
Patients giving consent for the procedure and agreeing for follow-up visits.
The exclusion criteria were as follows:
Patients younger than 18 years
Wound parallel to lines of Langer
Pregnant and lactating females
Lacerations near any neurovascular bundle or vital structures
Previous injection with botulinum toxin within 6 months
Allergy to botulinum toxin
Patients with myasthenia gravis
Patients on medications that interfere the neuromuscular conduction
Patients with multiple lacerations or with tissue loss
Patients not willing for the study
Patients were further randomized according to the odd–even method into Group A (BTX-A group)—odd serial number patients and Group B (control group)—even serial number patients.
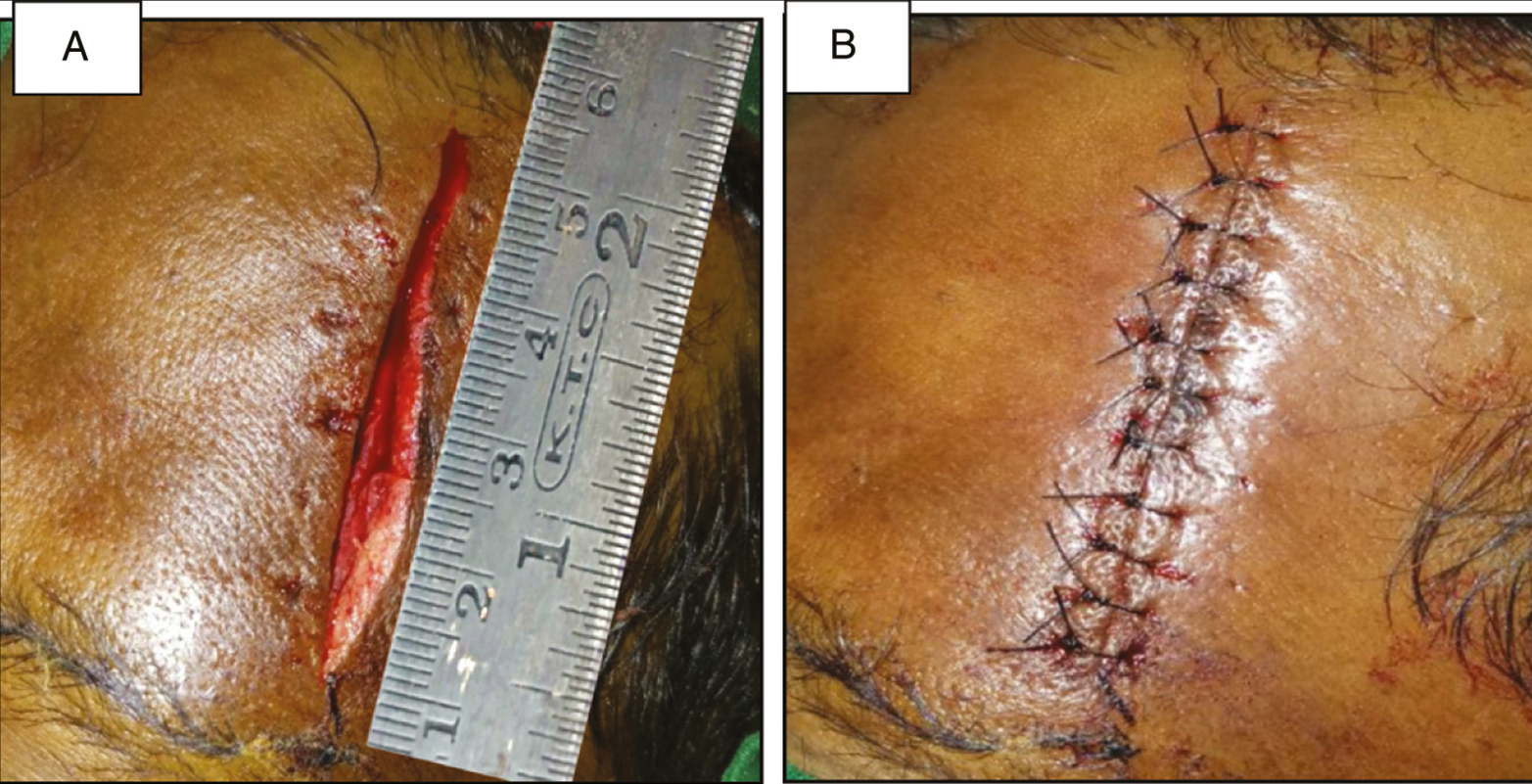
Every patient in Group A was subjected to an allergy skin test for BTX-A (SIAX; Aakar Pharmaceuticals, Mumbai, India). Debridement of wound was done, and the surrounding skin was prepared using the standard aseptic procedure using betadine solution and normal saline. Laceration length was measured using a measuring scale [Figure 1]. Study medication was formulated by mixing 100 U of BTX- A with 10 ml of 0.9% injectable saline + 3 mL of solution of 2% lidocaine with epinephrine 1: 200,000 per vial (LOX; NEON Laboratories, Chandigarh, India). This yielded 13 mL of solution per vial [Figure 2].
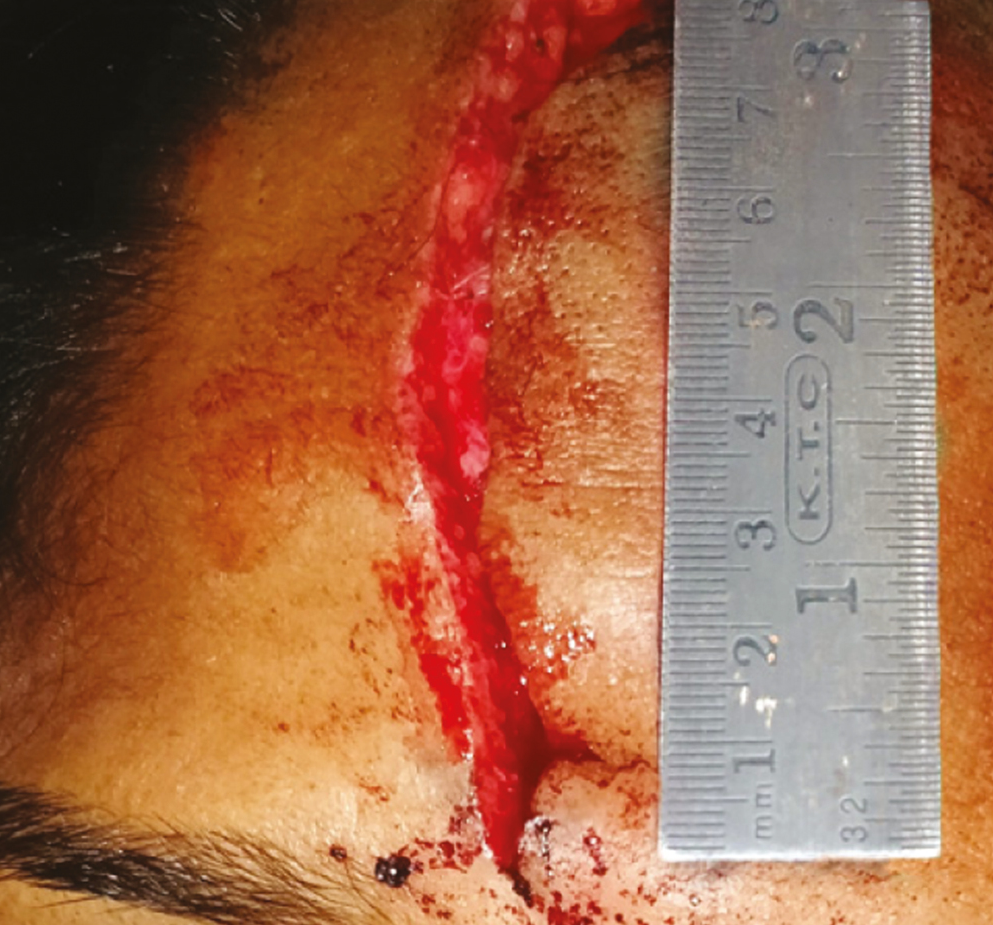
- Laceration length measurement using measuring scale for group A
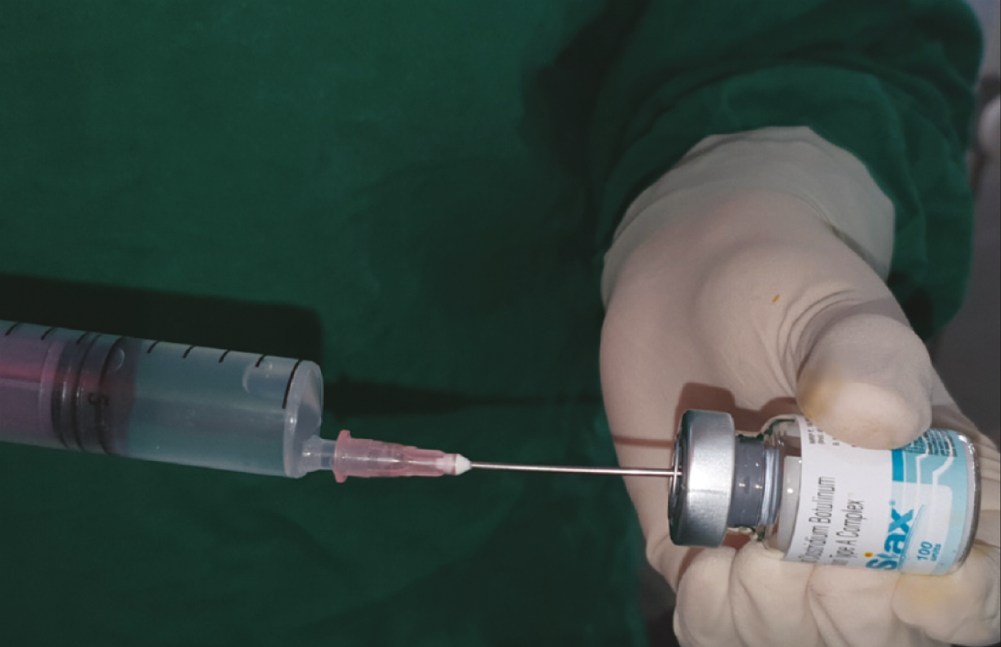
- Reconstitution of botulinum toxin
Local anesthesia was attained using adequate volume of infiltration using 2% lignocaine with 1:200,000 adrenaline (LOX). The BTX-A was injected adjacent the wound along the length of the laceration [Figure 3]. A volume of 1.5 U (0.19 mL) per cm of wound length was injected, with the needle prick placed approximately 3–4 mm from the edge of the wound to the depth of expected facial musculature around the specific anatomic site of the wound (approximately 5–8 mm). Lacerations was sutured in layers with vicryl 4-0 (1.5 m, 16 mm 1/2 circle, round bodied, absorbable surgical suture USP synthetic; Ethicon; Johnson & Johnson Pvt. Ltd., Aurangabad, India; subcutaneous layer) and ethilon 5-0 (1 m, 12 mm 3/8 circle, reverse cutting, nonabsorbable surgical suture USP; Ethicon, manufactured in India by Johnson & Johnson Pvt. Ltd.; cutaneous layer) in a simple interrupted manner [Figure 4].
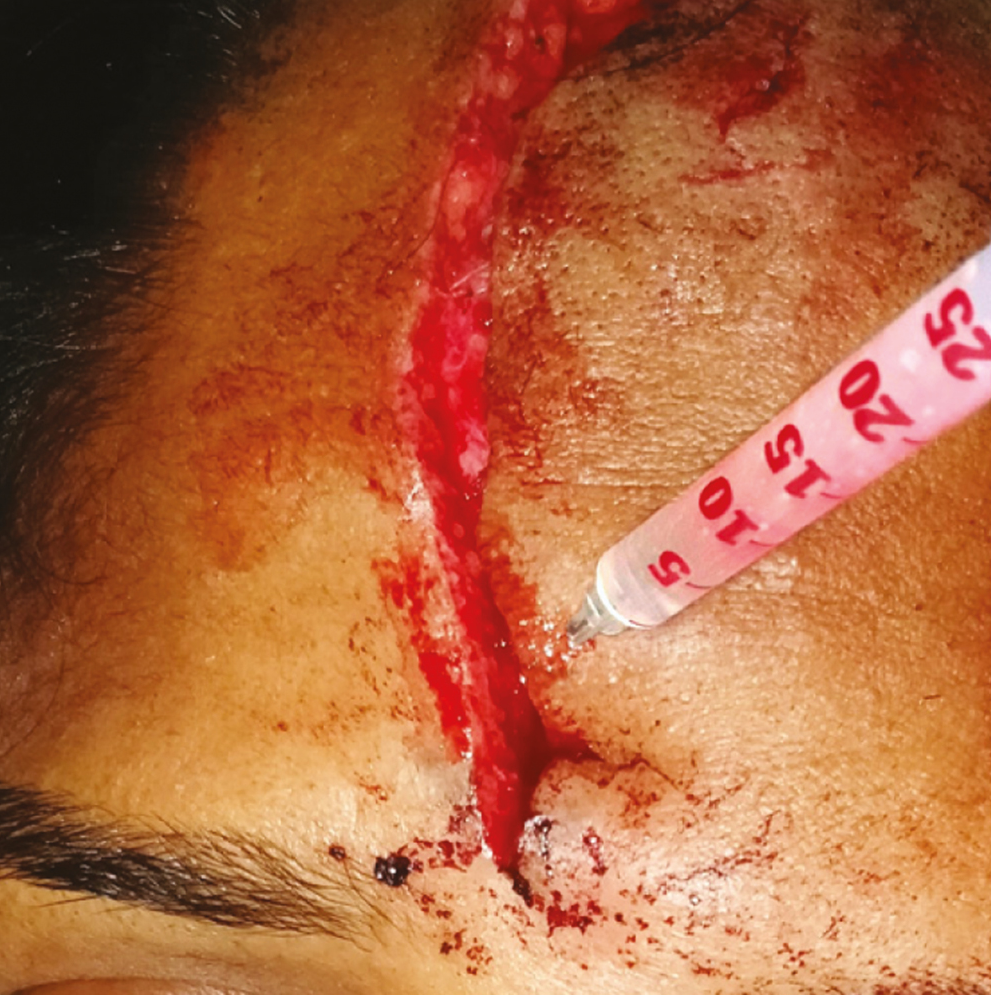
- Injecting botulinum toxin adjacent to the laceration
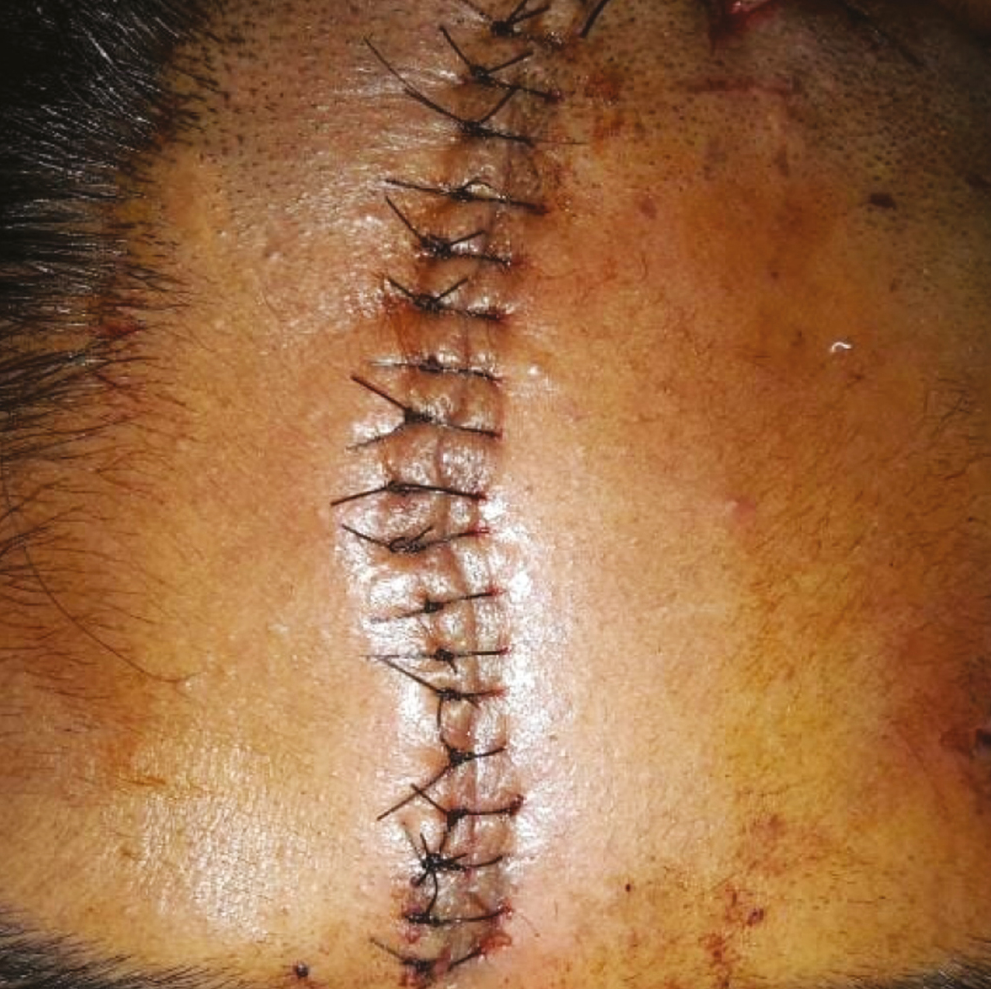
- Layered closure of laceration
For the patients in Group B, debridement of wound was done and the surrounding skin was prepared using the standard aseptic procedure using betadine solution and normal saline. Laceration length was measured using a measuring scale. Local anesthesia was attained using adequate volume of infiltration using 2% lignocaine with 1:200,000 adrenaline. Lacerations was sutured in layers with vicryl 4-0 (subcutaneous layer) and ethilon 5-0 (cutaneous layer) in a simple interrupted manner [Figure 5].
- (A) Laceration length measurement using measuring scale for group B. (B) Layered closure of laceration
Close-up photographs of the wounds were taken at a 1:1 ratio with a Samsung mobile phone camera, flash, 12-MP resolution prior to wound suturing and following wound suturing. All the patients were advised to report back to the department in case of any adverse reaction. All the patients were recalled after 7 days for suture removal, and photographs were repeated at 7 days and on 3- and 6-month follow-up visit with the same device and settings.
RESULTS
A total of 50 patients with traumatic facial lacerations were enrolled in this study. The patients were randomized into two groups. The total number of females and males enrolled in the BTX-A group was 9 and 16, respectively. The total number of females and males enrolled in the control group was 4 and 21, respectively [Chart 1]. At the end of the study, five patients failed to complete the 6-month follow-up (n = 2 in the BTX-A group and n = 3 in the control group). Therefore, those patients were completely eliminated from the study. The minimum wound size in the BTX-A group was 2 cm and the maximum was 14 cm, with mean wound size 4.27 cm (standard deviation of 2.81). The wound size in the control group was minimum 2 cm and maximum 9 cm, with a mean of 4.54 cm (standard deviation of 1.96).
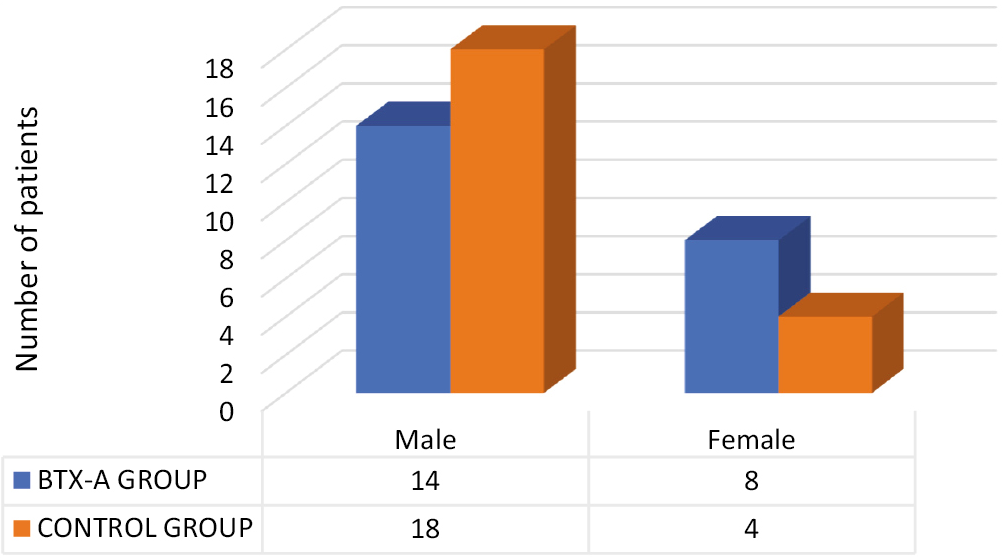
- Frequency distribution of the study participants according to gender in the BTX-A group and control group
The mean dose of botulinum toxin injected per patient in the BTX-A group was 6.40 U, with minimum of 3 U and maximum of 21 U. Wound was localized to the frontal bone in 63.6% (n = 14) of the cases in the BTX-A group and in 59% (n = 13) of the cases in the control group, followed by the chin region of mandible in 27.4% (n = 6) of the cases in the BTX-A group and 22.9% (n = 5) of the cases in the control group. The parameters evaluated included color change, width, elevation, and induration of the scar. The parameters were evaluated by two surgeons and scored from 0 to 10. Mean of both the scores was used for further analysis. The descriptive statistics were expressed as mean and standard deviations. The intragroup comparison of the visual analog scale (VAS) scores of different parameters was done at 1-week, 3-month, and 6-month follow-up using one-way analysis of variance. In the BTX-A group, this comparison was statistically significant for all the parameters: color change, width, elevation, and induration (P value < 0.05) [Table 1]. In the control group, this comparison was statistically insignificant for all the parameters from 1 week to 6 months except for width that was statistically significant (P value < 0.05) from 1 week to 6 months with P value = 0.021 [Table 2]. At different time intervals, the intergroup comparison of VAS scores for different parameters was assessed using the unpaired t-test among the BTX-A group and the control group. Statistically significant differences were seen at 1-week, 3-month, and 6-month follow-up [Table 3] (P value < 0.05*) between both the groups for color change, width, elevation, and induration [Figures 6 and 7]. The scars were evaluated by patients themselves for subjective satisfaction rating (very unsatisfied, unsatisfied, satisfied, and very satisfied). Frequency analysis and the chi-square test of proportion were done to find out statistically significant differences. The scars significantly improved over the period of 6 months with patients giving positive responses of very satisfied and satisfied in the BTX-A group. The results were statistically significant (P value < 0.05) with P = 0.031 at 1 week, P = 0.010 at 3 months, and P = 0.001 at 6 months [Chart 2]. Significant improvement was also seen for the scar in the control group with maximum responses received as satisfied [Chart 2]. At different time intervals, the intergroup comparison of frequency of patient self-assessment scale (PSAS) responses was assessed among the BTX-A group and the control group [Table 4]. The overall assessment of the PSAS responses states that approximately an average of 72% patients were very satisfied with the BTX-A treatment as compared to an average of 15% of patients who were very satisfied with the scar in the control group. Therefore, the overall assessment of the patients’ responses using PSAS scales for all the parameters states that the patients were more satisfied with BTX-A treatment than the control group.
| BTX-A group—VAS score | F value | P value | Control group—VAS score | F value | P value | ||
|---|---|---|---|---|---|---|---|
| Color change | 1 week | 7.133 | 0.001* | Color change | 1 week | 1.781 | 0.183 |
| 3 months | 3 months | ||||||
| 6 months | 6 months | ||||||
| Width | 1 week | 6.211 | 0.020* | Width | 1 week | 3.996 | 0.003* |
| 3 months | 3 months | ||||||
| 6 months | 6 months | ||||||
| Elevation | 1 week | 4.771 | 0.013* | Elevation | 1 week | 2.371 | 0.213 |
| 3 months | 3 months | ||||||
| 6 months | 6 months | ||||||
| Induration | 1 week | 3.527 | 0.081* | Induration | 1 week | 4.756 | 0.153 |
| 3 months | 3 months | ||||||
| 6 months | 6 months | ||||||
VAS = visual analog scale, BTX-A = botulinum toxin type A. *P value < 0.05
| Control group—VAS score | Follow-up at different intervals | Mean difference | P value | |
|---|---|---|---|---|
| Color change | 1-week follow-up | 3-month follow-up | −0.94 | 0.252 |
| 6-month follow-up | −1.29 | 0.078 | ||
| 3-month follow-up | 6-month follow-up | −0.35 | 0.823 | |
| Width | 1-week follow-up | 3-month follow-up | −1.08 | 0.17 |
| 6-month follow-up | −1.62000* | 0.021* | ||
| 3-month follow-up | 6-month follow-up | −0.54 | 0.635 | |
| Elevation | 1-week follow-up | 3-month follow-up | −0.76 | 0.417 |
| 6-month follow-up | −1.1 | 0.165 | ||
| 3-month follow-up | 6-month follow-up | −0.34 | 0.837 | |
| Induration | 1-week follow-up | 3-month follow-up | −1.01 | 0.227 |
| 6-month follow-up | −1.32 | 0.083 | ||
| 3-month follow-up | 6-month follow-up | −0.31 | 0.867 | |
VAS = visual analog scale, BTX-A = botulinum toxin type A. *P value < 0.05
| VAS score | 1 week | 3 months | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | t value | P value | Mean | t value | P value | Mean | t value | P value | ||
| Color change | BTX-A group | 1.31 | 4.04 | 0.000* | 1.3 | 6.09 | 0.000* | 1.64 | 4.31 | 0.000* |
| Control group | ||||||||||
| Width | BTX-A group | 1.8 | 4.89 | 0.000* | 1.61 | 5.89 | 0.02* | 1.67 | 4.3 | 0.000* |
| Control group | ||||||||||
| Elevation | BTX-A group | 0.68 | 2.27 | 0.028* | 0.81 | 3.32 | 0.001* | 1.11 | 2.47 | 0.017* |
| Control group | ||||||||||
| Induration | BTX-A group | 0.95 | 2.89 | 0.006* | 1.02 | 3.58 | 0.000* | 2.93 | 1.75 | 0.02* |
VAS = visual analog scale, BTX-A = botulinum toxin type A. *P value < 0.05
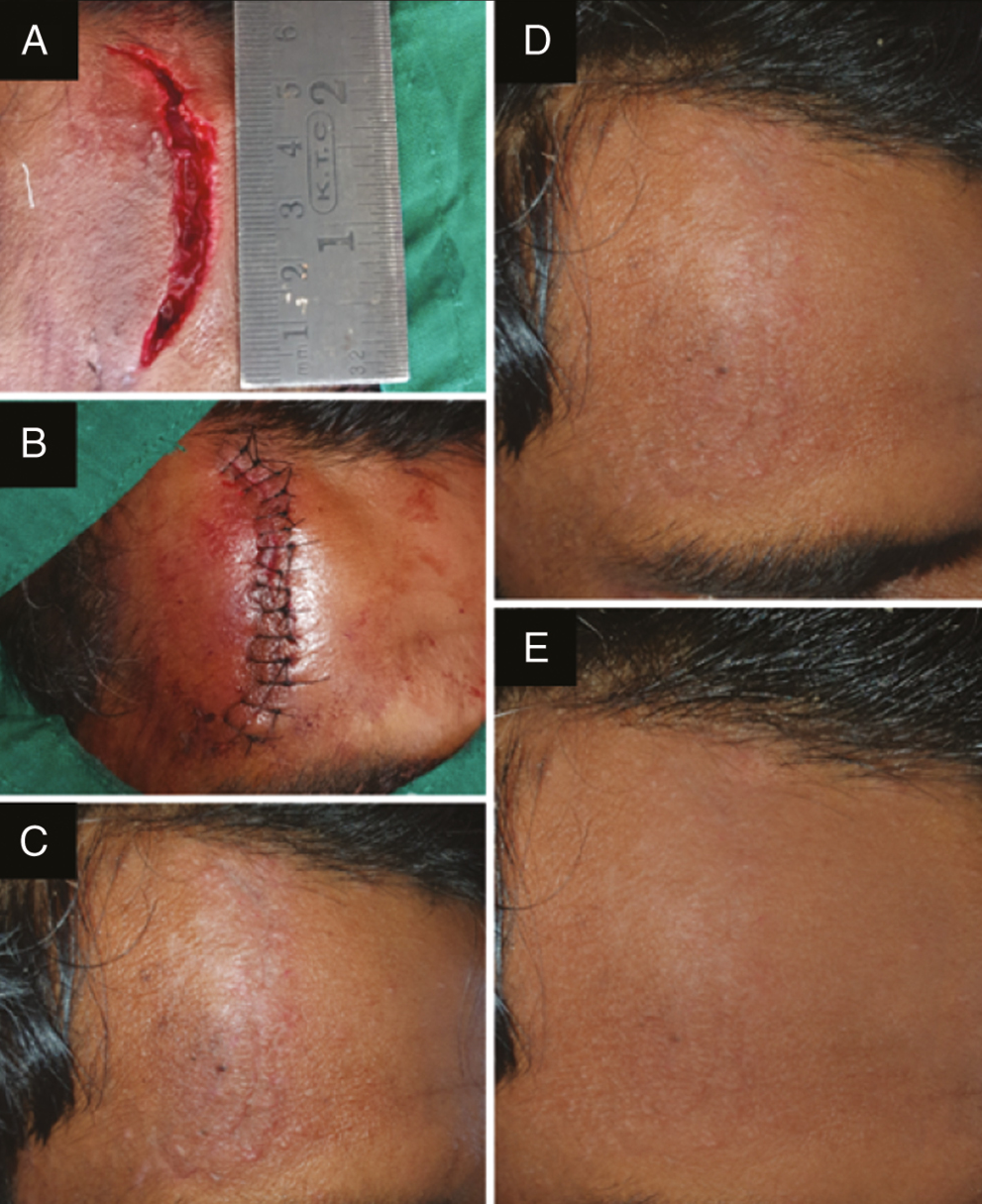
- Photograph showing (A) laceration over the right frontal bone treated with BTX-A, (B) sutured laceration, (C) scar at 1 week follow-up after suture removal, (D) scar at 3 months follow-up, and (E) scar at 6 month follow-up
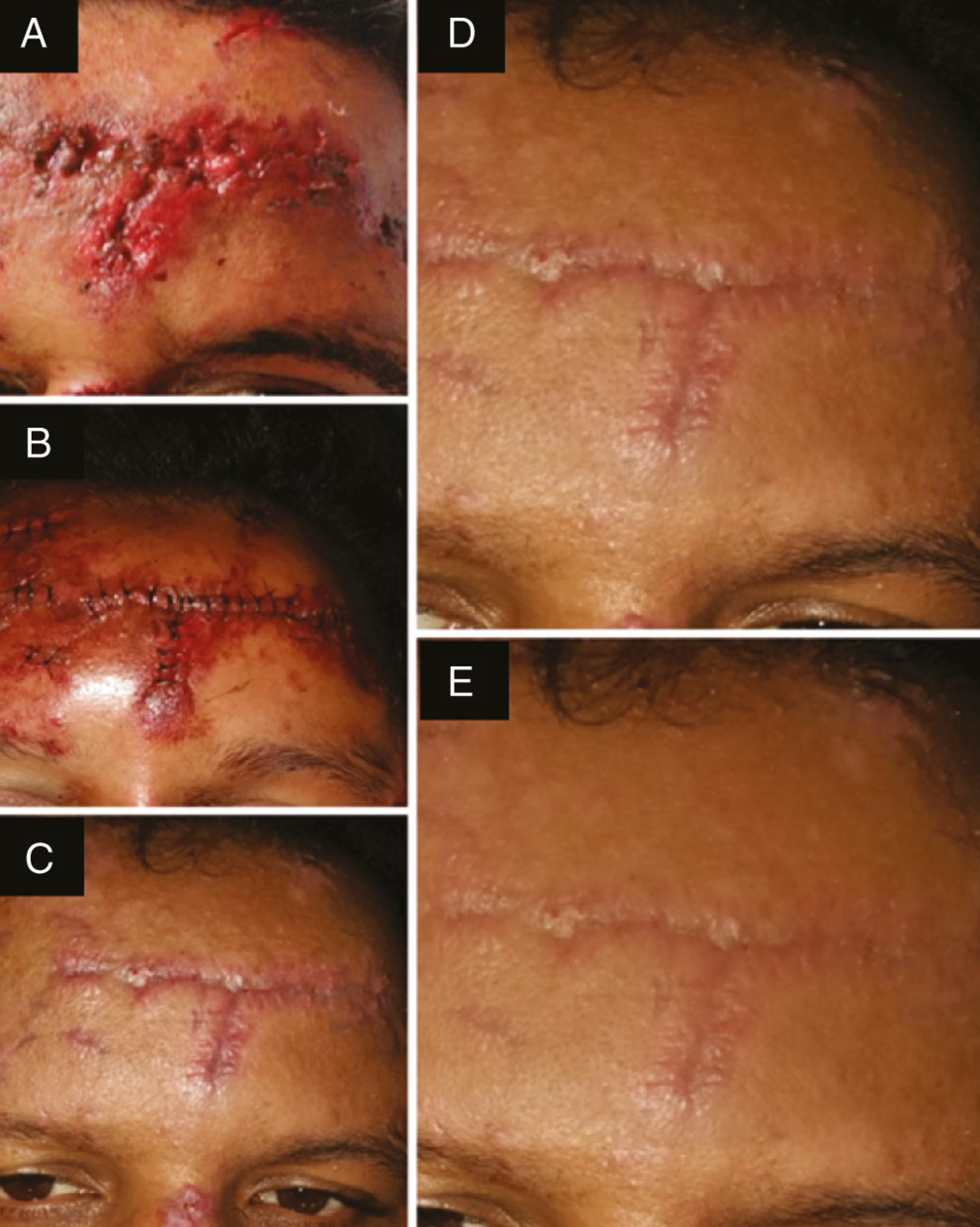
- Photograph showing (A) laceration over the left frontal bone treated without BTX-A, (B) sutured laceration, (C) scar at 1 week follow-up after suture removal, (D) scar at 3 months follow-up, and (E) scar at 6 month follow-up
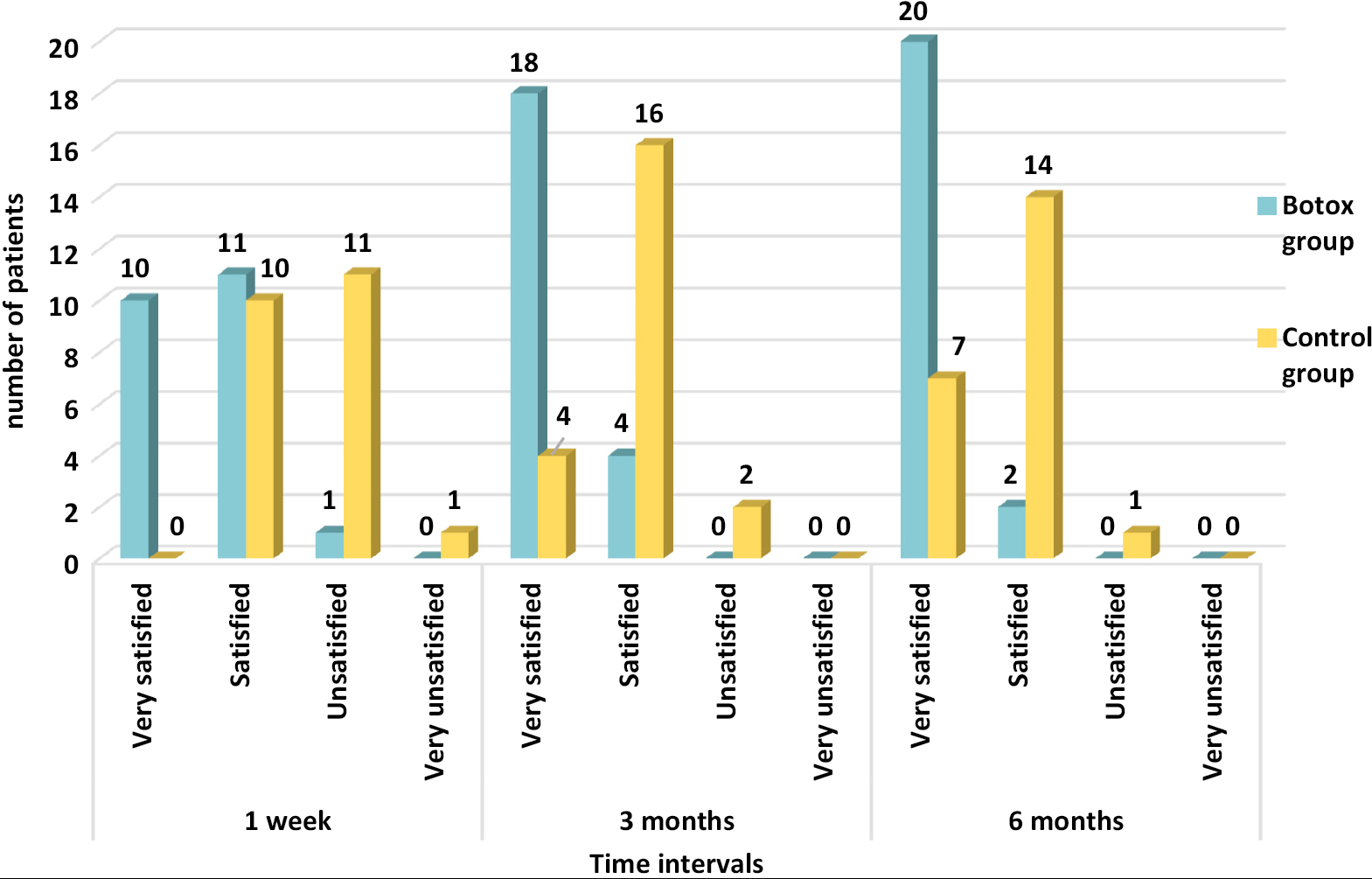
- Frequency distribution of patients according to PSAS for the resultant scar in the BTX-A group and control group at different time intervals
| Parameters | PSAS responses–1-week follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| Very satisfied (%) | Satisfied (%) | Unsatisfied (%) | Very unsatisfied (%) | |||||
| BTX-A group | Control group | BTX-A group | Control group | BTX-A group | Control group | BTX-A group | Control group | |
| Scar satisfaction at 1 week | 45.5 | - | 50 | 45.5 | 4.5 | 50 | - | 4.5 |
| Scar satisfaction at 3 months | 81.8 | 18.2 | 18.2 | 72.7 | - | 9.1 | - | - |
| Scar satisfaction at 6 months | 90.9 | 31.8 | 9.1 | 63.6 | - | 4.5 | - | - |
PSAS = patient self-assessment scale, BTX-A = botulinum toxin type A
DISCUSSION
Regardless of the era, appearance and beauty of oneself have always played a major aspect of human’s lives. The skin is an important visible element affecting the social interaction and quality of life. Wide ugly looking scars, especially on the visible areas of face, trigger varying levels of psychological distress and contribute to a lowered self-esteem and social awkwardness. Suturing of posttraumatic lacerations over face is usually followed by scarring and remains a frequent concern faced by the surgeons. Widening of scars is mainly a result of mechanical forces like the tensile distracting forces caused by elements such as muscle pull, elastic forces of adjacent skin, and external pressure that act on the resilient, immature collagen. Repeated cycles of microtrauma from the underlying muscles on the healing skin wound are believed to play a role in the prolongation of the inflammatory phase and the increased deposition of collagen and glycosaminoglycans within the wound, resulting in scar hypertrophy and hyperpigmentation.[67]
Operative techniques to minimize the effects of static tension on healing wounds include wide undermining, multilayered closure techniques; local flaps; external dressings; and stenting devices.[8] A key factor that determines the final cosmetic outcome of a cutaneous scar is the tension that acts on the wound edges during the healing phase.[9] One way to reduce the deleterious effects of dynamic tension caused by local muscle pull is to temporarily denervate the muscles that pull the wound edges apart through chemoimmobilization using botulinum toxin.
Botulinum toxin A allows near-complete elimination of dynamic muscle tension acting on the wound edges. Paralysis of the musculature beneath the wound minimizes the repetitive tensile forces on the wound edges. Botulinum toxin also represents an interesting modality to reduce the cycles of microtrauma in the early phases of wound healing to improve the overall scar quality.[35,610] It was recently reported that botulinum toxin inhibits the proliferation of fibroblasts, cell division, and transforming growth factor β1 expression.[111213] In addition, recent studies have also shown that botulinum toxin inhibits the formation of hypertrophic scars on the face.[58,14]
The use of botulinum toxin to improve facial wound scarring was first presented in a study in 1997.[15] Later, in 1999, Gassner et al. performed an animal trial on primates to study the cosmetic effects of botulinum toxin on cutaneous scars.[3] Results of this study showed significantly better cosmetic appearance of the cutaneous scars injected with botulinum toxin.
Recently, a few human studies have been reported on the effect of botulinum toxin in improving the outcomes of appearance of facial scars.[101516171819202122] In 2006, a study was performed by Gassner et al. to test whether the postoperative injections of BTX-A improved facial wound scarring in patients with forehead lacerations or after excisions of forehead masses in 31 patients.[6] Using previous studies as reference, the final cosmetic outcome of the study was assessed using the VAS at the 6-month follow-up visit.[2223] However other scoring systems can also be used like VSS (Vancouver Scar Score) which is more specific to scarring. The results using VAS were statistically significant, and clinically relevant improvement of the cosmetic appearance of facial scars was seen after treatment with botulinum toxin A. This study included 44 patients with posttraumatic facial lacerations. Those lacerations aligned favorably with RSTL were not included in the study. Unlike the study by Gassner et al. that was limited to forehead lacerations, in this study, lacerations over forehead, cheek, and chin were included. Injections in Gassner et al.’s study were performed postoperatively within 24 h, whereas injections in this study were performed just before suturing of the facial lacerations to get immediate effect. Lacerations managed by suturing along with botulinum toxin-induced chemodenervation of underlying muscles showed statistically significant healing with better cosmetic appearance when compared to those in the control group (with P value = 0.000 for color change and width, P value = 0.017 for elevation, and P value = 0.02 for induration). The results were in agreement with the findings of Wilson[14] who used BTX-A for muscle paralysis after revision of ugly facial scars. Similar result was also reported by Tollefson et al.[17] who used BTX-A in orbicularis oris muscle to decrease wound tension after closure of wide cleft lip repair and by Chang et al.[21] for cleft lip scar revision. Flynn[18] used BTX-A and BTX-B-induced chemodenervation in patients undergoing primary closure after surgical resection of skin cancer, which showed favorable results with no significant complications. Lee et al.[24] also reported significantly superior results over a period of 6-month follow-up in 30 patients with forehead lacerations treated with early postoperative injections of botulinum toxin A. Whereas Ziade et al. and Gassner et al. injected botulinum toxin within 24–72 h after suturing of posttraumatic laceration, in this study, BTX-A was injected just before suturing to achieve immediate effect.
Delay in onset of action of botulinum toxin may warrant additional injections during follow-up for complete paralyses around the wound. To overcome this, Gassner and Sherris performed a study in year 2000 to determine the paralyzing effect of BTX-A reconstituted in a solution of lidocaine with epinephrine.[5] Statistically significant difference (experimental side vs control side, P = 0.002) was noted after injection of the experimental drug when compared to the contralateral side of the forehead after the injection of the control drug in all patients. The simultaneous injection of a botulinum toxin and local anesthetic with vasoconstrictor reduces its local diffusion and provides the physician with immediate feedback on the desired treatment effect.[5] Additionally, the anesthetic agent lidocaine stabilizes the neuronal membrane and inhibits the ionic fluxes that are required for initiating and conducting the neuronal impulses.[25] This mechanism provided immediate muscle paralysis by blocking the efferent fibers. This novel formulation was used in this study for immediate feedback, thus avoiding the need for reinjection days or weeks later because of inadequate initial dosing.
In this study, besides assessment by specialists, scars were also rated by the patients individually (very satisfied, satisfied, unsatisfied, or very unsatisfied) using patient self-assessment score at 1 week, 3 months, and 6 months. At 6-month follow up, the outcome was considered as very satisfied by 90% of the patients in the BTX-A group compared to 31% of the patients in the control group. The wounds were injected with botulinum toxin in a linear pattern at the rate of approximately 1.5 U/cm of wound length. The injection was repeated every centimeter along the whole length of wound, with the needle prick placed approximately 3–4 mm from the edge of the wound, and the needle was inserted to the depth of expected facial musculature around the specific anatomic site of the wound. The similar method and dosage of BTX-A injection were described by Wilson[14] for better scar outcome of facial wounds and skin tumor excisions. However, the study was neither randomized nor controlled and mainly evaluated skin excisions that heal well. Moreover, children under 15 years of age were enrolled who might have different healing processes as compared to adults, which may alter the results. To eliminate the bias in this study, patients below 18 years of age were excluded from the study.
In this study, at 6-month follow-up, the VAS score assessed showed statistically significant improvements of scar in terms of color change (P = 0.000), width (P = 0.000), elevation (P = 0.01), and induration (P = 0.02). An average of 91% patients were very satisfied with the wound treated with botulinum toxin as compared to 32% in the control group at 6 months. This study along with the previous studies suggests better cosmetic outcome when posttraumatic facial lacerations are treated with botulinum toxin.
CONCLUSION
The ultimate goal of wound management is to achieve a functional and cosmetically acceptable scar. One way to improve the appearance of the scar is to eliminate the tension caused by pull of underlying muscles that exerts pull on the wound margins. Temporary denervation with a chemical agent is a convenient and useful option to achieve the desired effect of reducing the muscle pull, subsequent microtrauma, and eventually scar hypertrophy during wound healing, thereby improving the appearance of the scar. It was observed that botulinum toxin-induced chemoimmobilization significantly improved the cosmetic appearance of traumatic laceration over the face with no adverse effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Principles of facial reconstruction. Lippincott Williams & Wilkins; 1995.
- Aesthetic indications for botulinum toxin injections. Plast Reconstr Surg. 1995;95:427-8.
- [Google Scholar]
- Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg. 2000;105:1948-53; discussion 1954-5.
- [Google Scholar]
- Botox for the treatment of dynamic and hyperkinetic facial lines and furrows: adjunctive use in facial aesthetic surgery. Plast Reconstr Surg. 1999;103:701-13.
- [Google Scholar]
- Addition of an anesthetic agent to enhance the predictability of the effects of botulinum toxin type A injections: a randomized controlled study. Mayo Clin Proc. 2000;75:701-4.
- [Google Scholar]
- Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study. Mayo Clin Proc. 2006;81:1023-8.
- [Google Scholar]
- Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884-92.
- [Google Scholar]
- Management of scar contractures, hypertrophic scars, and keloids. Otolaryngol Clin North Am. 1995;28:1057-68.
- [Google Scholar]
- Chemoimmobilization: improving predictability in the treatment of facial scars. Plast Reconstr Surg. 2003;112:1464-6.
- [Google Scholar]
- Effect of botulinum toxin type A on transforming growth factor beta1 in fibroblasts derived from hypertrophic scar: a preliminary report. Aesthetic Plast Surg. 2010;34:424-7.
- [Google Scholar]
- Effects of botulinum toxin type A on collagen deposition in hypertrophic scars. Molecules. 2012;17:2169-77.
- [Google Scholar]
- Treatment of hypertrophic scars with intralesional botulinum toxin type A injections: a preliminary report. Aesthetic Plast Surg. 2009;33:409-12.
- [Google Scholar]
- Use of botulinum toxin type A to prevent widening of facial scars. Plast Reconstr Surg. 2006;117:1758-66; discussion 1767-8.
- [Google Scholar]
- Use of botulinum A toxin in patients at risk of wound complications following eyelid reconstruction. Ophthalmic Plast Reconstr Surg. 1997;13:259-64.
- [Google Scholar]
- Botulinum toxin to improve results in cleft lip repair. Arch Facial Plast Surg. 2006;8:221-2.
- [Google Scholar]
- Use of intraoperative botulinum toxin in facial reconstruction. Dermatol Surg. 2009;35:182-8.
- [Google Scholar]
- Botulinum toxin-induced immobilization of lower facial wounds. Arch Facial Plast Surg. 2009;11:140-2.
- [Google Scholar]
- Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg. 2013;66:209-14.
- [Google Scholar]
- Botulinum toxin to improve results in cleft lip repair: a double-blinded, randomized, vehicle-controlled clinical trial. PLoS One. 2014;9:e115690.
- [Google Scholar]
- Effects of botulinum toxin on improving facial surgical scars: a prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg. 2018;141:646-50.
- [Google Scholar]
- Clinical trial to evaluate the efficacy of botulinum toxin type A injection for reducing scars in patients with forehead laceration: a double-blinded, randomized controlled study. Medicine. 2019;98
- [Google Scholar]
- The efficacy and safety of early postoperative botulinum toxin A injection for facial scars. Aesthetic Plast Surg. 2018;42:530-7.
- [Google Scholar]
- The relationship between regional blood flow and absorption of lignocaine. Aust N Z J Surg. 1993;63:766-71.
- [Google Scholar]






