Translate this page into:
Giant PMMA Foreign Body Granulomas with Imaging
Address for correspondence: Dr. Sunthosh Sivam, Baylor College of Medicine, Houston, TX 77030, USA. E-mail: sunthosh.sivam@bcm.edu
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Filler complications have a wide array of presentations including early and late manifestations. A rare late complication is the foreign body granuloma or granulomatous foreign body reaction. We present a case of giant foreign body granulomas developing 7 years after polymethylmethacrylate (PMMA) filler injection. The patient had an excellent response to a single intralesional injection of triamcinolone acetonide and 5-fluorouracil. The unique opportunity to have pretreatment and posttreatment magnetic resonance imaging (MRI) allows for appreciation of the multidirectional expansion of these granulomas as well as the response in this case. Updated treatment recommendations based on the literature review support the use of oral antibiotics, oral steroids, and intralesional therapies. Surgical excision is reserved as an absolute last resort due to potential complications.
Keywords
Filler complication
foreign body granuloma
giant granuloma
polymethylmethacrylate complication
INTRODUCTION
Dermal filler complications have a wide array of presentations including early and late manifestations. Complications in general are demonstrably more common with permanent fillers such as polymethylmethacrylate (PMMA).[1] We present a case report of delayed complication from PMMA filler.
MATERIALS AND METHODS
Data regarding the patient’s presentation, workup, and interventions were collected prospectively. The data are de-identified in nature.
RESULTS
A 47-year-old woman presented with a complaint of bilateral cheek masses which developed one month earlier. The painful masses had grown rapidly. There was associated intermittent periorbital edema. The masses were refractory to multiple courses of oral antibiotics and steroids. The patient had undergone Artefill injections elsewhere in 2012 with no history of local complications at that time.
On exam, there was increased midface volume bilaterally without overlying skin changes. Palpation revealed large bilateral medial infraorbital cheek subcutaneous masses that were mobile, mildly tender, and nodular. Her cranial nerve exam was normal including the sensation of her midface.
A noncontrast maxillofacial computed tomography (CT) scan showed subtle and poorly defined premaxillary soft tissue masses. A subsequent magnetic resonance imaging (MRI) revealed bilateral (right 3.0 cm × 0.8 cm and left 2.6 cm × 0.8 cm) nodular irregular premalar soft-tissue masses [Figure 1]. Notably, there was no enhancement of the infraorbital nerve on either side though the masses were immediately adjacent to the foramina. Fine-needle aspiration was obtained bilaterally. Multinucleated giant cells were seen on cytopathology consistent with a granulomatous reaction.
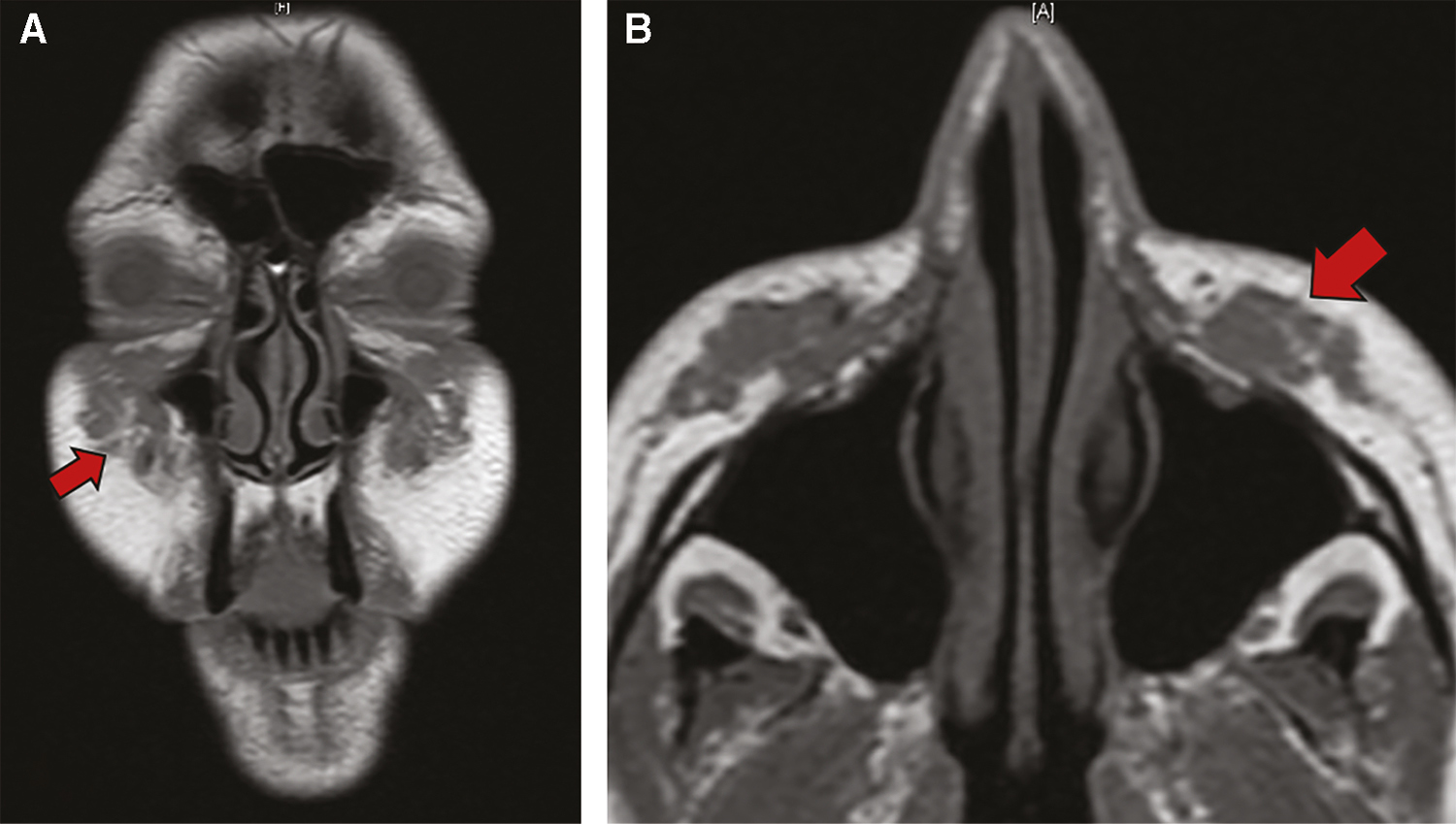
- Pretreatment non-contrasted MRI T1 coronal (A) and axial (B)
Treatment consisted of intralesional injections. A mixture of 2.5 mL of triamcinolone acetonide (10 mg/mL) and 0.5 mL of 5-fluorouracil (50 mg/mL) was injected into each granuloma. A total volume of 4.5 mL was ultimately injected into each granuloma. This was performed with aseptic technique under local anesthesia.
There was significant improvement at one week and very minor residual granuloma at just over one month from the single injection. We have now followed the patient for more than 1 year without exacerbation of the granulomatous reaction. Figure 2 is an MRI obtained post-treatment. Figure 3 shows pretreatment and posttreatment photos correlating with the time of the radiographic imaging.
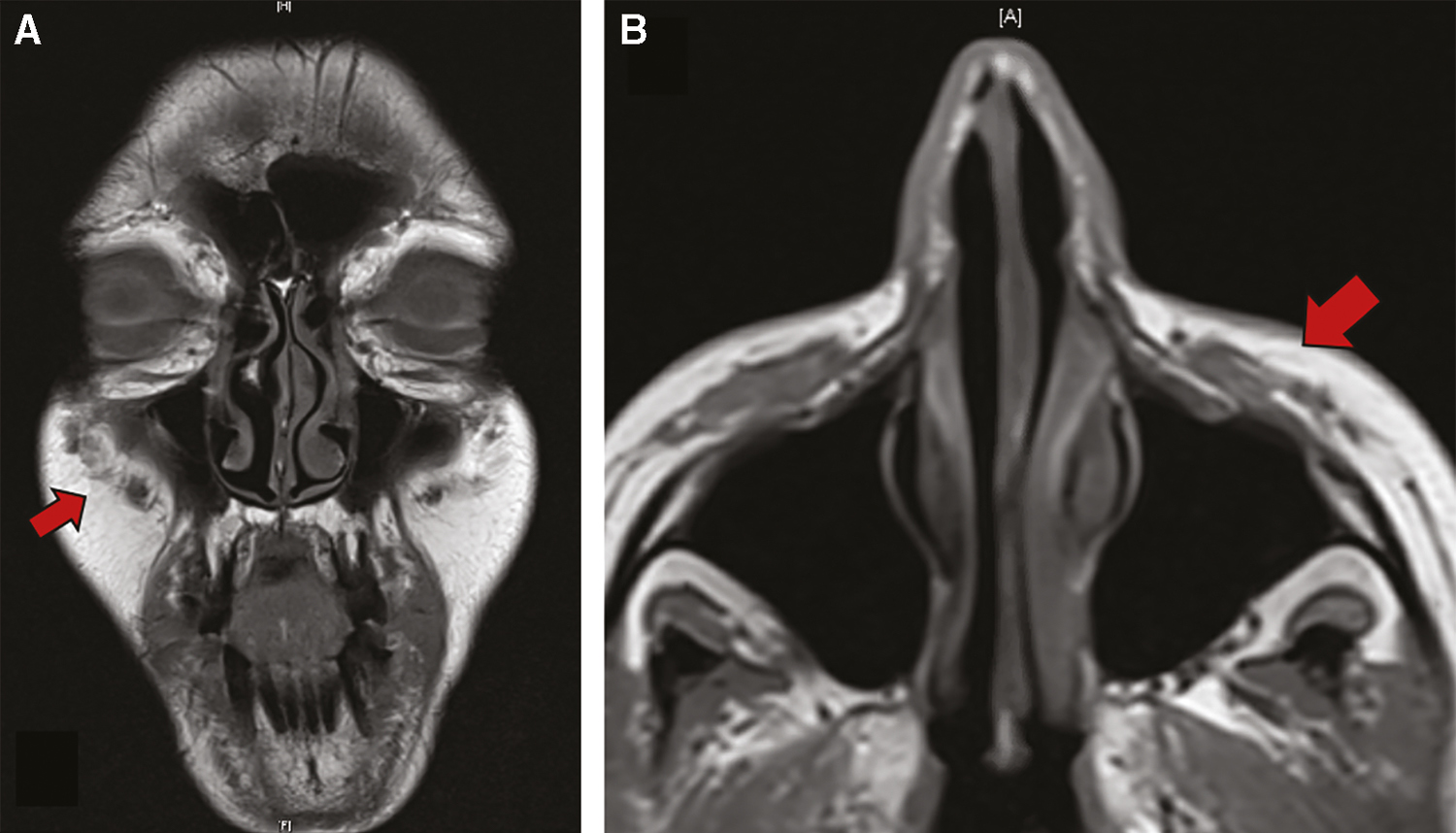
- Posttreatment non-contrasted MRI T1 coronal (A) and axial (B)
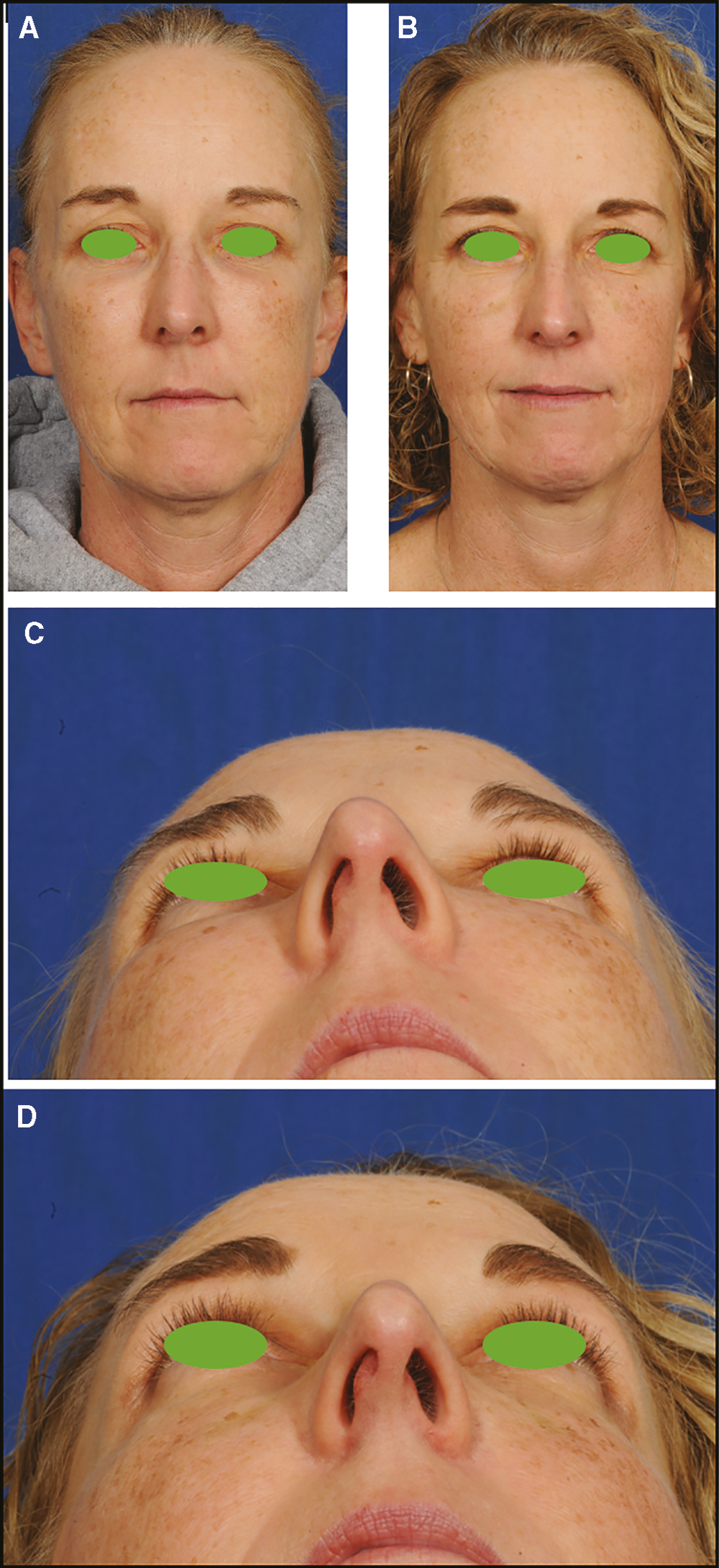
- Pretreatment (A, C) and posttreatment (B, D) photos
DISCUSSION
We describe Artefill injections for malar augmentation resulting in bilateral giant foreign body granulomas after a latent period of 7 years. After failing first-line therapy including oral antibiotics and steroids, the patient’s symptoms improved with a single session of intralesional triamcinolone and 5-fluorouracil injection. Pre- and posttreatment MRI imaging provided objective evidence of the dramatic treatment response [Figures 1 and 2].
Artefill is a permanent filler consisting of PMMA 32–40 µm microspheres suspended in a 3.5% solution of bovine collagen.[2] Permanent filler products have an increased risk of complications ranging from nodules to vascular occlusion.[1] Foreign body granulomas, a non-allergic inflammatory response composed of macrophages and foreign body giant cells, have been reported with use of dermal fillers.[3] Foreign body granulomas are rare yet significant and reported to occur within months to six years following filler injection.[4] PMMA served as the catalyst for the development of the granulomatous reaction in this case. As a non-degradable combination gel, the reactive nature of Artefill is by design to stimulate tissue fibrosis.[5] The problem can occur when this happens in an uncontrolled fashion resulting in a foreign body granuloma as opposed to aesthetically pleasing volume augmentation.
There is no clear consensus on treatment of PMMA-related granulomas; however, oral steroids, allopurinol, fumaric acid, and oral tetracycline have all been suggested as early measures in the literature.[15,6] The options for intralesional therapy include the use of triamcinolone and 5-fluorouracil to mitigate the immunologic reaction as was suggested by Wiest et al..[5] Synergistically, steroids inhibit fibroblast activity and giant cell formation, whereas 5-fluorouracil inhibits RNA synthesis which further inhibits fibroblast proliferation and related collagen production.[6]
Importantly, foreign body granulomas should be distinguished from the more subacute presentation of delayed onset nodules that occur weeks to months after injection. Biofilms may play a role in nodule development and for this reason prolonged treatment with oral antibiotics is recommended.[7] The use of multiple courses of oral antibiotics may be more appropriate for a delayed onset nodule than for foreign body granuloma.
Surgical excision is not recommended due to the possibility of scarring, deformity, and incomplete excision resulting in residual multifocal inflammation.[3] As illustrated in Figure 1, there is a distinct multidirectional progression of these granulomas that would present a hazard for complete surgical excision.
This case is illustrative of giant foreign body granuloma occurring with PMMA filler after a longer latency period than has been previously reported in the literature. Accurate diagnosis, avoidance of potentially deforming surgical excision, and administering appropriate treatment including intralesional steroid and 5-fluorouracil will allow for optimal care of similar patients.
Of note, Artefill branding was changed to Bellafill in 2014.[8]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
References
- Understanding, avoiding, and managing severe filler complications. Plast Reconstr Surg. 2015;136:196S-203S.
- [Google Scholar]
- Complications after polymethylmethacrylate injections: report of 32 cases. Plast Reconstr Surg. 2008;121: 1811-20.
- [Google Scholar]
- Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232-9.
- [Google Scholar]
- Delayed granulomatous reactions to facial cosmetic injections of polymethylmethacrylate microspheres and liquid injectable silicone: a case series. J Cosmet Laser Ther. 2016;18:170-3.
- [Google Scholar]
- Electron microscopic documentation of late changes in permanent fillers and clinical management of granulomas in affected patients. Dermatol Surg. 2009;35:1681-8.
- [Google Scholar]
- Adverse granulomatous reaction to artecoll treated by instralesional 5-fluorouracil and triamcinolone. Injections. 2006;32:1079-82.
- [Google Scholar]
- Available from: https://www.prnewswire.com/news-releases/bellafill-to-replace-artefill-as-new-brand-300001850.html. [Last accessed on 2020 Apr 30]






