Translate this page into:
Intralesional immunotherapy of cutaneous warts using tuberculin purified protein derivative and vitamin D3 – A randomized comparative study
*Corresponding author: Harris Ishtiyaq Shaafie, Department of Dermatology, Eras Lucknow Medical College and Hospital, Lucknow, Uttar Pradesh, India. harris.shaafie@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shaafie HI, Koti VR, Saxena K, Shukla P. Intralesional immunotherapy of cutaneous warts using tuberculin purified protein derivative and vitamin D3 – A randomized comparative study. J Cutan Aesthet Surg. 2025;18:86-91. doi: 10.25259/jcas_178_23
Abstract
Objectives:
Numerous studies have been conducted on the clinical efficacy of immunotherapeutic agents in the treatment of cutaneous warts showing variable results. The present study aimed to compare the clinical efficacy and safety of intralesional tuberculin purified protein derivative (PPD) and Vitamin D3 therapy in recurrent and recalcitrant extra-genital cutaneous warts.
Material and Methods:
This study was conducted as a prospective, randomized, comparative, and single-blind study. A total of 104 patients were randomly distributed into two treatment groups: Group A (Tuberculin PPD, n = 53) and Group B (Vitamin D3, n = 51). Each patient in Group A received an intralesional injection of 0.1 mL tuberculin PPD (5 Tuberculin Units), while each patient in Group B received an intralesional injection of 0.2 mL Vitamin D3 (Cholecalciferol containing 120,000 IU). The injections were given at the initial visit (week 0) and after every 2 weeks for a maximum of four sessions (weeks 2, 4, and 6). The largest wart was selected for intralesional therapy. The categorization of clinical response was done based on the percent reduction in the size of warts into complete (appearance of normal skin), marked (>50% reduction), moderate (<50% reduction), and inadequate (no change in warts) responses. Adverse effects (if any) were recorded during each patient visit. The final response was evaluated at 6 months follow-up from the last treatment session.
Results:
Regarding the response of patients to tuberculin PPD therapy, out of a total of 53 patients, 40 (75.5%) showed a complete response. Regarding the response of patients to Vitamin D3 therapy, out of a total of 51 patients, 36 (70.6%) showed a complete response. However, the difference in the response to the treatment between the two groups was statistically insignificant (P = 0.402).
Conclusion:
Both intralesional tuberculin PPD and Vitamin D3 are effective and safe in the treatment of all recurrent and recalcitrant extra-genital cutaneous warts.
Keywords
Intralesional
Immunotherapy
Recalcitrant Vitamin D3
Tuberculin PPD
INTRODUCTION
Cutaneous warts, caused by the Human Papillomavirus (HPV), represent one of the most prevalent dermatological conditions worldwide. While often considered benign, the impact of these seemingly innocuous growths on individuals’ physical and psychological well-being should not be underestimated. Warts tend to disappear spontaneously, recur after an apparent total clearance, or remain recalcitrant to all therapies. In general, a wart is considered recalcitrant if all the available conventional treatments fail.1 In healthy individuals, two-thirds of cutaneous warts are often self-limiting and regress spontaneously within 2 years.2 The condition may resolve slowly and persist for many years, especially in adults.3 Therefore, the management of cutaneous warts occasionally requires multiple modes of treatment. As cutaneous warts involve host immunity for eradication, immunocompromised individuals exhibit an elevated susceptibility to recalcitrant cutaneous warts, likely due to impaired viral clearance mediated by cellular and humoral immune responses.4 Multiple studies have been conducted on the use of immunotherapeutic agents in the treatment of warts. However, there is still a lack of high-quality, Level-1 evidence for the efficacy of this treatment.5 Even with the same agent used in different studies, the reported efficacy varies widely, depending on the patient’s age, compliance, and immunocompetence. Mycobacterial antigens have been popular for intralesional treatment of warts. Specific inoculations studied for this purpose include tuberculin purified protein derivative (PPD), Mycobacterium w vaccine, and Bacillus Calmette–Guerin. Of these three modalities, tuberculin PPD is by far the most frequently used for refractory cutaneous warts. The regression of warts from intralesional tuberculin PPD is associated with increased levels of interleukin (IL)-12 and IL-4, which contribute to cell-mediated immune reaction.6 Similarly, Vitamin D3 has numerous effects on cells within the immune system, including inhibition of B-cell proliferation, blocking B-cell differentiation, and immunoglobulin secretion.7 Vitamin D3 additionally suppresses T-cell proliferation and results in a shift from a Th1 to a Th2 phenotype.8 Furthermore, it affects T-cell maturation with a skewing away from the inflammatory Th17 phenotype and facilitates the induction of T regulatory cells.9 These effects result in decreased production of inflammatory cytokines (IL-17 and IL-21) and increased production of anti-inflammatory cytokines such as IL-10. Despite promising individual results for both, a paucity of studies directly compares the efficacy of intralesional tuberculin PPD and Vitamin D3 in treating extra-genital cutaneous warts. The present study is undertaken to gather more clinical evidence about the beneficial effects of tuberculin PPD and Vitamin D3 in cutaneous warts and compare the effectiveness of the two agents in recurrent and recalcitrant warts.
MATERIAL AND METHODS
This prospective, randomized, comparative, and single-blind study was approved by the Institutional Ethics Committee at Era’s Lucknow Medical College and Hospital, Human Research Cell, ERA University, Lucknow, India, and was carried out as per the guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from all patients before they participated in the study.
Participants
A total of 104 patients, both male and female, in the age group of 18–70 years with two or more extra-genital cutaneous warts attending the Dermatology Outpatient department and not responding to any previous conventional wart treatment were included in the study. Patients with genital, perianal, and mucosal warts on current systemic or topical treatment for warts, on immunosuppressant medications, and pregnant and lactating women were excluded from the study. Randomization was performed using the Sequentially Numbered Opaque Sealed Envelope technique to ensure allocation concealment and minimize selection bias. Fifty-three participants were assigned to Group A, designated for tuberculin PPD treatment, and 51 participants were assigned to Group B, designated for Vitamin D3 treatment.
Intervention
To ensure consistency, all treatment sessions were conducted by a single, trained dermatologist (H.I.S.). The patients in each group were blinded to the study interventions. Each patient in Group A received an intralesional injection of 0.1 mL of tuberculin PPD (5 tuberculin units), while each patient in Group B received an intralesional injection of 0.2 mL of Vitamin D3 (cholecalciferol containing 120,000 IU). The injections were given at the first visit (week 0) and every 2 weeks (weeks 2, 4, and 6) for a maximum of four sessions. The largest wart (target wart) was selected for intralesional therapy. To ensure patient comfort, a topical anesthetic, a mixture of 2.5% lidocaine and 2.5% prilocaine cream EMLA (eutectic mixture of local anesthetics (EMLA); AstraZeneca LP), was applied to the treatment area 60 min before the procedure. At each visit, the patients were evaluated for the size of the target wart, the appearance of any new lesions, and the adverse effects of the therapy. In the case of the resolution of the largest wart before the completion of injection therapy at 6 weeks (4th session), subsequent warts in the patient were considered for treatment based on their size. In the case of complete clearance of all warts before four injections, the treatment was stopped, and patients were followed up for any recurrence. All patients were advised against the use of any oral or topical medications specified in the exclusion criteria throughout the entire duration of the study.
Evaluation
Photographic documentation was acquired under controlled conditions at specific time points, including baseline (week 0), weeks 2, 4, and 6, and at 6 months following the last treatment session. The categorization of clinical response was done based on the percent reduction in the size of warts into complete (appearance of normal skin), marked (>50% reduction in size), moderate (<50% reduction in size), and inadequate (no change in warts) responses. Safety evaluations, including the documentation of adverse effects, were done during treatment, after treatment, and at every follow-up session.
Statistical analysis
All collected data were analyzed using IBM® Statistical Package for the Social Sciences® Version 25.0. (IBM Corp). Descriptive analysis was used for demographic data. The Chi-square test, independent samples t-test, and Mann–Whitney U-tests were used to compare the results between the two groups. Kaplan–Meier survival analysis was done to compare the resolution time between the two groups. Numerical variables were shown as mean ± Standard deviation of the mean and as a percentage of change. P < 0.05 was considered statistically significant.
RESULTS
Age and gender distribution of patients and the treatment given
As shown in Table 1, the age range of patients varied between 18 and 70 years in Group A (Tuberculin PPD) and between 18 and 65 years in Group B (Vitamin D3). The mean age for Group A patients was 28.09 ± 11.96 years, while it was 26.63 ± 9.46 years for Group B patients. However, the difference in the mean age of patients in both groups was statistically insignificant (P = 0.490). The table also shows that most of the patients in both groups were in the younger age group of up to 30 years (Group A: 75.5% and Group B: 68.6%). The study group of 104 patients included 71 males and 33 females, making the male-to-female ratio in the study 2.1:1. Out of these, 53 patients (51%) were randomized for Group A and received tuberculin PPD therapy, while 51 patients (49%) were randomized for Group B and received Vitamin D3 therapy. The male-to-female ratio was 3.8:1 in Group A and 1.3:1 in Group B, showing that male patients were dominant in both types of treatment. The proportion of males in Group A (79.2%) was significantly higher (P = 0.014) when compared to Group B (56.9%). However, the proportion of females in Group B was significantly higher than in Group A (P = 0.014).
| S. No | Age group | Group A-(PPD, n=53) (%) | Group B-(Vitamin D3, n=51) (%) | Total (n=104) (%) |
|---|---|---|---|---|
| 1. | ≤20 Years | 17 (32.1) | 20 (39.2) | 37 (35.6) |
| 2. | 21–30 Years | 23 (43.4) | 15 (29.4) | 38 (36.5) |
| 3. | 31–40 Years | 4 (7.5) | 11 (21.6) | 15 (14.4) |
| 4. | 41–50 Years | 6 (11.3) | 4 (7.8) | 10 (9.6) |
| 5. | ≥50 years | 3 (5.7) | 1 (2.0) | 4 (3.8) |
| Mean±SD (Range) | 28.09±11.96 (18–70 years) |
26.63±9.46 (18–65 years) |
27.38±10.78 |
“t”=0.692, P=0.490. PPD: Purified protein derivative, SD: Standard deviation
Clinical types of cutaneous warts in two treatment groups
As shown in Table 2, almost an equal number of different clinical types of cutaneous warts received either tuberculin PPD or Vitamin D3 therapy. The difference in the treatment of warts in both groups is statistically insignificant (P = 0.926).
| S. No | Type of wart | Group A (PPD, n=53) (%) | Group B (Vitamin D3, n=51) (%) | Total (n=104) (%) |
|---|---|---|---|---|
| 1 | Verruca filiformis | 3 (5.7) | 2 (3.9) | 5 (4.8) |
| 2 | Verruca palmaris | 3 (5.7) | 5 (9.8) | 8 (7.7) |
| 3 | Periungual warts | 4 (7.5) | 5 (9.8) | 9 (8.7) |
| 4 | Verruca plantaris | 11 (20.8) | 10 (19.6) | 21 (20.2) |
| 5 | Verruca plana | 17 (32.1) | 13 (25.5) | 30 (28.8) |
| 6 | Verruca vulgaris | 15 (28.3) | 16 (31.4) | 31 (29.9) |
(Chi-square)=1.366, P=0.926. PPD: Purified protein derivative
The overall response to treatment
As shown in Table 3, a total of 75.5% of cases in the tuberculin PPD group and 70.7% in the Vitamin D3 group showed complete resolution of warts. At the 6-month follow-up, complete resolution of warts was achieved in 75.5% (40/53) of patients in Group A (tuberculin PPD) and 70.6% (36/51) of those in Group B (Vitamin D3). Taking into account both the treatment groups, complete response of the warts after intralesional immunotherapy was observed in 76 out of 104 cases (73.1%). Further, analysis revealed a statistically insignificant difference in treatment response between the two groups (P = 0.402). Analyzing response by wart type [Table 4], verruca plantaris demonstrated the highest complete resolution rate at 81%, followed closely by verruca plana (80%) and verruca vulgaris (77.4%). Notably, no cases of verruca filiformis achieved a complete response. As shown in Table 5, comparing the response of different types of warts to tuberculin PPD (Group A) and Vitamin D3 (Group B) therapy, it was observed that verruca plana and verruca vulgaris [Figure 1a and b] showed around 94% response following tuberculin PPD therapy. In comparison, verruca palmaris and plantaris showed a 100% response following Vitamin D3 therapy [Figure 2a and b]. These findings suggest potential morphological specificity in treatment response for these modalities. The response of other clinical types to treatment was varied for the two therapies. The mean time taken for complete resolution of warts was 5.849 ± 0.280 weeks (median 6 weeks) in the tuberculin PPD group and 6.118 ± 0.260 weeks (median 6 weeks) in the Vitamin D3 group. Data evaluation showed that the time difference in the resolution of warts in the two groups was statistically insignificant (P = 0.552). The patient data showed that there was no recurrence of cutaneous warts after 3 months of intralesional therapy with tuberculin PPD or Vitamin D3. However, out of 40 patients in Group A and 36 patients in Group B who had achieved complete response at the end of treatment, 7 patients (17.5%) in Group A and 4 patients (11.1%) in Group B reported recurrence of warts at 6 months follow-up.
| S. No | Outcome | Group A (PPD, n=53) | Group B (Vitamin D3, n=51) | Total (n=104) (%) |
|---|---|---|---|---|
| 1 | Complete response | 40 (75.5) | 36 (70.6) | 76 (73.1) |
| 2 | Marked response | 5 (9.4) | 2 (3.9) | 7 (6.7) |
| 3 | Moderate response | 4 (7.5) | 4 (7.8) | 8 (7.7) |
| 4 | Inadequate response | 4 (7.5) | 9 (17.6) | 13 (12.5) |
z=0.893, P=0.402. PPD: Purified protein derivative
| Type of wart | No. of cases | No. of cases showing complete response (%) |
|---|---|---|
| Verruca filiformis | 5 | 0 |
| Verruca palmaris | 8 | 5 (62.5) |
| Periungual warts | 9 | 6 (66.7) |
| Verruca vulgaris | 31 | 24 (77.4) |
| Verruca plana | 30 | 24 (80) |
| Verruca plantaris | 21 | 17 (81) |
| Total cases | 104 | 76 (73.1) |
| Type of wart | Group A (PPD, n=53) | Group B (Vitamin D3, n=51) | Statistical significance (Fisher exact test) “P” value | ||
|---|---|---|---|---|---|
| Total no. | No. resolved (%) | Total no. | No. resolved (%) | ||
| Verruca filiformis | 3 | 0 | 2 | 0 | 1.000 |
| Verruca palmaris | 3 | 0 | 5 | 5 (100) | 0.018 |
| Periungual warts | 4 | 3 (75) | 5 | 3 (60) | 1.000 |
| Verruca plantaris | 11 | 7 (63.6) | 10 | 10 (100) | 0.090 |
| Verruca plana | 17 | 16 (94.1) | 13 | 8 (61.5) | 0.061 |
| Verruca vulgaris | 15 | 14 (93.3) | 16 | 10 (62.5) | 0.083 |
PPD: Purified protein derivative
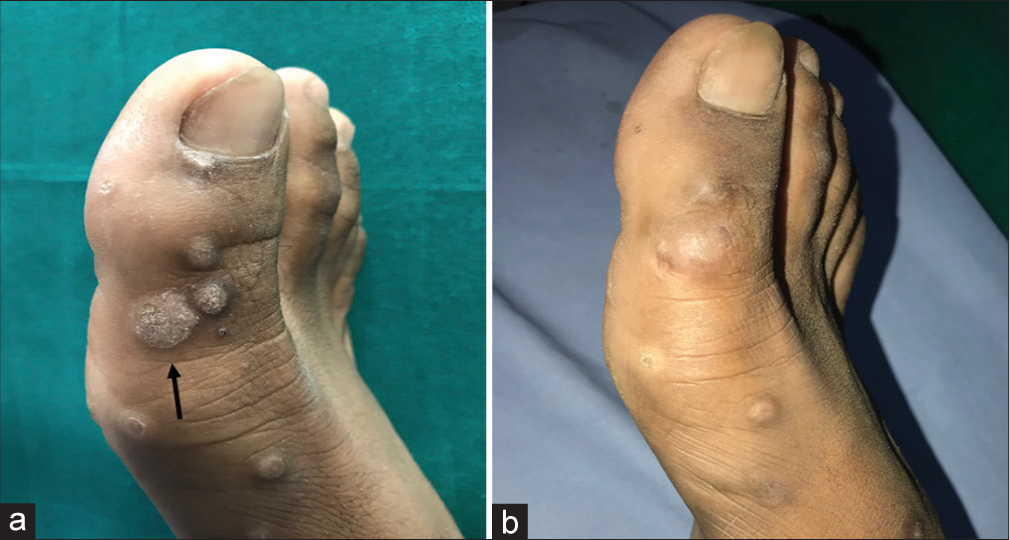
- (a) A 35-year-old male patient presenting with verruca vulgaris on the dorsal aspect of right great toe. The target lesion is indicated by the black arrow. On the first session (week 0) of treatment with intralesional tuberculin purified protein derivative (PPD). (b) At 6 months follow-up, the warts showed a marked response to treatment with intralesional tuberculin PPD.
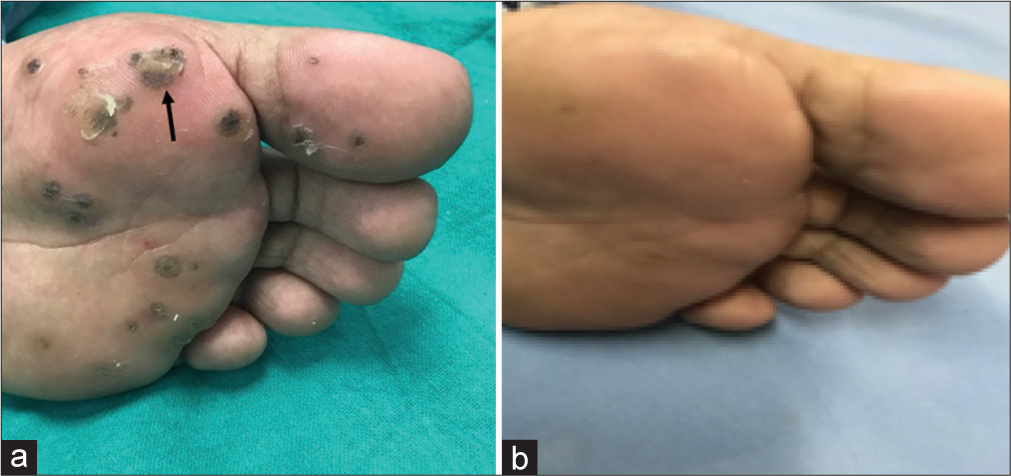
- (a) A 28-year-old male patient presenting with a cluster of verruca plantaris on the left foot. The target lesion is indicated by the black arrow. On the first session (week 0) of treatment with intralesional Vitamin D3. (b) At 6 months follow-up, the warts showed a complete response to treatment with intralesional Vitamin D3.
DISCUSSION
Due to the immune system’s pivotal role in restricting wart proliferation, intralesional immunotherapy has emerged as a potential therapeutic approach for patients with cutaneous warts. Its action is based on the activation of the patient’s immune system, which enhances the recognition and eradication of the virus, leading to the clearance of primary and distant warts. Very few studies have been conducted comparing the efficacy of intralesional tuberculin PPD with intralesional Vitamin D3 in extragenital cutaneous warts. The characteristic feature of young age observed in most of our patients is in agreement with the study on warts carried out by Berth-Jones and Hutchinson,10 who showed that more than 54% of their cases were in the young age group of 11–25 years. The high prevalence of cutaneous warts observed in the young age group of our patients seems related to their increased susceptibility to skin trauma secondary to physical activity, the effect of cosmetic procedures, close contact with wart patients, and overcrowding. The majority of patients in our study were male (68.3%), with a male-to-female ratio of 2.15:1. Johnson and Roberts11 showed in their study that cutaneous warts affect more males as compared to females. This seems related to the increasing trend of cosmetic concern among males. According to the study conducted by Liu et al.,12 males are physically more active compared to females and, therefore, at a higher risk of damage to the stratum corneum that serves as an entry point of HPV. Verruca vulgaris was the most commonly observed wart in the present study (29.9%), followed by verruca plana (28.8%) and verruca plantaris (20.2%). Periungual warts (8.7%), verruca palmaris (7.7%), and filiform warts (4.8%) accounted for the remaining smaller percentage of lesions. Similar results were obtained by Liu et al.12 and Ghadgepatil et al.13 regarding the frequency of verruca vulgaris. Comparing the complete response of our patients in the tuberculin PPD group, our results were in agreement with several studies conducted on the response of cutaneous warts to tuberculin PPD therapy.14-16 However, the response obtained in our patients was greater than in the study conducted by Shaheen et al.,17 who reported only a 60% complete response to both target and distant warts with tuberculin PPD therapy. Comparing the response to Vitamin D3 therapy using the same dosage and time interval of 2 weeks between sessions, our results are in agreement with Kavya et al.,18 who achieved a 78.6% complete response. However, using a higher dose of Vitamin D3 and a time interval of 3 weeks between sessions, Raghukumar et al.19 achieved complete clearance of warts in 90% of patients. The results suggest that there is a direct dose-response relationship between warts and Vitamin D3. We observed a response of 93.3% and 94.1% in verruca vulgaris and verruca plana, respectively, after tuberculin PPD therapy. The response obtained for verruca vulgaris was 90.9% in the study conducted by Akula et al.,20 which is closer to our results. However, our response for verruca plana was quite higher than that obtained by Fawzy et al.,21 Jaiswal et al.,22 and Saoji et al.23 who achieved a final response of 55%, 60%, and 67%, respectively, at the end of their study. Both verruca palmaris and verruca plantaris showed 100% response to Vitamin D3 therapy in our study. However, Kavya et al.18 obtained only 82.6% response to Vitamin D3 for both these types of warts. Using a higher dose of 0.4 mL Vitamin D3 at an interval of 3 weeks between sessions, Raghukumar et al.19 obtained a response of 100% for both verruca palmaris and verruca plantaris. A varied response to Vitamin D3 has been shown by other researchers such as Shaldoum et al.,24 Abou-Taleb et al.,25 and Aktaş et al.26 in palmoplantar warts who achieved a final response of 40%, 43.5%, and 80%, respectively. The difference in the response of warts to tuberculin PPD and Vitamin D3 therapy seems related to the individual variations and dose of the drug given. No adverse reactions were observed in patients of both study groups. However, mild pain and swelling at the injection site were observed in three patients with facial warts (5.88%) following Vitamin D3 therapy. Abou-Taleb et al.25 reported edema and erythema in 9.80% of patients following intralesional Vitamin D3 therapy and 5.66% of patients following intralesional tuberculin PPD therapy. One of the limitations of our study was the absence of a placebo control group in the study design. A placebo group would have provided a better baseline for comparison.
CONCLUSION
In light of the present study’s findings, intralesional tuberculin PPD and Vitamin D3 emerge as potential first-line treatments for the management of recurrent and recalcitrant extragenital cutaneous warts, given their evident effectiveness and safety profile. However, further large-scale comparative investigations may be necessary to elucidate potential variations in efficacy across specific wart morphologies.
Authors’ contributions
Conceptualization: Harris I Shaafie, VR Koti, Kshitij Saxena. Methodology: Harris I Shaafie, VR Koti, Kshitij Saxena. Validation: VR Koti. Formal analysis: Harris I Shaafie, VR Koti. Resources: Harris I Shaafie, Priyanka Shukla. Data curation: Harris I Shaafie. Original draft preparation: Harris I Shaafie. Review and editing: Harris I Shaafie, VR Koti, Kshitij Saxena. Visualization: VR Koti, Kshitij Saxena, Priyanka Shukla.
Ethical approval
The study was approved by the Institutional Ethics Committee at Era’s Lucknow Medical College and Hospital, Human Research Cell, ERA University, Lucknow, India, Approval No- IRB/R cell/105/2019, dated-April 2019 and was carried out as per the guidelines of the 1975 Declaration of Helsinki.
Declaration of patients consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- What's new in human papillomavirus infection. Curr Opin Pediatr. 2000;12:365-9.
- [CrossRef] [PubMed] [Google Scholar]
- British Association of Dermatologists' guidelines for the management of cutaneous warts 2014. Br J Dermatol. 2014;171:696-712.
- [CrossRef] [PubMed] [Google Scholar]
- Verrucas. Guidelines for management. Am J Clin Dermatol. 2000;1:143-9.
- [CrossRef] [PubMed] [Google Scholar]
- Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2003;3:CD001781.
- [CrossRef] [Google Scholar]
- Intralesional tuberculin for treatment of refractory warts. J Eur Acad Dermatol Venereol. 2005;19:515-6.
- [CrossRef] [PubMed] [Google Scholar]
- Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634-47.
- [CrossRef] [PubMed] [Google Scholar]
- 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974-80.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490-7.
- [CrossRef] [PubMed] [Google Scholar]
- Modern treatment of warts: Cure rates at 3 and 6 months. Br J Dermatol. 1992;127:262-5.
- [CrossRef] [PubMed] [Google Scholar]
- Skin conditions and related need for medical care among persons 1-74 years. United States, 1971-1974. Vital Health Stat 11. 1978;212:i-v, 1-72
- [Google Scholar]
- Epidemiology and clinical profile of cutaneous warts in Chinese college students: A cross-sectional and follow-up study. Sci Rep. 2018;8:15450.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicoepidemiological study of different types of warts. Dermatol Res Pract. 2016;2016:7989817.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of IL-12 serum level in patients with recalcitrant multiple common warts. Treated by intralesional tuberculin antigen. J Dermatolog Treat. 2014;25:264-7.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculin purified protein derivative immunotherapy in the treatment of viral warts. Indian J Drugs Dermatol. 2016;2:19-23.
- [CrossRef] [Google Scholar]
- Intralesional immunotherapy with tuberculin purified protein derivative (PPD) in recalcitrant wart: A randomized, placebo-controlled, double-blind clinical trial including an extra group of candidates for cryotherapy. J Dermatolog Treat. 2016;27:173-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2015;28:194-200.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of intralesional vitamin D3 in cutaneous warts: An open uncontrolled trial. J Cutan Aesthet Surg. 2017;10:90-4.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional vitamin D3 injection in the treatment of recalcitrant warts: A novel proposition. J Cutan Med Surg. 2017;21:320-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study of therapeutic efficacy of intralesional vitamin D3 versus intralesional purified protein derivative in the treatment of warts. IP Indian J Clin Exp Dermatol. 2018;4:226-31.
- [CrossRef] [Google Scholar]
- Intralesional antigen immunotherapy for the treatment of plane warts: A comparative study. Dermatol Ther. 2020;33:e13807.
- [CrossRef] [Google Scholar]
- Immunotherapy with PPD in treatment of warts: An open labelled study from western Uttar Pradesh. IP Indian J Clin Exp Dermatol. 2019;5:41-5.
- [CrossRef] [Google Scholar]
- Immunotherapy using purified protein derivative in the treatment of warts: An open uncontrolled trial. Indian J Dermatol Venereol Leprol. 2016;82:42-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative clinical study of the efficacy of intralesional MMR vaccine vs intralesional vitamin D injection in treatment of warts. J Cosmet Dermatol. 2020;19:2033-40.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional vitamin D3 versus intralesional purified protein derivative in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2019;9:13034.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional vitamin D injection may be an effective treatment option for warts. J Cutan Med Surg. 2016;20:118-22.
- [CrossRef] [PubMed] [Google Scholar]






