Translate this page into:
Intradermal Platelet-Rich Plasma for the Treatment of Melasma: A Clinical and Dermoscopic Evaluation in Dark Skin
Address for correspondence: Dr. Aradhana Rout, Department of Dermatology, Military Hospital, Satwari, Jammu, Jammu and Kashmir 180003, India. E-mail: serenity.aradhana@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Melasma is a common dermatosis in both men and women showing varying degrees of success with treatment. Relapse of melasma is high in dark skin types, which necessitates the need for finding a modality of treatment, which not only treats but also prevents relapse.
Aims:
To study the effectiveness of platelet-rich plasma (PRP) in patients of melasma both clinically and dermoscopically in dark skin types.
Materials and Methods:
A prospective study of 20 female patients of Fitzpatrick skin type IV–V with mixed type of melasma and bilateral involvement of the face were enrolled for the study. PRP was injected intradermally at 4 weeks interval for three sittings, and the results were assessed clinically (by modified melasma area and severity score) and dermoscopically. Patients were counselled to ensure strict sun protection measures. Patient satisfaction was noted at baseline, 4 weeks, 8 weeks, and 12 weeks. Patients were followed up for 3 months to see for any relapse of the pigmentation. The follow-up showed no relapse of melasma in these patients.
Statistical Analysis:
Analysis of variance was used with Bonferroni correction for modified melasma area and severity score at various time interval. Subject global aesthetic improvement scale (SGAIS) and physician global esthetic improvement scale (PGAIS) were expressed in counts. P-value ≤ 0.05 was considered significant.
Results:
Modified melasma area and severity score and dermoscopic changes showed statistically significant improvement compared at the end of study in mild to severe cases. The subjective assessment was made by PGAIS. Patient satisfaction levels (assessed by SGAIS) also showed significant improvement in successive weeks of treatment. Few patients had mild redness and burning post procedure, which resolved spontaneously after few hours.
Conclusion:
From this study we concluded that PRP shows a significant improvement in melasma after 12 weeks of treatment with no relapse even after 3 months. Hence, PRP may be used not only as an adjuvant but also as a first line treatment in the view of longer sustained results when combined with strict sun protection. There is a paucity of studies showing results of PRP treatment in dark skin types, which is more resistant to treatment than lighter skin. Moreover, clinical improvement should not be the only parameter to decide on stopping treatment as chances of relapse can be higher. Dermoscopic evaluation helps in determining the changes in vasculature (telangiectasias) and pigmentation (dots and globules), which are better indicators of success of treatment.
Keywords
Intradermal
melasma
platelet-rich plasma
Intradermal platelet-rich plasma (PRP) is an effective treatment modality for melasma either as an adjuvant or standalone treatment.

INTRODUCTION
Melasma is a common skin pigmentation disorder characterized by hyperpigmented macules in sun-exposed areas of the face such as the forehead, cheeks, lips, and nose.[1] Melasma commonly affects women (4:1 ratio of women–men) with Fitzpatrick skin types IV–VI.[2] The prevalence of melasma is about 40% in Southeast Asian population. The common risk factors for melasma include genetics, endocrine abnormalities (thyroid hormone), ultraviolet (UV) light exposure, pregnancy, and use of oral contraceptives, and anticonvulsants such as phenytoin.[3]
Recent pathogenesis of melasma has shown that inciting stimulus such as UV exposure, genetic predisposition, hormones, pregnancy, or drugs acts as a triggering factor leading to increased p53 expression.[4] Propriomelanocortin hormone levels increase with an increase in p53 levels further leading to the stimulation of alpha-melanocyte stimulating hormone (α-MSH). α-MSH binds to Melanocortin 1 receptor, thereby inducing microphthalmia-induced transcription factor (MITF) transduction. The resulting increase in tyrosinase activity causes melanin deposition in the skin. PRP is an amalgamation of multiple growth factors of which transforming growth factor beta (TGF-β) is primarily involved in the treatment of melasma. TGF-β reduces the signal transduction of MITF, thereby reducing tyrosinase activity and pigmentation in melasma.[5]
PRP is plasma with concentrated platelets derived from blood, which has found extensive use in dermatology. Few of the widely used indications include facial rejuvenation, acne scars, androgenetic alopecia, and wound healing.[6] The mechanism of action of PRP in melasma has not been completely elucidated. However, studies have shown that TGF-β has a predominant role in treatment of melasma.[7] TGF-β decreases signal transduction of MITF and thus decreases tyrosinase and melanin synthesis. Additionally, PRP also induces collagen synthesis, thereby improving the quality and texture of the skin.[8]
Melasma is a difficult to treat cosmetic problem. Various topical therapies, which have been used for the treatment of melasma include hydroquinone, topical retinoids, topical corticosteroids, vitamin C, and licorice extract. Though chemical peels and lasers have yielded satisfactory results, they still carry a significant risk of relapse of the dermatoses by postinflammatory hyperpigmentation.
Studies have shown PRP to be an excellent treatment option in melasma in combination treatment as well as standalone treatment.[9] However, significant data on its efficacy in treating melasma in dark skin types are still inadequate. Dermoscopy of melasma consists of diffuse light to dark brown pseudoreticular network with multiple brown dots and globules in an arcuate distribution and sparing of perifollicular areas. Reduction in dots and globules and pigmentation in the pseudoreticular network can be seen post treatment in these patients.[10]
However, available sparse literature demands a larger number of studies are needed to assess the effectiveness of PRP in treating melasma in dark skin types.
SUBJECTS AND METHODS
Patient selection
This study was performed with 20 female patients of Fitzpatrick skin type IV–V from November 2021 to March 2022 after approval by the local institutional ethics committee. We included female patients with bilateral mixed-type melasma of the face who were 18–50 years of age. Pregnant or lactating women, use of oral contraceptive pills or isotretinoin, bleeding tendencies, any recent laser treatment, any concurrent melasma treatments, and any bleaching or whitening cream were excluded. Written informed consent of individual subjects was obtained before starting the treatment procedure. Dermoscopy was performed on all patients on beginning of treatment and on serial follow-up.
Procedure
About 5 mL of subjects’ blood was withdrawn with aseptic precaution into the kit and was processed to give final outcome of about 03 mL of PRP. PRP was prepared by manual rotation PRP 1600g for 6 min, then second spin of 2000g for 5 min to obtain platelet concentration of 1200 × 106 platelets/μL.
Patients were seated comfortably in inclined position, the face was cleaned and topical analgesic cream (7% lidocaine and 7% tetracaine, Viveta) was applied under occlusion onto the areas to be treated. PRP thus prepared was loaded in multiple 30–31 gauge needles. PRP (0.1 mL/cm2) was then injected intradermally into melasma-affected areas on both sides of the face with the injection sites were spaced out from each other by 1 cm. This treatment was administered over 4 weeks interval for 4 sittings. Patients were also instructed to apply a broad-spectrum sunscreen and refrain from using any other topical preparations on the face during the study period.
Study outcome parameters
The results were assessed by mean changes in modified melasma area and severity (mMASI) score at baseline and at the end of the study. mMASI score was used to assess the severity of melasma and calculated by the following equation:
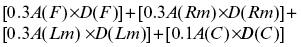
D—darkness, A—area, F—forehead, C—chin, Rm—right malar, and Lm—left malar. The darkness was scored from 0 to 4 (0—absent, 1—mild, 2—moderate, 3—moderately severe and 4—severe) and area of involvement was scored from 0 to 6 (0 = absent, 1 ≤ 10%, 2 = 10%–29%, 3 = 30%–49%, 4 = 50%–69%, 5 = 70%–89% and 6 = 90%–100%). On the basis of modified MASI scoring melasma was categorized into mild (0–8), moderate (8–16), and severe (16–24).
Dermoscopic changes were noted at the beginning, at every visit, and at the end of treatment to see for corroboration with clinical results.
Physician and subjects assessed the overall esthetic improvement from baseline with PGAIS and SGAIS, respectively.
RESULTS
Study population included 20 adult melasma patients with Fitzpatrick skin types IV and V. Total 20 subjects received three sessions of PRP monotherapy (no lost to follow-up) and all subjects completed 3 months follow-up after completion of treatment. Following PRP therapy, we found a statistically significant decrease in the mean mMASI scores at all visits compared to baseline (P < 0.05 for each visit) [Figure 1 and Table 1].
| Time | Mean | Std. Dev. |
|---|---|---|
| Baseline | 7.52 | 6.11 |
| 4 week | 5.75 | 4.68 |
| 8 week | 4.32 | 3.88 |
| 12 week | 3.04 | 3.44 |
| 5.15 | 4.84 (P = 0.02) |
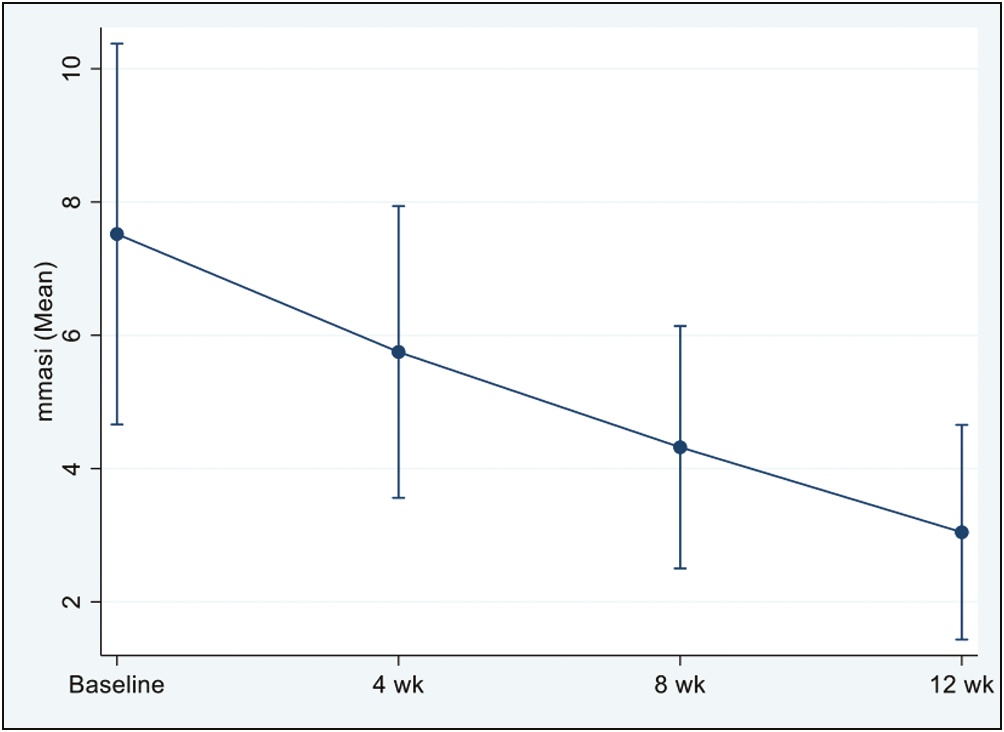
- Mean mMASI scores at all visits compared to baseline (P < 0.05 for each visit)
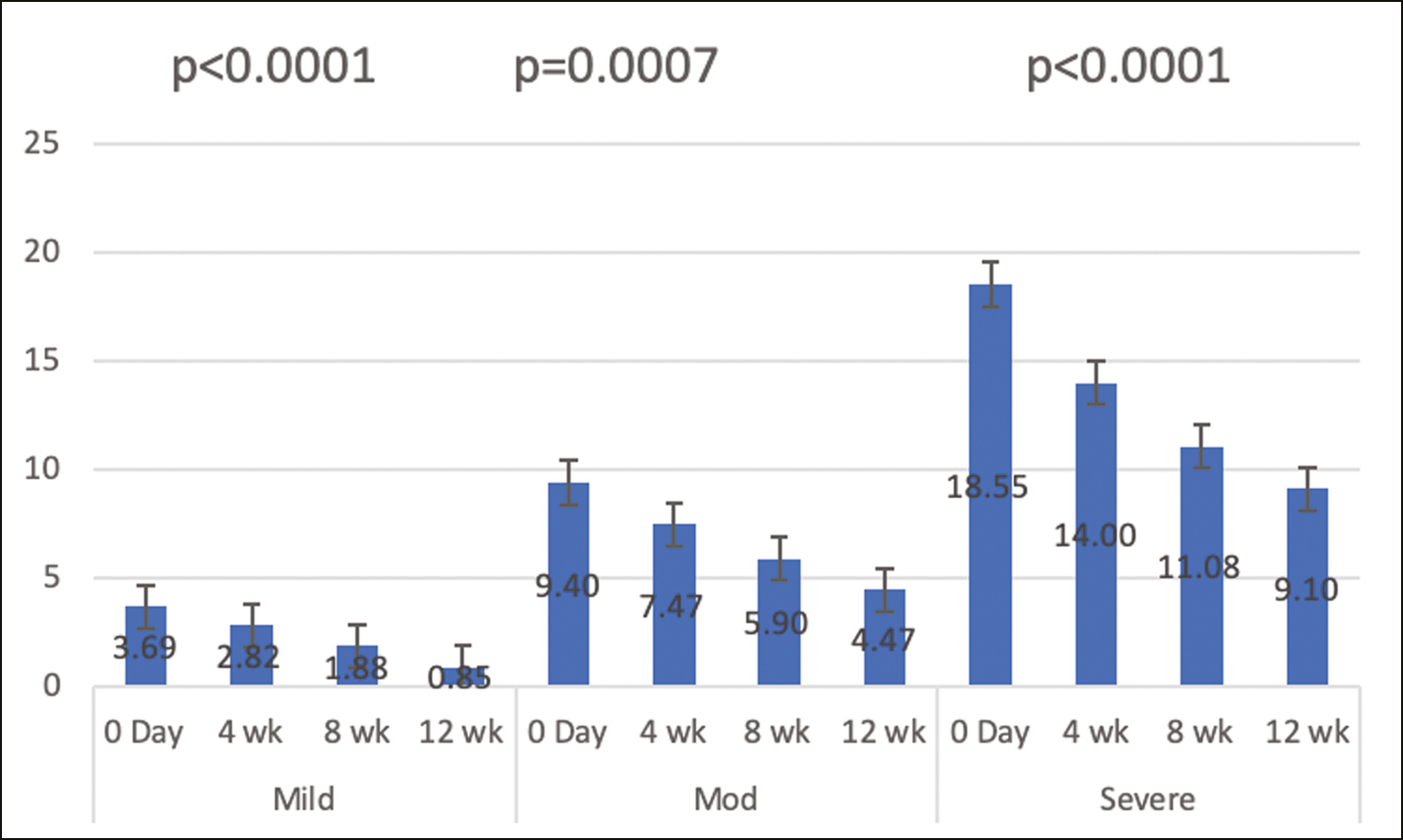
After classification of severity of melasma based on mMASI, it was found that significant reduction in melasma was seen at the two poles (mild and severe cases) at the end of treatment (P < 0.05) [Figure 2].
- Classification of severity of melasma based on mMASI, it was found that significant reduction in melasma was seen at the two poles (mild and severe cases) at the end of treatment (P < 0.05)
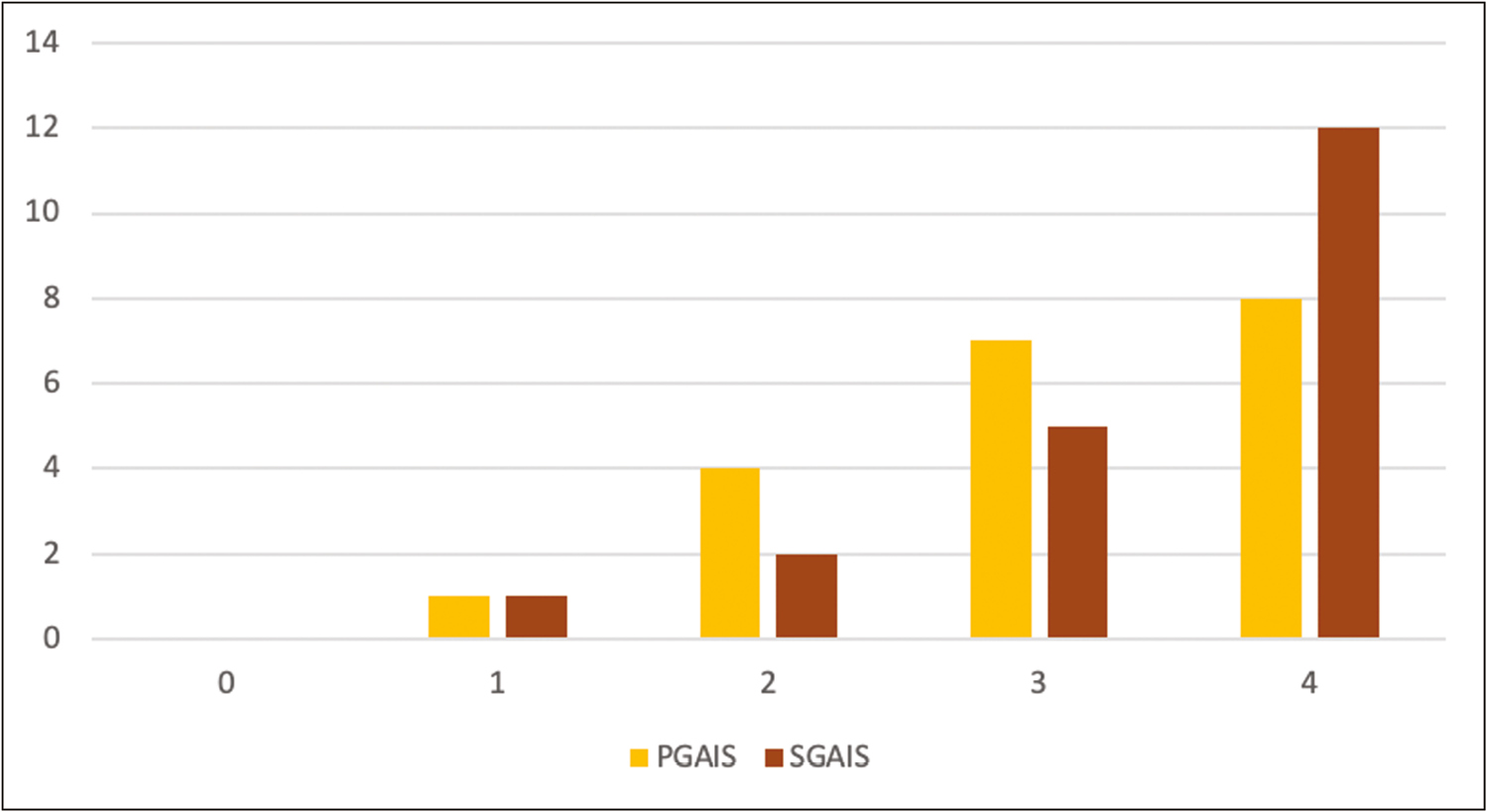
Clinician and subject assessment using PGAIS and SGAIS showed an overall esthetic improvement at the end of the study. Improvement of PGAIS from baseline was noticeable earlier as compared to SGAIS, which showed dramatic improvement at the end of the study [Figure 3]. No increase in pigmentation was seen in any of the subjects. All side effects reported by subjects were mild such as injection site pain, erythema, oedema, and bruising, and resolved spontaneously within a few hours of onset. Two patients had mild redness and watering of eyes postprocedure, for which they were evaluated by an ophthalmologist and symptoms related to adverse feature of the treatment were ruled out. Clinical changes in melasma at baseline and the end of 12 weeks are shown in mild case [Figure 4A and B], moderate case [Figure 4C and D] and severe case [Figure 4E and F], respectively.
- Improvement of PGAIS and SGAIS from baseline was noticeable earlier as compared to SGAIS, which showed dramatic improvement at the end of the study
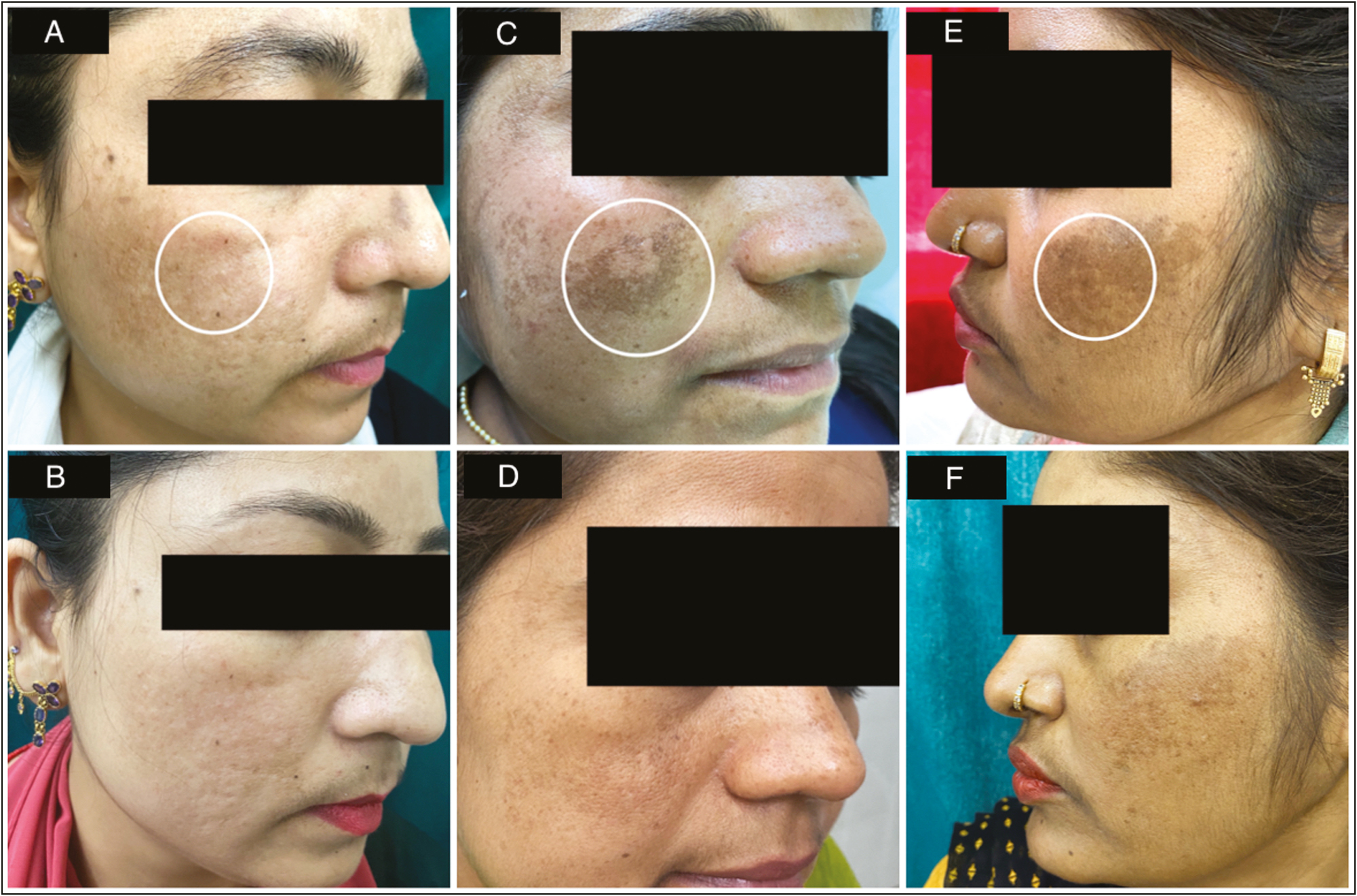
- (A) Skin appearance at baseline in a mild case of melasma showing area of dermoscopic marking in white circle. (B) Skin appearance at end of 12 weeks post treatment in a mild case of melasma (C) Skin appearance at baseline in a moderate case of melasma showing area of dermoscopic marking in white circle. (D) Skin appearance at end of 12 weeks post treatment in a moderate case of melasma. (E) Skin appearance at baseline in a severe case of melasma showing area of dermoscopic marking in white circle. (F) Skin appearance at end of 12 weeks post treatment in a severe case of melasma
Dermoscopic changes, which were seen in melasma included dots and globules, pseudoreticular pattern with arcuate structures and telangiectasia. Posttreatment patients showed resolution of pseudoreticular pattern in arcuate distribution (at 4 weeks) [Figure 5A and B], and dots and globules (12 weeks) [Figure 5C and D]. Telangiectasias were reduced in number and perifollicular clearing increased significantly at the end of treatment [Figure 5E and F].
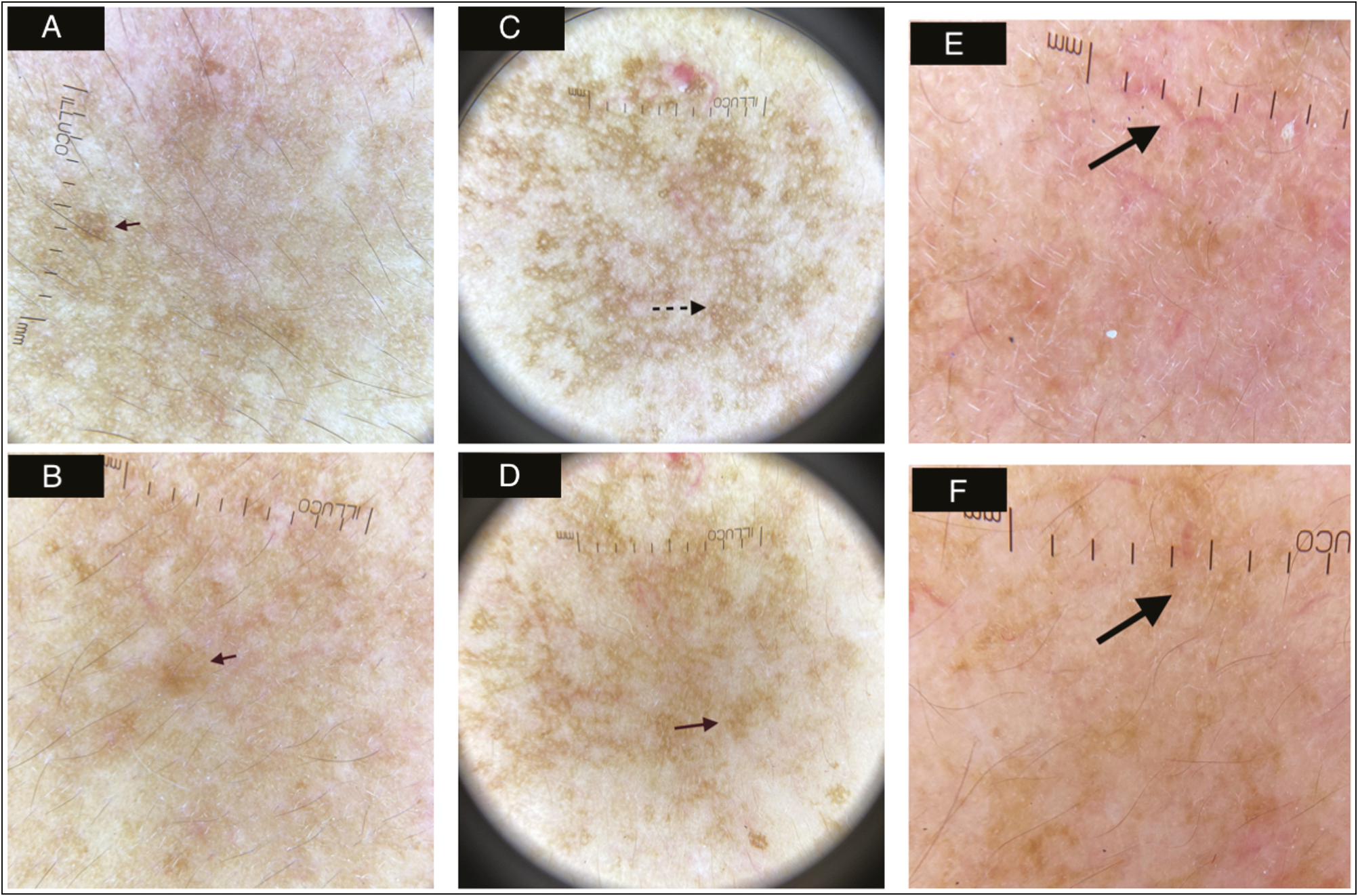
- (A) Skin appearance at baseline in a moderate case of melasma showing area of dermoscopic marking in white circle. (B) Skin appearance at end of 12 weeks posttreatment in a moderate case of melasma (C) Dermoscopy (X10, ILLUCO,Korea) at baseline showing dots and globules marked by black dotted arrow. (D) Dermoscopy (X10, ILLUCO,Korea)after 12 weeks of treatment showing resolution of dots and globules marked by red solid arrow. (E) Dermoscopy (X10, ILLUCO,Korea) at baseline showing telengiectasias marked by black solid arrow. (F) Dermoscopy (X10, ILLUCO,Korea) after 12 weeks of treatment showing resolution of telengiectasias marked by black solid arrow
DISCUSSION
Melasma, like other pigmentary dermatological conditions, has a varied clinical presentation and course of treatment. Apart from adversely affecting the morale of the affected person, it also affects the individual’s quality of life and social interactions. It has been found in various studies that PRP has potential in reducing pigmentation in melasma.[11] PRP essentially works by degranulation of the intracellular alpha-granules of the platelets to the release of various growth factors such as platelet-derived growth factor, epidermal growth factor (EGF), vascular endothelial growth factor, insulin growth factor, fibroblast growth factor, and TGF-β. It is found that TGF-β1 exerts its action by reducing MITF promoter activity, thereby reducing tyrosinase activity and melanin synthesis. EGF was seen to decrease melanin production in melanocytes by inhibiting prostaglandin E2 expression.[1213] These growth factors also stimulate collagen synthesis and epidermal regeneration, which reduces the chances of repigmentation in these areas.[14] However, there is a lack of substantial evidence of use of PRP as a monotherapy for melasma and its efficacy in treating melasma in Fitzpatrick skin types IV–VI.
We aimed to study PRP as a possible therapeutic option for the treatment of melasma showing significant decrease in mMASI scores from baseline in all severities of melasma. Most of our melasma cases had a mixed type of melasma, which are challenging to treat and require a more aggressive approach. Our study showed 77% reduction in mMASI in mild melasma, 52% reduction in mMASI in moderate melasma and 50% mMASI reduction in severe melasma. Severe melasma cases also benefit significantly from PRP treatment, a finding that was not confirmed with previous studies. In mild cases, PRP was found to be an excellent standalone treatment with very less relapse.
Limitations of the study were the loss of follow-up of few patients and requirement of a larger sample size. Mild pain during injections was the common but well tolerated side effect and lasted a maximum of up to 1 h.
Hofny et al.[15] treated melasma with three monthly PRP sessions and observed a statistically significant decrease in mean mMASI score (28% reduction in mMASI in mixed type of melasma). Amini et al.[16] reported two cases of melasma treated with three monthly sessions of PRP. They had lower percentage of improvement in case 2, which was attributed to the higher Fitzpatrick skin photo type (V) and the more resistant mixed type of melasma. However, in our study, we found a good improvement in moderate and severe melasma in Fitzpatrick skin type V–VI. We concluded that severe melasma in dark skin types require a good photoprotection and require larger number of sessions to yield the same results as compared to the lighter skin types (melasma in darker skin types is difficult to treat but is not resistant to treatment). Latest split-face pilot study conducted in Thailand, injected PRP and normal saline intradermally in 10 patients every 2 weeks for four sessions and showed a significant improvement in mMASI score.[17]
We assessed PRP as a standalone treatment in melasma in patients who had not been started on any other form of treatment. Though standalone treatment yielded excellent results in mild cases, combining PRP with other modes of treatment such as topical depigmenting agents, Nd-YAG laser or chemical peels in moderate and severe cases may achieve higher and faster clearance rates. As compared to other modalities of treatment where the chances of relapse are more, PRP was found to have lesser chances of relapse. Patients also had a higher satisfaction rate and reported an overall “better feel” of the face apart from resolution of the hyperpigmentation.
Dermoscopic evaluation of the results pretreatment and posttreatment showed a resolution of the pseudoreticular pigment pattern as well as a reduction in dots and globules. No loss of pigmentation of facial hair was observed as is seen with Nd-YAG laser.
PRP therapy can be a safe, effective, and new option in the armamentarium of melasma management. We recommend it as a standalone treatment as well as an adjuvant therapy in moderate to severe cases in dark skin types. We aim to highlight the importance of dermoscopic evaluation as an add on to clinical assessment in deciding the duration of treatment to reduce chances of relapse in the treatment of melasma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Platelet-rich plasma is a promising therapy for melasma. J Cosmet Dermatol. 2021;20:2431-6.
- [Google Scholar]
- Regression of melasma with platelet-rich plasma treatment. Ann Dermatol. 2014;26:401-2.
- [Google Scholar]
- Efficacy and safety of platelet-rich plasma in melasma: A systematic review and meta-analysis. Dermatol Therapy. 2021;11:1587-97.
- [Google Scholar]
- Comparative study between topical tranexamic acid alone versus its combination with autologous platelet rich plasma for treatment of melasma. J Dermatolog Treat. 2022;33:798-804.
- [Google Scholar]
- Platelet-rich plasma therapy in the treatment of recalcitrant melasma. Dermatol Surg. 2019;45:482-4.
- [Google Scholar]
- Clinical, dermoscopic, and histopathologic evaluation of vitamin C versus PRP, with microneedling in the treatment of mixed melasma: A split-face, comparative study. Dermatol Ther. 2022;35:e15239.
- [Google Scholar]
- Role of platelet-rich plasma therapy as an adjuvant in treatment of melasma. Dermatol Surg. 2022;48:429-34.
- [Google Scholar]
- Comparison of clinical results of oral tranexamic acid and platelet rich plasma therapies in melasma treatment. Dermatol Ther. 2022;8:e15499.
- [Google Scholar]
- Comparing the efficacy of platelet-rich plasma (PRP) versus tranexamic acid (4 mg/mL) as intradermal treatments of melasma. Age (years). 2021;24:23-94.
- [Google Scholar]
- Efficacy of the combination of Q-switched Nd: YAG laser and microneedling for melasma. J Cosmet Dermatol. 2021;20:75-6.
- [Google Scholar]
- The efficacy of energy-based devices combination therapy for melasma. Dermatol Ther. 2021;34:e14927.
- [Google Scholar]
- Micro needling vs micro needling combined with autologous topical platelet rich plasma in the treatment of melasma: A prospective randomized comparative study. Int J Res. 2022;8:78.
- [Google Scholar]
- Platelet-rich plasma is a useful therapeutic option in melasma. J Dermatolog Treat. 2019;30:396-401.
- [Google Scholar]
- Response to intradermal autologous platelet rich plasma injection in refractory dermal melasma: Report of two cases. J Health Transl Med. 2015;18:1-6.
- [Google Scholar]
- Platelet-rich plasma treatment for melasma: A pilot study. J Cosmet Dermatol. 2020;19:1321-7.
- [Google Scholar]





