Translate this page into:
Microneedling combined with concentrated adipose-derived mesenchymal stem cells secretome alleviate facial skin aging features: A double-blind, randomized controlled trial
*Corresponding author: Lis Surachmiati Suseno, Department of Dermatology and Venereology, Dr. Cipto Mangunkusumo Hospital, Jakarta Pusat, Indonesia. lissuseno04@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Suseno LS, Japranata VV, Legiawati L, Sitohang IB, Yusharyahya SN, Liem IK, et al. Microneedling combined with concentrated adipose-derived mesenchymal stem cells secretome alleviate facial skin aging features: A double-blind, randomized controlled trial. J Cutan Aesthet Surg. doi: 10.25259/JCAS_78_2025
Abstract
Objectives:
Adipose-derived mesenchymal stem cell secretome is recognized for its anti-aging properties. This study investigated the optimal secretome administration for this purpose.
Material and Methods:
For 2 months at 2-week intervals, a total of 57 Indonesian women aged 35–59 years old were assigned to microneedling and one of these substrates: normal saline as control (C), unconcentrated secretome (S), or four-fold concentrated secretome (CS). The participants’ facial skin aging status was evaluated with the Janus-II measurement system and dermoscopy photoaging scale (DPAS). Statistical tests were used to analyze the data at the significance level of 0.05.
Results:
The CS group witnessed a significant decrement of ultraviolet spots at week 8 (P = 0.035). Intergroup comparison with control demonstrated spot reduction in subjects receiving CS at week 4 (P = 0.031) and porphyrin at week 6 (P = 0.007) and week 8 (P = 0.019). No marked discrepancies existed between the C and S groups in all Janus-II parameters. The total DPAS declined within all study groups (P < 0.001), but their average was comparable.
Conclusion:
CS treatment displayed positive responses for spots, porphyrin, and ultraviolet spots in senescent facial skin.
Keywords
Adipose-derived mesenchymal stem cells
Microneedling
Secretome
Skin aging
INTRODUCTION
As life expectancy substantially improves, the human population is moving toward the elevation of aging, which is a gradual process due to the diminution of living organisms’ structural and functional integrity.1,2 It is precipitated by the exposure of intrinsic and extrinsic factors during a lifetime. It negatively affects multiple organ systems, resulting in an increased incidence of degenerative diseases in older adults.2,3 Skin, as the outermost body organ, is heavily influenced by these factors, and cutaneous changes related to senescence directly determine cosmetic appearance, which has a major impact on individual quality of life.4,5 The distinctive features of skin aging, including dryness, thinning, wrinkles, and hyperpigmentation,4 have prompted the implementation of a healthy lifestyle, development of topical and systemic agents, invasive procedures, and extensive research to relieve such issues, thus potentially promoting industrial market growth in the field of esthetic dermatology.5
Latest studies and experiments have unveiled the efficacy of stem cells as auspicious contenders for therapeutic modality in regenerative medicine and cosmetic dermatology, given their capability of self-renewal and pluripotency to differentiate into other cell types. Adipose-derived mesenchymal stem cells (ADMSCs), which are aspirated from fat tissue and proliferated in vitro, have been broadly utilized to treat numerous cutaneous diseases (for instance, psoriasis, scleroderma, burn wounds, alopecia, and skin aging).6,7 Under optimal conditions, ADMSCs release a tremendous amount of immunomodulatory cytokines, growth and trophic factors, and matrix proteins into extracellular space, notably known as the secretome.8 For skin rejuvenation purposes, it regulates various biological processes that lead to fibroblast proliferation and suppression of apoptosis triggered by deleterious ultraviolet radiation (photoaging), collagen synthesis, and neovascularization.9
However, despite proper implantation of the cells, several reports have compromised stem cell-based therapy due to the risk of tumorigenesis, immune response evocation, and infection. On the other hand, secretome-based treatment is considered a safer and preferable option due to the lower possibility of contamination and uncomplicated maintenance, though repetitive applications are required to exhibit its virtue and higher manufacturing costs.8,10 Therefore, the knowledge of the optimal secretome delivery method for curative measures is essential to ensure the cost-effectiveness of its application. In the present study, we aimed to investigate the effect of unconcentrated and concentrated ADMSC secretome administered by microneedling for attenuating skin aging manifestations.
MATERIAL AND METHODS
The complete protocol of this study is visualized in Figure 1.
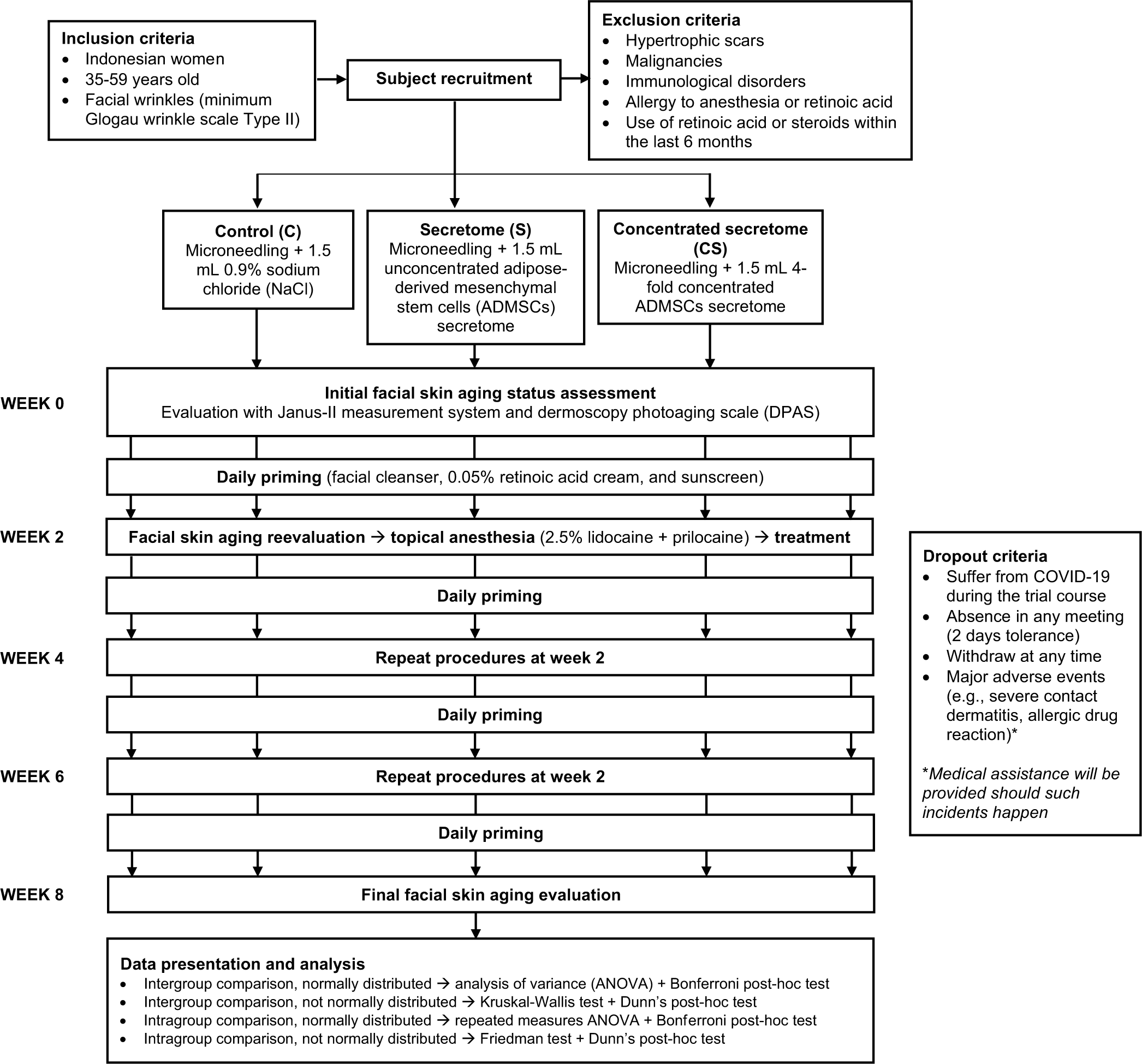
- Study protocol.
Selection and randomization of study participants
We recruited middle-aged Indonesian women (35–59 years old) with observable facial skin aging features (specifically the presence of wrinkles with the minimum Glogau wrinkle scale Type II) to participate in this study. Preliminary screening was commenced to exclude subjects meeting one of these criteria: history of hypertrophic or keloidal scars; malignant neoplasms; immunological disorders; allergic reaction to topical anesthetic agents or retinoic acid; and prior application of retinoic acid-containing products or immunosuppressants within the past 6 months. The remaining applicants subsequently consented to an 8-week-long clinical trial with an appointment biweekly. They were also informed regarding the dropout criteria during the study, including suffering from coronavirus disease 2019, failing to present in any designated meetings with 2-day tolerance, intentionally abstaining before the trial conclusion, and occurrence of major adverse events due to the received treatments (such as severe contact dermatitis or allergic drug reaction). Should such incidents happen, they would acquire medical assistance.
The eligible study participants were then equally randomized into one of three arms: The control (C) group that would receive 0.9% NaCl, the unconcentrated secretome (S) group, and the concentrated secretome (CS) group. The randomization was generated through a computer program, and it remained confidential to the research team and patients until the end of the trial.
ADMSCs secretome
The allogeneic, unconcentrated, and four-fold concentrated ADMSC secretome used in this clinical study were developed by Stem Cell Medical Technology Integrated Service Installation, Dr. Cipto Mangunkusumo Hospital, Jakarta. We selected the four-fold concentration for this study because our previous in vitro study with the unconcentrated conditioned medium revealed that it only contained ¼ of the minimal concentration of growth differentiation factor-9 (GDF-9) to exert a clinically significant effect.11 Their production adhered to Good Manufacturing Practice principles in a facility certified by the Indonesian Food and Drug Authority. Strict production procedures and quality control of raw materials were applied to get an equal amount of constituents. Each secretome had the same production batch and passed the sterility test for aerobic and anaerobic bacteria, fungi, endotoxins, and Mycoplasma. The total protein concentration was measured by Bradford protein colorimetric assay and spectrophotometry under 595 nm wavelength. Identification of the protein constituents was then done using Luminex multiplex assay and enzyme-linked immunosorbent assay, which include growth factors beta nerve growth factor (β-NGF), epidermal growth factor (EGF), fibroblast growth factor-4 (FGF-4), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), platelet-derived growth factor AB (PDGF-AB), growth differentiation factor-9 (GDF-9), interleukins (IL-6, IL-10), interferon-gamma (IFN-γ), bone morphogenetic proteins (BMP-2), BMP-7, and tumor necrosis factor-alpha. The final products were kept in a freezer at −80°C to maintain stability and freshly thawed before use.
Clinical trial
The entire clinical trial was conducted at Dermatovenereology Clinic in our hospital for 8 weeks (from October 04, 2021 to December 10, 2021). To establish the baseline for our study, we initially assessed seven facial skin aging variables (pore, wrinkle, sebum, porphyrin, spots, tone, and ultraviolet [UV] spots) in the subjects utilizing the Janus-II measurement system. The precedent total dermoscopy photoaging scale (DPAS) was also calculated, with which we looked for the existence of 11 dermoscopic features (yellowish discoloration, skin atrophy/white lines, lentigo, hypo-hyperpigmented macules, telangiectasia, yellowish papules, senile comedones, actinic keratosis, deep wrinkles, superficial wrinkles, and criss-cross wrinkles) on four facial areas (forehead, left cheek, right cheek, and mandible) and gave one point for each finding, giving the maximum total DPAS possible of 44. Afterward, the participants were directed to perform a daily priming routine at home with facial cleanser, 0.05% retinoic acid cream, and sunscreen with a sun protection factor of 45.
On the following appointment (week 2), their skin aging status was reevaluated with the same methods. They were then subjected to topical anesthesia using 2.5% lidocaine + 2.5% prilocaine cream for 30 min before receiving microneedling treatment with 36-fine needled dermapen and a depth of 1.5 µm until diffuse erythema was achieved. A total of 1.5 mL of the randomly assigned substances (C, S, or CS) were thereupon evenly distributed to the entire face. Succeeding the treatments, facial cleanser, sunscreen, and retinoic acid cream application were resumed after 3 days. A similar protocol would later be replicated in weeks 4 and 6. The final skin aging parameters were evaluated in the 8th week, and no ensuing treatments were given.
Data curation and analysis
Descriptive data concerning the characteristics of participants and their values of Janus-II skin aging parameters and total DPAS were recorded with the Statistical Package for the Social Sciences 23.0 software for Microsoft Windows. Normally distributed numerical data are expressed in mean ± standard deviation, or else they are reported in median (interquartile range). Intergroup analysis was conducted using one-way analysis of variance (ANOVA) or Kruskal–Wallis test, while intragroup analysis was performed with repeated ANOVA or Friedman test, followed by appropriate post hoc analysis. A significance level of 0.05 was used throughout the statistical analysis.
Data availability
The subjects’ data including pictures are properties of Dr. Cipto Mangunkusumo Hospital medical records and therefore subject to the Ministry of Health of the Republic of Indonesia regulation number 18 (2022) on Information System, prohibiting the authors to share individual participant data. Anonymized version of the data, however, could be obtained upon reasonable request to the corresponding author.
RESULTS
Participants in the clinical trial
During the recruitment period (from September 27, 2021, to October 01, 2021), 70 registrants were evaluated for eligibility; 10 were excluded for not meeting inclusion criteria. None of the prospective subjects refused to engage in the study. The remaining 60 participants were arbitrarily allocated into control (C; n = 20), unconcentrated secretome (S; n = 20), and concentrated secretome (CS; n = 20) groups. At the end of the trial, one patient from each experimental group was eliminated due to fulfilling the dropout criteria [Figure 2]. The profiles of subjects included in the final analysis are presented in Table 1.
| Parameter | Study group | ||
|---|---|---|---|
| C (n=19) | S (n=19) | CS (n=19) | |
| Age (years) | 48.32±1.40 | 48.74±1.40 | 47.05±1.68 |
| Daily sun exposure (hours) | 1.68±0.48 | 1.84±0.31 | 1.91±0.36 |
| Family history of wrinkles (%) | 15 (78.9) | 16 (84.2) | 14 (73.7) |
| Prior pregnancy (%) | 18 (94.7) | 17 (89.5) | 19 (100.0) |
| Hormonal contraception (%) | 4 (21.1) | 6 (31.6) | 5 (26.3) |
| Oral medication (%) | |||
| Hypertension | 2 (10.5) | 2 (10.5) | 4 (21.1) |
| Diabetes mellitus | 2 (10.5) | 0 (0.0) | 0 (0.0) |
| Dyslipidemia | 1 (5.3) | 2 (10.5) | 1 (5.3) |
| Glogau wrinkle scale (%) | |||
| Type I (no wrinkles) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Type II (wrinkles in motion) | 7 (36.8) | 8 (42.1) | 6 (31.6) |
| Type III (wrinkles at rest) | 12 (63.2) | 11 (57.9) | 12 (63.2) |
| Type IV (only wrinkles) | 0 (0.0) | 0 (0.0) | 1 (5.3) |
Values are presented in mean±standard deviation or frequency (percentage within group). CS: Concentrated secretome, C: Control, S: Unconcentrated secretome
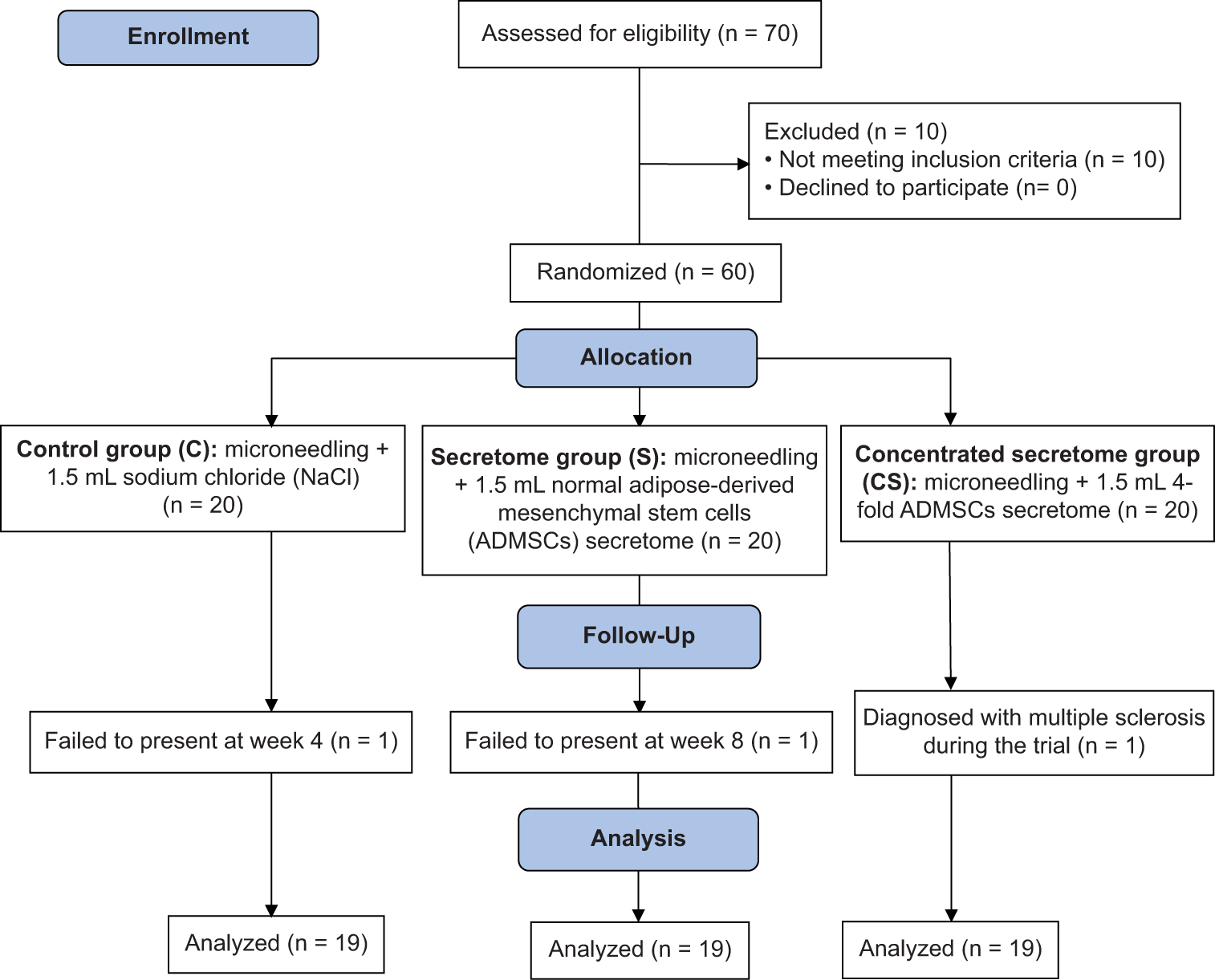
- Workflow of this randomized clinical trial.
Janus-II skin aging variables
We performed intragroup analysis of facial cutaneous senescence features assessed by the Janus-II measurement system, as shown in Table 2. A remarkable difference in UV spots was evident in those acquiring CS treatment (P = 0.035). Correspondingly, intergroup analysis at each observational week indicated that notable disparities were found on spots at week 4 (P = 0.031) and porphyrin at week 6 (P = 0.007) and week 8 (P = 0.019) [Table 3]. Furthermore, Bonferroni post hoc tests on variables of interest are displayed in Figure 3, which includes (a) Evaluation within CS group for UV spots at week 6 and week 8 (−7.00 [8.00] vs. −9.00 (6.50); P = 0.034); (b) intergroup evaluation of spots between C and CS at week 4 (12.11 ± 7.62 vs. 6.16 ± 6.65; P = 0.034); and (c) intergroup evaluation of porphyrin between C and CS at week 6 (23.42 ± 15.47 vs. 8.84 ± 11.46; P = 0.005) and week 8 (24.95 ± 20.35 vs. 9.37 ± 9.71; P = 0.017). On the contrary, comparisons of all skin aging parameters between unconcentrated secretome (S) and 0.9% NaCl (C) groups yielded no significance.
| Group | Time | Janus-II parameter | ||||||
|---|---|---|---|---|---|---|---|---|
| Pore | Wrinkle | Sebum | Porphyrin | Spots | Tone | UV spots | ||
| C | Week 2 | −0.05±5.18 | −2.00 (7.50) | −24.00 (62.50) | 20.63±16.75 | 10.79±7.79 | −19.89±3.91 | −3.16±9.39 |
| Week 4 | −0.74±5.37 | −4.00 (14.00) | −16.00 (55.50) | 20.58±14.57 | 12.11±7.62 | −20.05±3.99 | −3.89±8.35 | |
| Week 6 | −0.21±4.96 | −6.00 (7.50) | −6.00 (57.50) | 23.42±15.47 | 11.16±6.52 | −19.95±4.02 | −3.16±8.28 | |
| Week 8 | −1.74±5.39 | −2.00 (11.00) | −23.00 (66.50) | 24.95±20.35 | 10.16±6.08 | −20.37±3.67 | −5.37±7.67 | |
| P-value | NS | NS | NS | NS | NS | NS | NS | |
| S | Week 2 | −3.00 (4.50) | −4.53±9.09 | −12.00 (45.50) | 7.00 (9.50) | 8.58±7.54 | −22.00 (4.50) | −4.58±10.31 |
| Week 4 | −3.00 (6.50) | −2.21±12.33 | −16.00 (77.50) | 9.00 (22.50) | 10.63±6.68 | −21.00 (4.50) | −2.11±11.84 | |
| Week 6 | −4.00 (7.50) | −4.37±10.12 | 8.00 (80.00) | 13.00 (17.50) | 9.58±5.50 | −20.00 (4.00) | −2.68±11.78 | |
| Week 8 | −3.00 (5.00) | −5.16±9.15 | 0.00 (133.00) | 10.00 (21.00) | 8.74±7.58 | −22.00 (2.00) | −2.11±11.59 | |
| P-value | NS | NS | NS | NS | NS | NS | NS | |
| CS | Week 2 | −1.58±6.69 | −5.47±7.86 | −1.00 (87.00) | 13.21±13.66 | 6.21±6.77 | −21.58±3.31 | −7.00 (8.00) |
| Week 4 | −3.95±10.30 | −6.79±8.46 | −18.00 (44.00) | 13.00±10.20 | 6.16±6.65 | −21.68±3.43 | −7.00 (8.00) | |
| Week 6 | −1.63±6.40 | −5.11±6.58 | −20.00 (54.50) | 8.84±11.46 | 7.37±6.10 | −21.21±2.78 | −7.00 (8.00) | |
| Week 8 | −2.21±8.25 | −6.37±8.69 | −25.00 (38.00) | 9.37±9.71 | 6.84±5.80 | −21.47±2.48 | −9.00 (6.50) | |
| P-value | NS | NS | NS | NS | NS | NS | 0.035* | |
All values are shown in arbitrary units (a.u.). Normally distributed data are presented in mean±standard deviation, otherwise they are expressed in median (interquartile range). *: P≤0.05, NS: Not significant (P>0.05). CS: Concentrated secretome, UV: Ultraviolet, C: Control, S: Unconcentrated secretome
| Time | Group | Janus-II parameter | ||||||
|---|---|---|---|---|---|---|---|---|
| Pore | Wrinkle | Sebum | Porphyrin | Spots | Tone | UV spots | ||
| Week 2 | C | −0.05±5.18 | −3.37±7.24 | −24.00 (62.50) | 14.00 (23.00) | 10.79±7.79 | −19.89±3.91 | −3.16±9.39 |
| S | −3.05±5.76 | −4.53±9.09 | −12.00 (45.50) | 7.00 (9.50) | 8.58±7.54 | −21.53±3.42 | −4.58±10.31 | |
| CS | −1.58±6.69 | −5.47±7.86 | −1.00 (87.00) | 12.00 (9.50) | 6.21±6.77 | −21.58±3.31 | −6.47±7.76 | |
| P-value | NS | NS | NS | NS | NS | NS | NS | |
| Week 4 | C | −0.74±5.37 | −0.37±13.36 | −16.00 (55.50) | 20.00 (16.00) | 12.11±7.62 | −20.05±3.99 | −3.89±8.35 |
| S | −3.16±6.07 | −2.21±12.33 | −16.00 (77.50) | 9.00 (22.50) | 10.63±6.68 | −20.47±3.58 | −2.11±11.84 | |
| CS | −3.95±10.30 | −6.79±8.46 | −18.00 (44.00) | 13.00 (14.00) | 6.16±6.65 | −21.68±3.43 | −7.11±6.38 | |
| P-value | Ns | NS | NS | NS | 0.031* | NS | NS | |
| Week 6 | C | −0.21±4.96 | −6.00 (7.50) | −6.00 (57.50) | 23.42±15.47 | 11.16±6.52 | −19.95±4.02 | −3.00 (10.00) |
| S | −2.95±5.89 | −5.00 (14.00) | 8.00 (80.00) | 14.53±13.90 | 9.58±5.50 | −20.42±2.95 | −2.00 (15.00) | |
| CS | −1.63±6.40 | −7.00 (7.50) | −20.00 (54.50) | 8.84±11.46 | 7.37±6.10 | −21.21±2.78 | −7.00 (8.00) | |
| P-value | NS | NS | NS | 0.007** | NS | NS | NS | |
| Week 8 | C | −2.00 (7.50) | −1.79±7.99 | −23.00 (66.50) | 24.95±20.35 | 10.16±6.08 | −20.00 (5.50) | −5.00 (12.50) |
| S | −3.00 (5.00) | −5.16±9.15 | 0.00 (133.00) | 15.00±18.10 | 8.74±7.58 | −22.00 (2.00) | −3.00 (10.00) | |
| CS | −1.00 (8.00) | −6.37±8.69 | −25.00 (38.00) | 9.37±9.71 | 6.84±5.80 | −22.00 (2.50) | −9.00 (6.50) | |
| P-value | NS | NS | NS | 0.019* | NS | NS | NS | |
All values are shown in arbitrary units (a.u.). Normally distributed data are presented in mean±standard deviation, otherwise they are expressed in median (interquartile range). *: P≤0.05, **: P≤0.01, NS: Not significant (P>0.05). UV: Ultraviolet, CS: Concentrated secretome,C: Control, S: Unconcentrated secretome
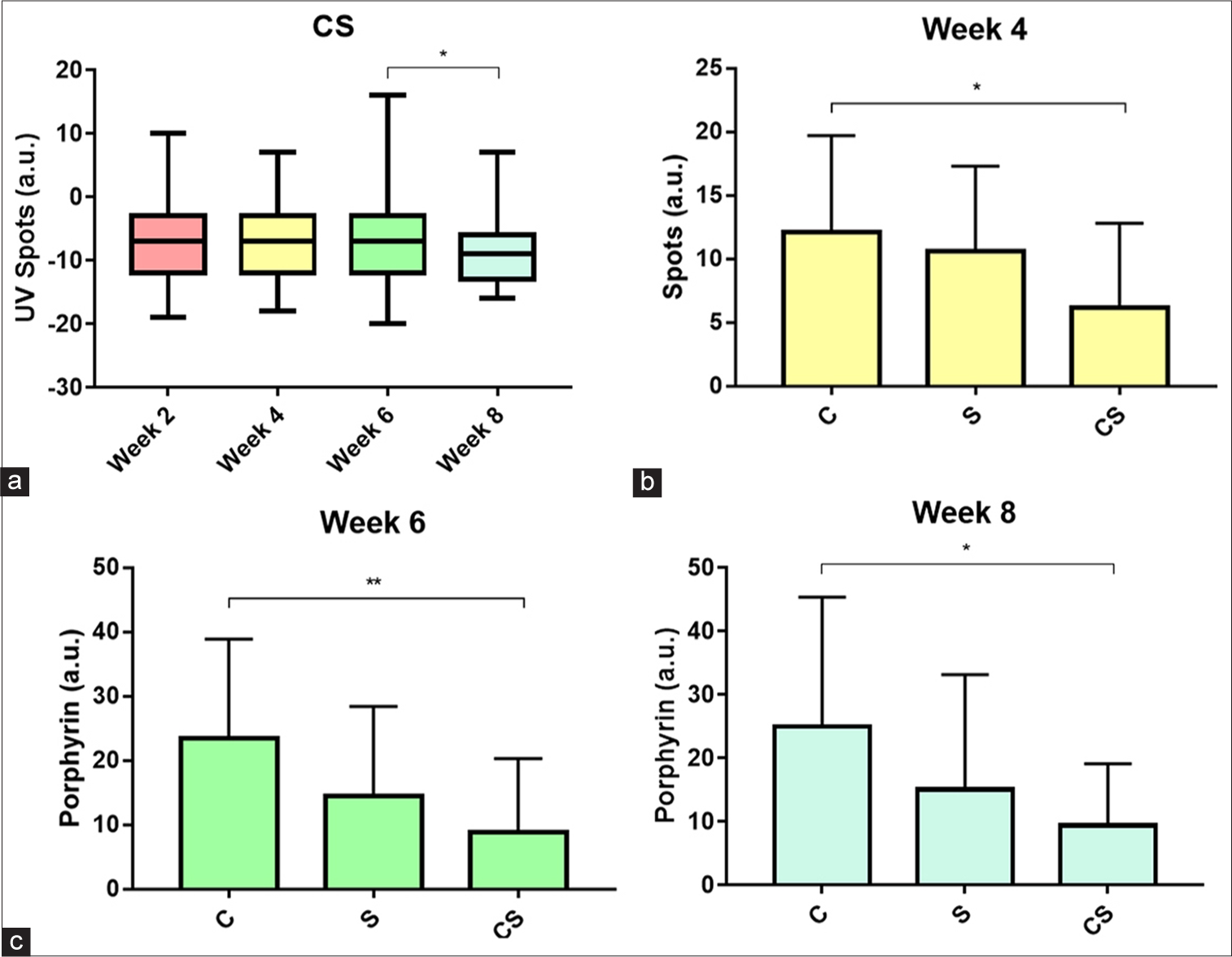
- Bonferroni post hoc analysis of Janus-II parameters with significant P-values. (a) Intragroup comparison of ultraviolet spots parameter in concentrated secretome group. (b) Intergroup comparison of spots parameter at week 4. (c) Intergroup comparison of porphyrin parameter at weeks 6 and 8. All values are in arbitrary units (a.u.). *: P ≤ 0.05; **: P ≤ 0.01, CS: Concentrated secretome, C: Control, S: Unconcentrated secretome.
Dermoscopy photoaging scale (DPAS)
Table 4 summarizes the total DPAS of all study groups during 8 weeks of trial. Statistically significant declines in total DPAS were noticed in all study groups at the final observation (week 8) compared to their respective initial threshold (week 2). As illustrated in Figure 4, total DPAS was decreased from 9.79 ± 1.51 to 5.58 ± 1.80 in C group (P < 0.001), from 10.74 ± 2.23 to 6.63 ± 2.83 in S group (P < 0.001), and from 11.00 (2.50) to 6.00 (2.50) in CS group (P < 0.001), even though the average DPAS was similar among the cohorts. A successive examination of dermoscopic [Figure 5] and clinical [Figure 6] features disclosed that hyperpigmentation and superficial wrinkles diminished remarkably, especially in experimental groups (S and CS).
| Time | Group | P-value | |||||
|---|---|---|---|---|---|---|---|
| C | S | CS | |||||
| M±SD | Med (IQR) | M±SD | Med (IQR) | M±SD | Med (IQR) | ||
| Week 2 | 9.79±1.51 | 10.00 (1.50) | 10.74±2.23 | 11.00 (3.00) | 10.74±2.81 | 11.00 (2.50) | NS |
| Week 4 | 8.58±1.43 | 8.00 (1.50) | 10.00±2.26 | 10.00 (2.50) | 9.47±3.29 | 8.00 (3.00) | NS |
| Week 6 | 6.63±1.95 | 6.00 (3.00) | 8.00±2.45 | 8.00 (2.50) | 7.95±3.12 | 7.00 (3.00) | NS |
| Week 8 | 5.58±1.80 | 5.00 (2.50) | 6.63±2.83 | 6.00 (5.00) | 6.42±3.15 | 6.00 (2.50) | NS |
| P-value | <0.001* | <0.001* | <0.001* | ||||
M: Mean, SD: Standard deviation, Med: Median, IQR: Interquartile range, CS: Concentrated secretome, C: Control, S: Unconcentrated secretome, DPAS: Dermoscopy photoaging scale, *: P≤0.05, NS: Not significant (P>0.05)
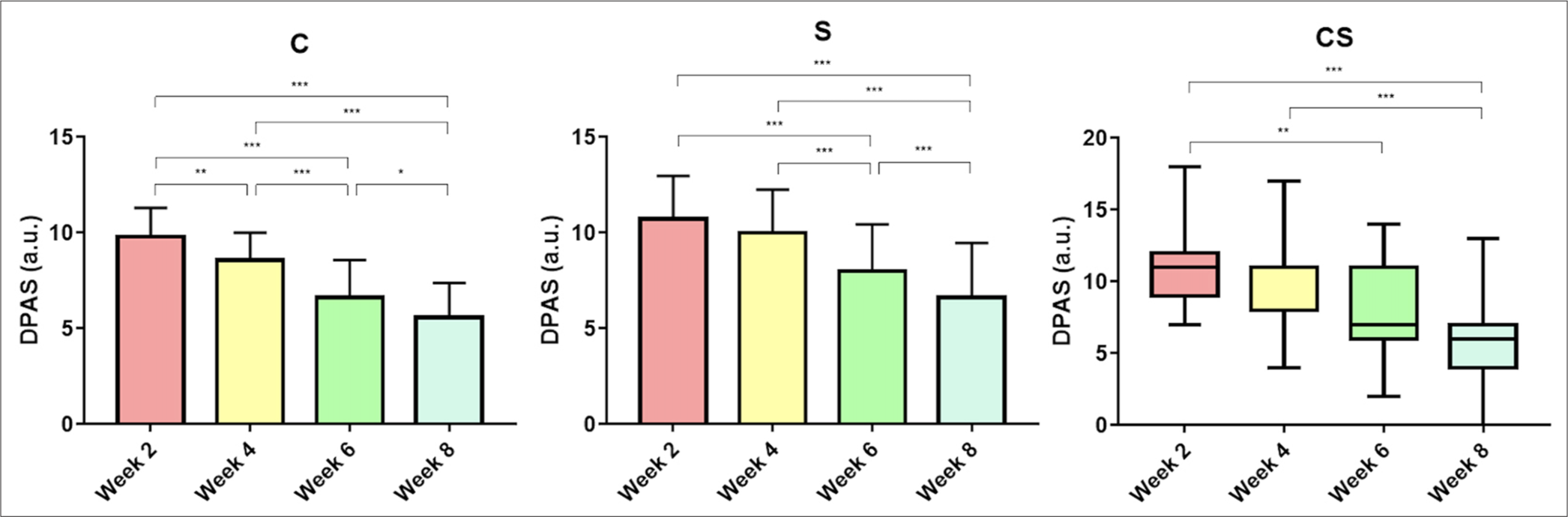
- Intragroup comparison of dermoscopy photoaging scale within all study groups. All values are in arbitrary units (a.u.). *: P ≤ 0.05; **: P ≤ 0.01; ***: P ≤ 0.001. C: Control, S: Unconcentrated secretome, CS: Concentrated secretome. DPAS: Dermoscopy photoaging scale
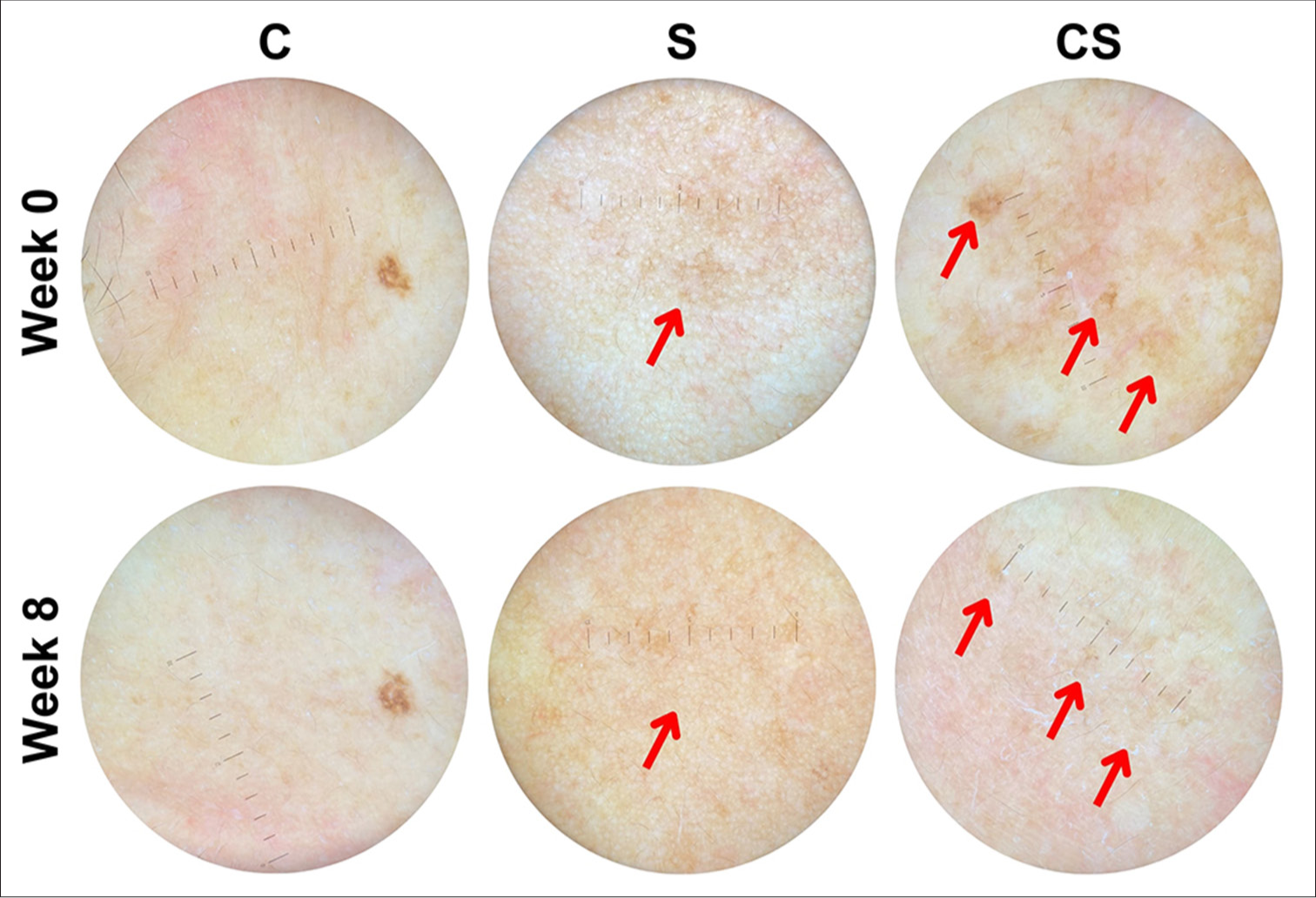
- Dermoscopic representation of hyperpigmented macules (one of the observed skin aging signs) in subjects receiving 0.9% NaCl control (C), unconcentrated secretome (S), and concentrated secretome (CS) under ×10 magnifying power. Note the qualitative reduction of hyperpigmentation (
 ).
).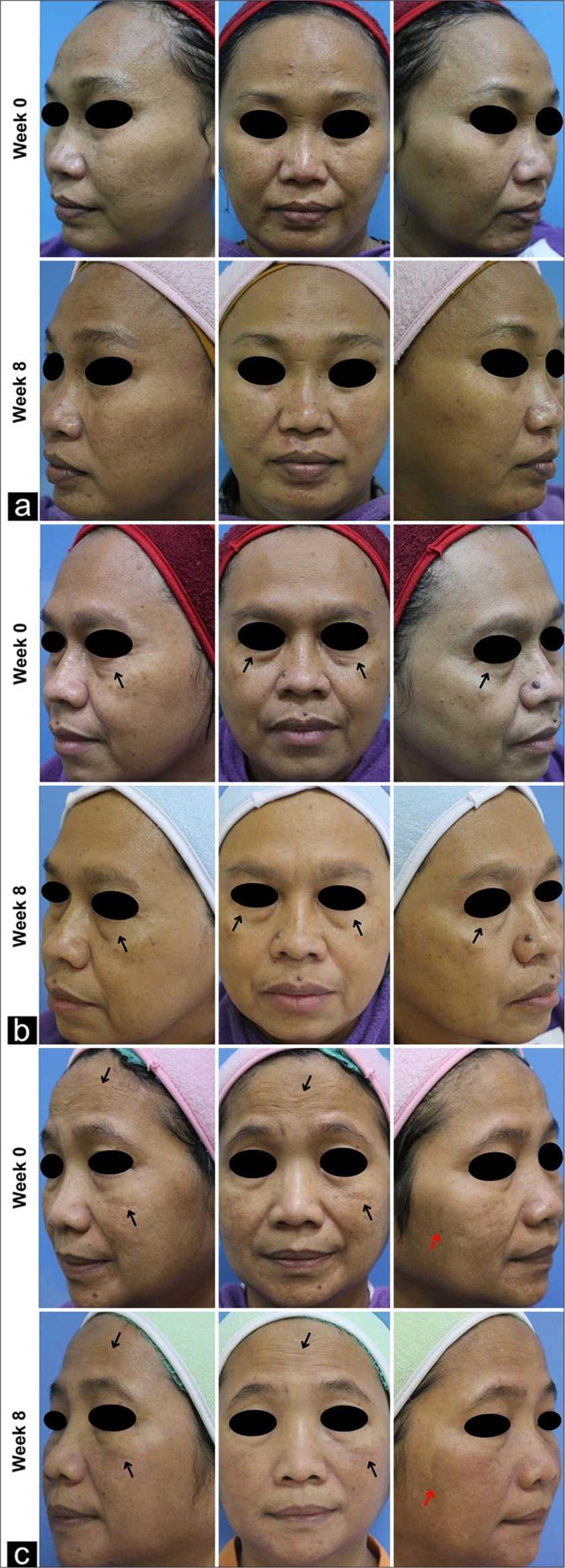
- Clinical representation of skin aging features in subjects treated with (a) control (C), (b) unconcentrated secretome (S), and (c) concentrated secretome (CS). Note the qualitative diminution of hyperpigmentation (
 ) and observable wrinkles (
) and observable wrinkles ( ).
).DISCUSSION
In the effort to combat the contemporary skin aging trend, multiple approaches in esthetic dermatology have been addressed to restore the alterations, ranging from topical products (retinoic acid, ascorbic acid, glycolic acid, and peptides), energy-based devices (ablative or non-ablative laser, radiofrequency, and ultrasound), and dermal fillers or injectables.12 While the previous studies have successfully demonstrated the effectiveness of those substrates and modalities, advancements in regenerative medicine offer cell-based and cell-free therapies derived from mesenchymal stem cells (MSCs) as alternatives.13 The secretory components of MSCs, namely secretome, contain cytokines, growth factors, and extracellular vesicles capable of skin rejuvenation through complex paracrine signaling responsible for collagen synthesis and rearrangement, hence ameliorating wrinkles in aged skin.14,15 Moreover, MSCs secretome preserves fibroblasts from UV-induced apoptosis by scavenging free radicals14 and inhibits melanogenesis through downregulation of tyrosinase-related protein 1 (TYRP1) and tyrosinase15,16 – properties that protect against hyperpigmentation.
This present randomized clinical trial further supported the ADMSC’s role in diminishing the signs of skin aging. We utilized the microneedling technique combined with the topical administration of the products (0.9% NaCl, unconcentrated or concentrated ADMSCs secretome), creating microtunnels into the dermis (±1.5 µm from the skin surface) in an organized manner, thus enabling efficacious transdermal substrates transfer, especially for large-sized molecules (>500 Dalton) to penetrate the skin barrier.17 Initial characterization of the study participants showed that the mean age and daily sunlight exposure, and the distribution of those with a family history of wrinkles, former pregnancies, receiving hormonal contraceptives, oral medications for systemic diseases, and Glogau wrinkle scale (for weighing the severity of wrinkles and photoaging) were comparable among experimental groups, warranting their balanced state at the beginning of the trial. In addition, obtained data from week 2 served as the baseline in this study (instead of week 0) to abolish the skin priming influence, as retinoic acid may have several consequences for skin aging variables.18,19 Similar to their base profiles, the discrepancies of all Janus-II parameters in the C, S, and CS groups were negligible at week 2.
We found that the absolute values of skin aging variables generally decreased during the clinical trial, although statistically insignificant. The exception goes to the sebum and UV spots parameters, which exhibit relatively high variances of the former and a marked diminution of the latter in the CS group at week 8. The variation of skin sebum may be explained by sebocyte activity being a complicated interplay of genetics (particularly WNT10A gene expression),20 dietary,21 ethnicity, age, and biological sex22 factors, even under the controlled experimental environment. As for the UV spot decrement observed in those treated with CS, it may be inferred that the amount of trophic factors in CS is sufficient to exert its anti-melanogenic effect through TYRP1 and tyrosinase silencing, as mentioned earlier.15,16 Intergroup comparisons also disclosed the potential of CS in reducing spots (at week 4) and porphyrin (starting at week 6), though the exact mechanism of the latter remains unknown. A probable explanation of this phenomenon is that the concentrated secretome, augmented by the retinoic acid application during skin priming, regulates the sebum production, which subsequently interferes with the primary porphyrin-synthetizing commensal bacterium in the skin (Cutibacterium acnes).23
Apart from the Janus-II measurement system, this study also adapted modified DPAS from Isik et al. that aimed to assess extrinsic skin aging features using a dermoscope due to several signs not visible to the unaided eye.24 Treatment with microneedle alone turned out to have an effect of total DPAS attenuation, specifically superficial wrinkles, as evidenced by the notable reduction of which at week 8 compared to week 2 in the control group. This may be due to microtrauma created by microneedling, which induces dermal remodeling through stimulation of fibroblasts in producing collagen and elastin.25 The addition of unconcentrated or concentrated secretome did not seem to augment the microneedling effect in decreasing total DPAS. Nevertheless, the frequency of application, volume, and concentration of ADMSCs secretome must be considered, as they may be insufficient to generate efficacies in this current trial. DPAS is also a subjective instrument that depends on the evaluator and cannot distinguish cutaneous senescence qualitatively. For example, hyperpigmented macules were partially improved in some participants upon treatment with microneedling and ADMSCs secretome. Yet, they contributed one point to total DPAS as the macules were still apparent, regardless of their quality.
In spite of our results, we are aware that the small sample number and the brief duration of this study (8 weeks) may hinder the extrapolation to the larger scale and longer term. However, our findings may provide insights for manufacturers to produce concentrated stem cell products since treatment with unconcentrated secretome had no substantial benefits in alleviating skin aging compared to the control group. Furthermore, we encourage clinicians and researchers to conduct parallel studies investigating the issues with various ADMSC secretome concentrations and volumes to discover the optimal and cost-effective treatment for skin rejuvenation. While we harnessed a practical Janus-II measurement system to evaluate facial skin aging features simultaneously in this study, assessment of such parameters utilizing other devices with high validity and reliability (e.g., Mexameter and Chromameter to quantify melanin and erythema values)26 may also be a viable option for further studies.
CONCLUSION
Microneedling combined with concentrated ADMSC secretome application upon facial aging skin may improve spots, porphyrin, and UV spots after 8 weeks of treatment. Future research is prompted to uncover the minimum concentration and volume required for skin rejuvenation, as it would minimize the production cost and provide advantages to both manufacturers and consumers.
Acknowledgments:
Our sincere gratitude goes to Prof. Ismail Hadisoebroto Dilogo, MD, PhD, and the affiliated institution for providing the ADSCMs secretome used in this study. We also offer thanks to Sekar Ayu Kinanti Tistia, MD; Witri Widiati Ningrum, MD; Karin Rachmani, MD; Riris Asti Respati, MD; Vashty Amanda Hosfiar, MD; Agung Mohamad Rheza, MD; Firman Parrol, MD; Joses Saputra, MD for assisting with the clinical trial; Prof. Muchtarrudin Mansyur, MD, PhD for insights in statistical analysis.
Authors’ contributions:
Lis Surachmiati Suseno contributed to the conceptualization of the research design, conducting the trial and data curation, and writing/editing the manuscript. V. V. Japranata contributed to the data analysis and visualization, as well as writing the manuscript. L. Legiawati, I. B. S. Sitohang, and S. N. Yusharyahya participated in the writing/editing of the manuscript. I. K. Liem, J. A. Pawitan, I. S. Putri, and T. Kurniawati participated in the quality control of the trial and editing of the manuscript.
Ethical approval:
This clinical study was conducted according to the principles of the Declaration of Helsinki (2013), and the study procedure was designed based on the Consolidated Standards of Reporting Trials (CONSORT) guideline. As for the ethical matter, this study obtained prior approval from the Health Research Ethics Committee of the Faculty of Medicine, University of Indonesia on August 09, 2021, with letter number ND-629/UN2.F1/ETIK/PPM.00.02/2021, and Dr. Cipto Mangunkusumo Hospital on September 22, 2021, with letter number LB.02.01/2.6.1/0921/2021.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Financial support for this study was sourced from the research grant provided by the National Research and Innovation Agency (Badan Riset dan Inovasi Nasional) with letter number Ristek/BRIN 661.328.460 in 2021.
References
- Lifespan and Healthspan: Past, present, and promise. Gerontologist. 2015;55:901-11.
- [CrossRef] [PubMed] [Google Scholar]
- Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology. 2021;22:165-87.
- [CrossRef] [PubMed] [Google Scholar]
- Physiology, aging In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. Available from: www.ncbi.nlm.nih.gov/books/NBK556106 [Last accessed on 2025 Apr 01]
- [Google Scholar]
- Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018;27:729-38.
- [CrossRef] [PubMed] [Google Scholar]
- Skin ageing: Pathophysiology and current market treatment approaches. Curr Aging Sci. 2020;13:22-30.
- [CrossRef] [PubMed] [Google Scholar]
- Rise of stem cell therapies in aesthetics. Clin Dermatol. 2022;40:49-56.
- [CrossRef] [PubMed] [Google Scholar]
- The use of stem cells in aesthetic dermatology and plastic surgery procedures. A compact review of experimental and clinical applications. Postepy Dermatol Alergol. 2017;34:526-34.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int J Mol Sci. 2020;21:7038.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal stem cell secretome for dermatology application: A review. Clin Cosmet Investig Dermatol. 2021;14:1401-12.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cell secretome, regeneration, and clinical translation: A narrative review. Ann Transl Med. 2021;9:70.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of conditioned medium of umbilical cord-derived mesenchymal stem cells as a culture medium for human granulosa cells: An experimental study. Int J Reprod Biomed. 2021;19:1037-44.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20:2126.
- [CrossRef] [PubMed] [Google Scholar]
- Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan. Regen Ther. 2022;21:73-80.
- [CrossRef] [PubMed] [Google Scholar]
- Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther. 2020;11:264.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: A randomized, controlled, blinded split-face study. Ann Dermatol. 2014;26:584-91.
- [CrossRef] [PubMed] [Google Scholar]
- Application of adipose-derived stem cells in photoaging: Basic science and literature review. Stem Cell Res Ther. 2020;11:491.
- [CrossRef] [PubMed] [Google Scholar]
- Microneedles in action: Microneedling and microneedles-assisted transdermal delivery. Polymers (Basel). 2022;14:1608.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular basis of retinol anti-ageing properties in naturally aged human skin in vivo. Int J Cosmet Sci. 2017;39:56-65.
- [CrossRef] [PubMed] [Google Scholar]
- Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019;36:392-7.
- [CrossRef] [PubMed] [Google Scholar]
- Gene variants associated with acne vulgaris presentation and severity: A systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary patterns associated with sebum content, skin hydration and pH, and their sex-dependent differences in healthy Korean adults. Nutrients. 2019;11:619.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of ethnicity, gender and age on the amount and composition of residual skin surface components derived from sebum, sweat and epidermal lipids. Skin Res Technol. 2014;20:97-107.
- [CrossRef] [PubMed] [Google Scholar]
- Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms. 2021;9:303.
- [CrossRef] [PubMed] [Google Scholar]
- Development of skin aging scale by using dermoscopy. Skin Res Technol. 2013;19:69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of ageingthe role of micro-needling in neck and cleavage rejuvenation: A narrative review. Int J Environ Res Public Health. 2022;19:9055.
- [CrossRef] [PubMed] [Google Scholar]
- Skin measurement devices to assess skin quality: A systematic review on reliability and validity. Skin Res Technol. 2022;28:212-24.
- [CrossRef] [PubMed] [Google Scholar]







