Translate this page into:
Non-invasive system delivering microwaves energy for localized adiposity reduction, skin laxity, and cellulite: Clinical evidence
*Corresponding author: Irene Fusco, El.En. Group, Calenzano, Italy. i.fusco@deka.it
-
Received: ,
Accepted: ,
How to cite this article: Arrigoni F, Fusco I, Zingoni T, Massirone A. Non-invasive system delivering microwaves energy for localized adiposity reduction, skin laxity, and cellulite: Clinical evidence. J Cutan Aesthet Surg. doi: 10.25259/JCAS_7_2024
Abstract
Localized adiposities are the accumulation of subcutaneous adipose tissue resulting in an alteration of the body silhouette. This study evaluates the efficacy and safety of a non-invasive microwaves (MWs) device for the treatment of localized adiposities of the thighs and abdomen. Sixteen women enrolled underwent four sessions with the study device at intervals of 30 days. Clinical evaluation, anthropometric evaluation, ultrasound evaluations, and 3D photographs were performed. Cellulite and skin laxity were evaluated. Patients filled 4-points Global Aesthetic Improvement Scale (GAIS) and 10-points Visual Analog Scale. The mean abdominal circumference significantly (P <0.05) decreased 4 weeks after the last treatment, and the mean culotte/thighs circumference significantly (P < 0.05) decreased 4 weeks after the last treatment. Both cellulite and skin laxity were improved. The GAIS scores showed satisfactory results. No adverse effects were observed. This MWs system has been found to be safe and effective in the reduction of body circumferences.
Keywords
Microwaves system
Localized adiposities management
Cellulite
Skin laxity
INTRODUCTION
A recently, among the panorama of new technologies, microwaves (MWs) have shown a promising role in body remodelling.1-5 A non-invasive procedure can improve the abdominal appearance, reducing subcutaneous adipose tissue, maintaining and/or improving skin tightening, and avoiding adverse reactions and the requirement to prolong the patient’s recovery time. Based on these scientific findings, this study provided information to assess the effectiveness and safety of a non-invasive, localized MWs device for the treatment of localized adiposities in the thighs and abdomen.
SUBJECTS AND METHODS
In this study, we presented the clinical findings from a group of 16 female patients treated with the ONDA system (DEKA M.E.L.A, Florence, Italy): 10/16 patients display an android fat distribution, and 7/16 patients display a gynoid fat distribution. The mean age was 40.12 (± 8.25), range between 26 and 49 years, the mean weight was 63.73 (± 11.61) kg, the mean body mass index was 24.12 (± 2.67) kg/m2, the mean height was 162.3 (± 7.97) cm, the mean body fat was 27.10 (± 6.39) %, the mean water was 46.63 (± 5.13) %, the mean muscles was 32.22 (± 3.68) %, and the mean bones was 2.83 (± 0.46) kg. These body parameters were measured with the use of an impedance meter weighing scale (Beurer – BF 105). All patients completed all scheduled visits.
Before starting the treatment, all the patients read the treatment information and filled out informed consent and photographic release form.
Each patient was subjected to four treatment sessions with the ONDA system (DEKA, M.E.L.A Florence, Italy) at intervals of 30 days (1 session every 4 weeks for 4 months) on the abdominal and thigh’s areas. A total of ten abdomens and seven culotte/thighs were treated.
The area to be treated was divided into two adjacent squares 15 × 15 cm2. The power ranged between 130 and 190 Watts. The typical treatment time for each square was approximately 15 min.
The evaluation timing was as follows: T0 – study start (0 sessions), Tm – mid-study (2 sessions – before 3rd session), and Tf – 4 weeks from the end of the study.
All the patients filled out an anamnestic questionnaire and underwent a medical interview.
Clinical evaluation, anthropometric evaluation, and 3D photographs were performed before the first treatment, before the third treatment, and 4 weeks after the last treatment. 3D photographs assessment was performed with QuantifiCare LifeViz® Infinity 3D photographic system.
Three randomized patients also underwent ultrasound evaluations before the first treatment, before the third treatment, and after 4 weeks after the last treatment. For Ultrasound evaluation, the differential diagnosis between adiposity and Edemato-Fibro-Sclerotic Panniculopathy, the possible presence of lipomas, nodules, pre-existing fibrotic tissue, and other possible abnormalities, and tissue thickness subcutaneous adipose were considered. Body waist circumferences were measured for abdomens and culotte/thighs.
For Cellulite evaluation, the Nürnberger–Müller and Cellulite Severity Scale was selected; skin laxity changes were also observed 4 weeks after the last treatment with the following degrees: None, mild, moderate, and severe.
Pain experienced by the patient during the treatment was evaluated by the 10-point visual analogue scale (VAS). Four points Global Aesthetic Improvement Scale (GAIS) and patient satisfaction questionnaire were recorded.
All possible adverse events were monitored during 24 hours and after 4 weeks from the past treatment session.
Student’s t-test and Statistical Package for the Social Sciences 26.0 (IBM Corp., New York, NY, USA) were used to perform statistical analysis.
RESULTS
For ten patients examined, mean abdominal circumference significantly (P < 0.05) decreased from baseline 96.59 (± 5.74) cm to 94.55 (± 5.03) cm, 4 weeks after the last treatment (Tf). For seven patients, the mean culotte/thighs circumference significantly (P < 0.05) decreased from baseline 98.35 (± 4.78) cm to 96.59 (± 3.64) cm, 4 weeks after the last treatment (Tf). All measurements are reported in Table 1.
| Treated area | T0 (mean±SD) | Tm (mean±SD) | Tf (mean±SD) | P-value T0 vs. Tf | |
|---|---|---|---|---|---|
| Circumference (cm) | Abdomen | 96.59±5.74 | 95.70±5.69 | 94.55±5.03 | P<0.05 |
| Culotte/thighs | 98.35±4.78 | 96.52±4.81 | 96.59±3.64 | P<0.05 |
SD: Standard deviation
The ultrasound investigation of three patients revealed a significantly (P < 0.05) reduction in the mean thickness values of the abdominal subcutaneous adipose tissue as reported in Table 2.
| Side | T0(mean±SD) | Tf(mean±SD) | P-value T0 vsTf | |
|---|---|---|---|---|
| Subcutaneous adipose tissue (cm) | Left | 1.32±0.03 | 0.85±0.55 | P<0.05 |
| Right | 1.23±0.62 | 0.92±0.56 | P<0.05 |
SD: Standard deviation, T0: baseline, Tf: 4 weeks from the end of the study
Cellulite in the culotte areas was improved, as shown in Tables 3 and 4, Figures 1 and 2. Furthermore, skin laxity for all patient’s abdomen and culotte areas was improved, as shown in Table 5 and Figure 3. The GAIS scores showed satisfactory results: 6/16 patients were slightly improved, 4/16 patients were improved, and 6/16 patients were slightly improved. The mean value of pain assessed by the 10-point VAS experienced by the patients was 3.47 (±1.28). From the satisfaction questionnaire, all the patients recommend the treatment to a friend. No adverse effects were observed. 3D photograph evaluation reports optimal esthetic results, as shown in Figures 4-8.
| Nürnberger–Müller classification (grade 0-3) | ||||
|---|---|---|---|---|
| % of patients | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
| T0 (%) | 0 | 42.85 | 28.57 | 28.57 |
| Tf (%) | 57.14 | 28.57 | 14.28 | 0 |
T0: baseline, Tf: 4 weeks from the end of the study
| CSS index | ||||
|---|---|---|---|---|
| % of patients | None | Mild | Moderate | Severe |
| T0 (%) | 0 | 71.42 | 28.57 | 0 |
| Tf (%) | 42.85 | 42.85 | 14.28 | 0 |
CSS: Cellulite severity scale, T0: baseline, Tf: 4 weeks from the end of the study
| Skin Laxity | ||||
|---|---|---|---|---|
| % of patients | None | Mild | Moderate | Severe |
| T0 (%) | 0 | 47.05 | 47.05 | 5.88 |
| Tf (%) | 41.17 | 47.05 | 11.76 | 0 |
T0: baseline, Tf: 4 weeks from the end of the study
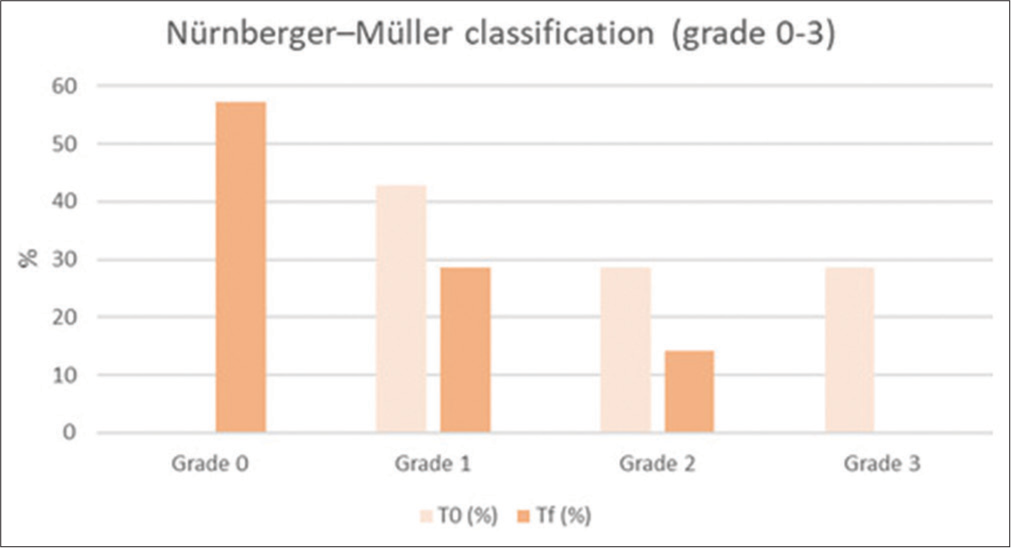
- Histogram representation of Nürnberger–Müller classification results. T0: baseline, Tf: 4 weeks from the end of the study
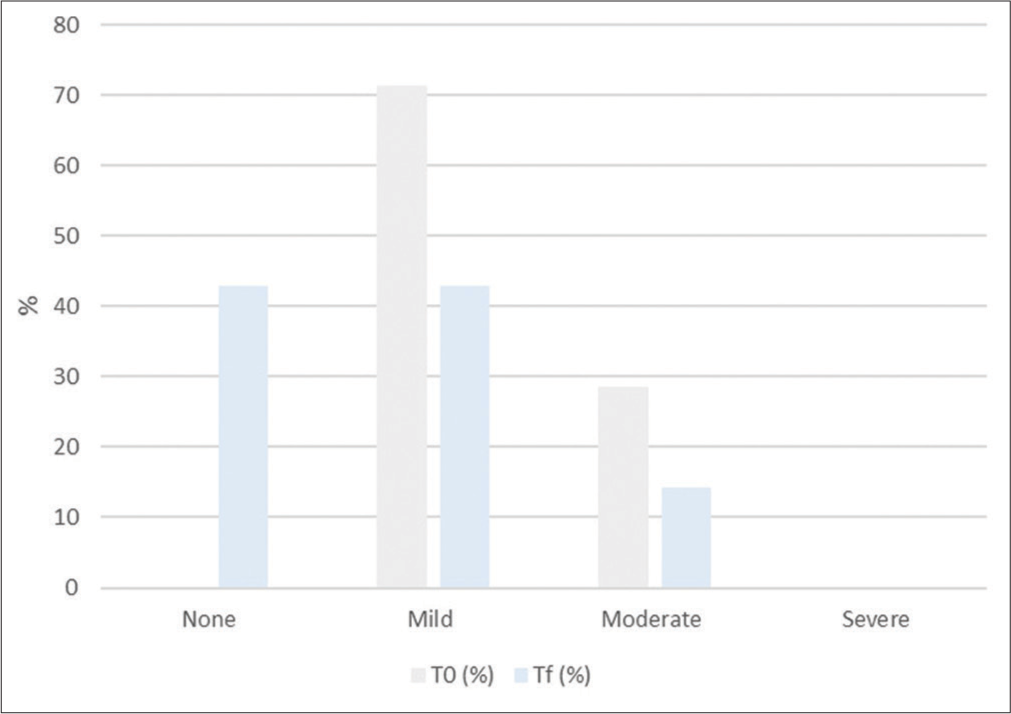
- Histogram representation of Cellulite Severity Scale results. T0: baseline, Tf: 4 weeks from the end of the study.
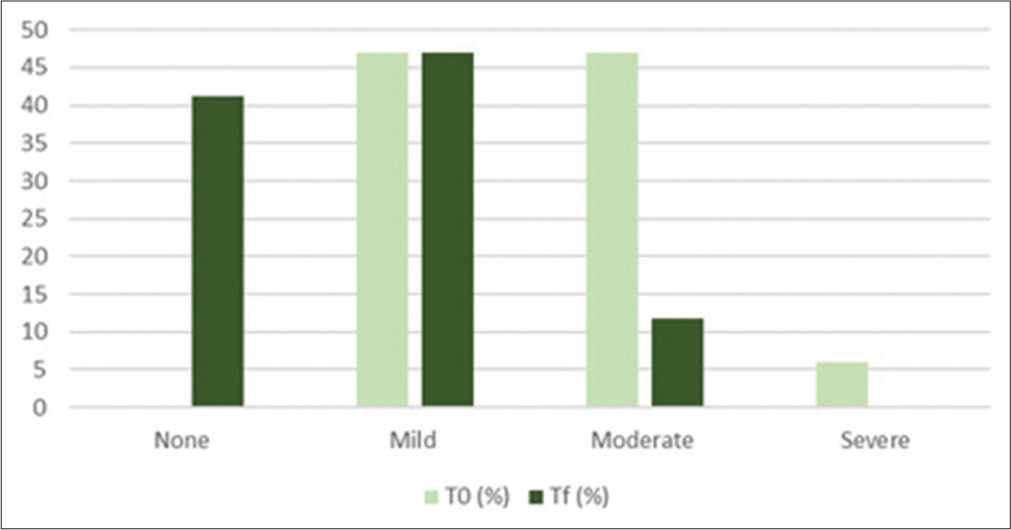
- Histogram representation of skin laxity results. T0: baseline, Tf: 4 weeks from the end of the study.
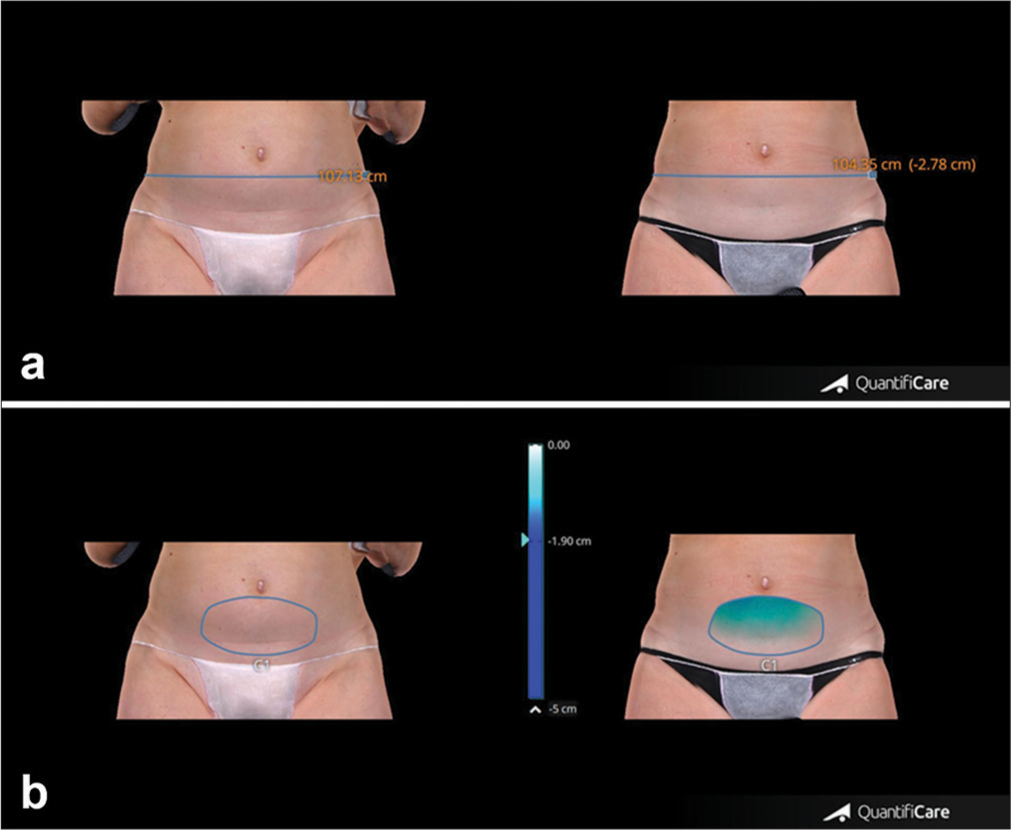
- (a) Abdominal circumference measurements of patient at baseline and after 4 weeks from the last treatment session. (b) Abdominal volume change of the same patient at baseline and after 4 weeks from the last treatment session. Blue line: Thigh circumference, Blue circle: Thigh volume.
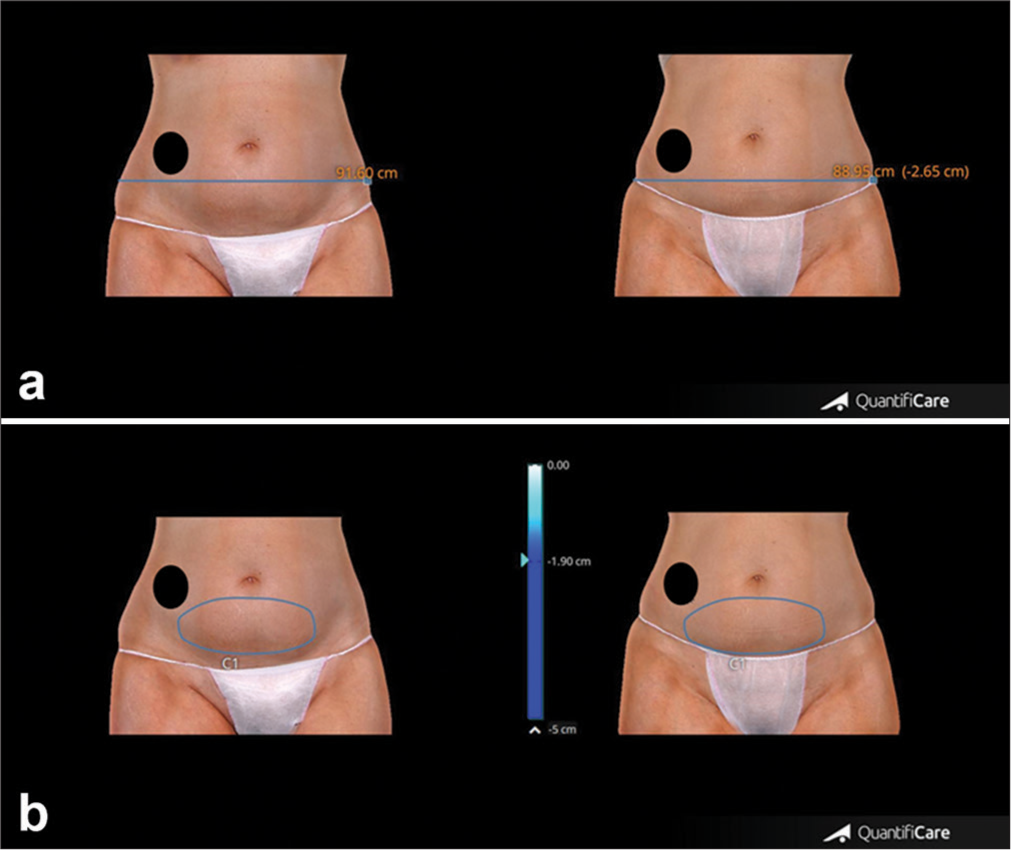
- (a) Upper panel. Abdominal representative case showing circumference reduction, clinical improvement, and morphological changes of abdominal appearance before and after 4 weeks from the last treatment session. (b) Lower panel. Change of abdominal volume in the same patient before and after 4 weeks from the last treatment session. A black circle was added to the image to cover the patient’s tattoo for privacy protection. Blue line: Thigh circumference, Blue circle: Thigh volume.
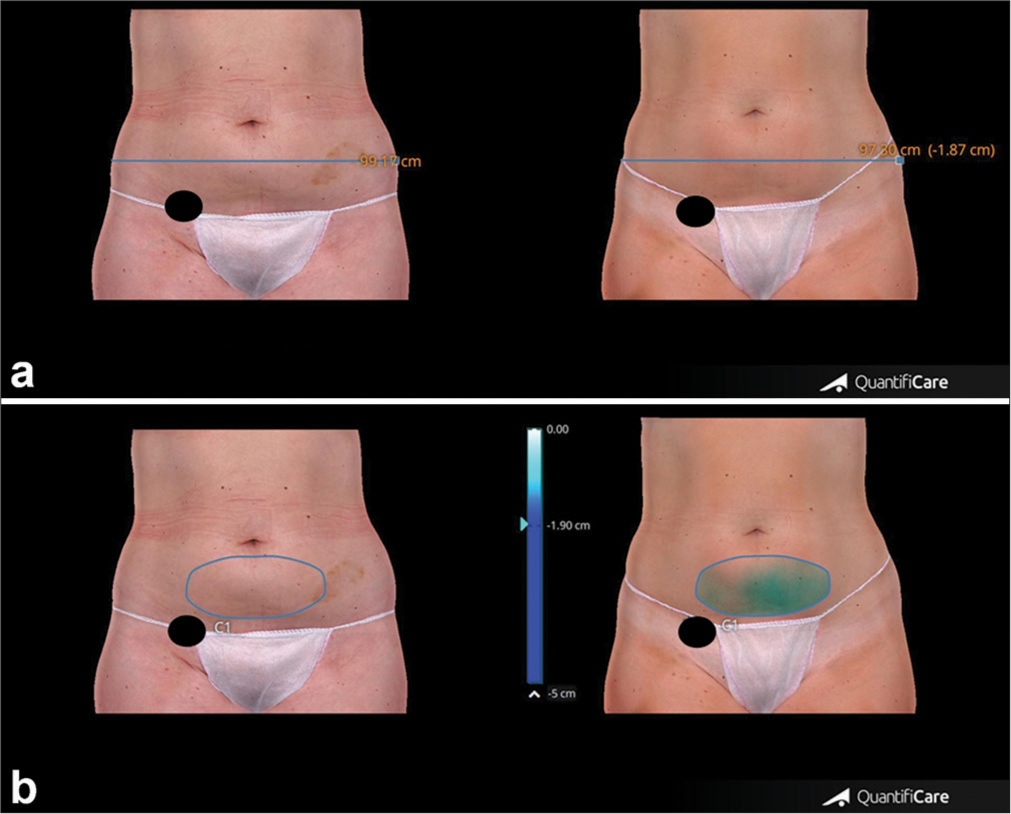
- (a) Measurements of abdomen circumference reduction were taken before and after 4 weeks from the last treatment session. (b) Changes in abdominal volume measured before and after 4 weeks from the last treatment session. The black circle covers the patient’s tattoo for privacy protection. Blue line: Thigh circumference, Blue circle: Thigh volume.
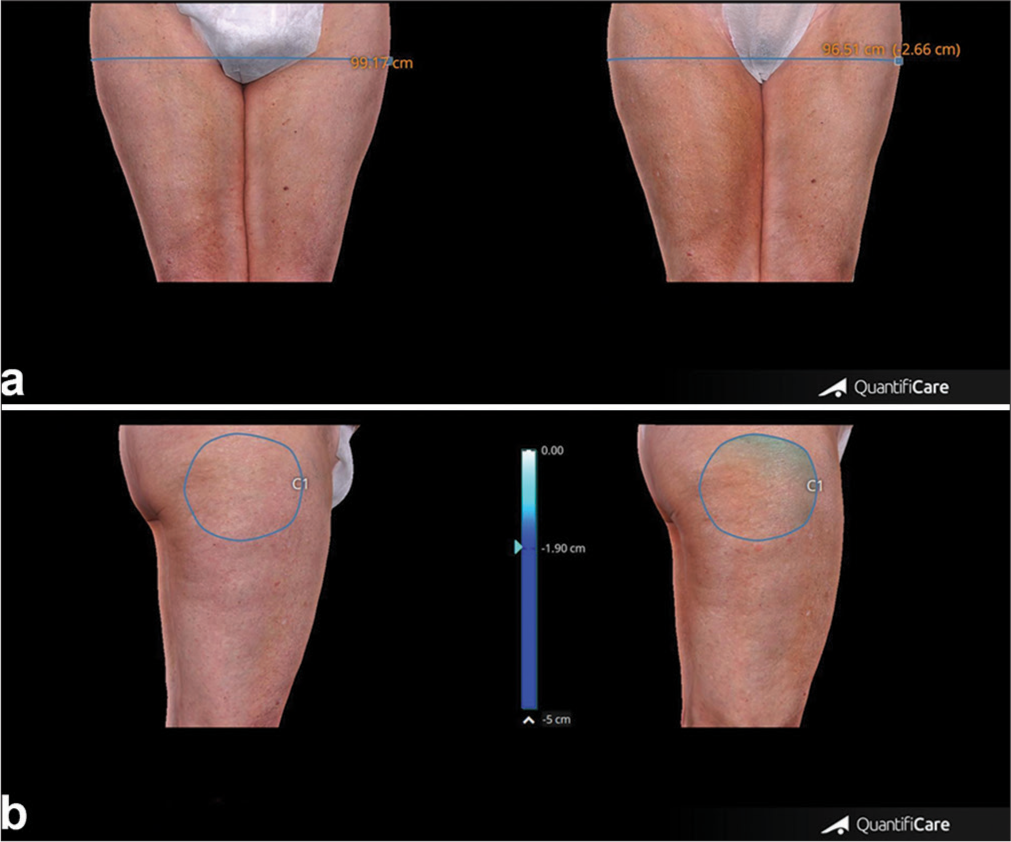
- (a) Thigh circumference measures before and after 4 weeks from the last treatment session. (b) Thigh volume measures taken before and after 4 weeks from the last treatment session. Blue line: Thigh circumference, Blue circle: Thigh volume.
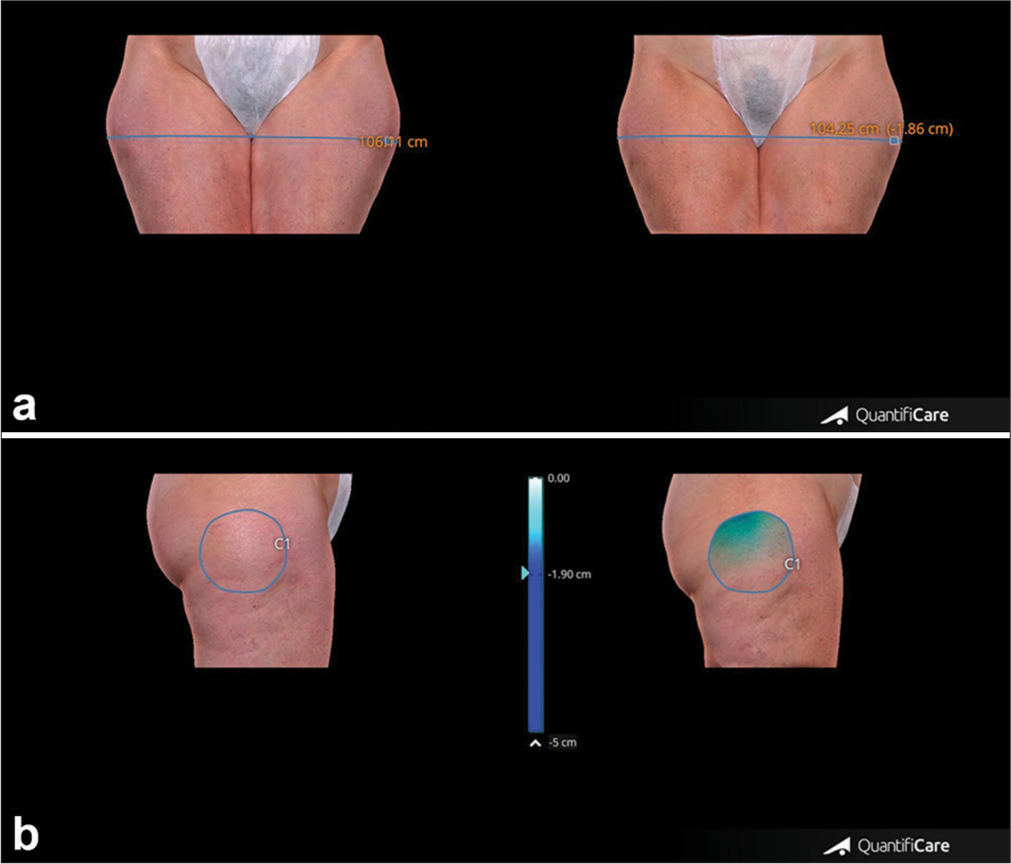
- (a) Upper panel. Representative case showing circumference reduction, clinical improvement and morphological changes of thighs appearance before and after 4 weeks from the last treatment session. (b) Lower panel. Change of thigh volume in the same patient before and after 4 weeks from the last treatment session. Blue line: Thigh circumference, Blue circle: Thigh volume.
DISCUSSION
Our findings demonstrated a significant reduction in abdominal and thigh circumferences and volume associated with the removal of subcutaneous fat in that region, as well as a restoration of the skin collagen’s normal elastic tension, which improved skin laxity and cellulite in treated patients. Along with being extremely tolerable and having no complications, the non-invasive technique used in this study may provide a fresh, intriguing alternative to conventional lipoplasty.
All patients indicated that they recommend the same treatment and patient satisfaction rates matched clinical improvement levels.
LEARNING POINTS
The microwaves device leads to a significant reduction in abdominal and thigh circumferences.
The study device leads to a significant reduction of volume associated with the removal of subcutaneous fat in that region.
A restoration of the skin collagen’s normal elastic tension and an improvement of skin laxity and cellulite were observed.
No complications or side effects were detected.
The non-invasive technique used in this study may provide an alternative approach to conventional lipoplasty.
CONCLUSION
The study outcomes are expected to suggest that MWs technology can be used in a safe and effective manner for non-invasive body shaping treatments.
Authors’ contributions
Concepts: Francesca Arrigoni, Tiziano Zingoni, Alberto Massirone; Design: Francesca Arrigoni, Tiziano Zingoni, Alberto Massirone; Definition of intellectual content: Francesca Arrigoni, Tiziano Zingoni, Alberto Massirone; Literature search: Irene Fusco; Clinical studies: Francesca Arrigoni, Tiziano Zingoni, Alberto Massirone; Experimental studies: Francesca Arrigoni, Alberto Massirone; Data acquisition: Francesca Arrigoni, Tiziano Zingoni, Alberto Massirone; Data analysis: Francesca Arrigoni, Tiziano Zingoni, Alberto Massirone; Statistical analysis: Francesca Arrigoni, Alberto Massirone; Manuscript preparation: Francesca Arrigoni, Irene Fusco; Manuscript editing: Francesca Arrigoni, Irene Fusco, Tiziano Zingoni, Alberto Massirone; Manuscript review: Francesca Arrigoni, Irene Fusco, Tiziano Zingoni, Alberto Massirone; Guarantor: Tiziano Zingoni, Alberto Massirone.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study as the Onda device is already a CE-marked device since 2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Irene Fusco and Tiziano Zingoni are employed at El.En. Group.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Microwave therapy for cellulite: An effective non-invasive treatment. J Clin Med. 2022;11:515.
- [CrossRef] [Google Scholar]
- Combined microwaves and fractional microablative CO2 laser treatment for postpartum abdominal laxity. J Cosmet Dermatol. 2021;20:124-31.
- [CrossRef] [Google Scholar]
- A new protocol to treat abdominal subcutaneous fat combining microwaves and flat magnetic stimulation. Bioengineering. 2022;9:182.
- [CrossRef] [Google Scholar]
- Non-invasive system delivering microwaves energy for unwanted fat reduction and submental skin tightening: Clinical evidence. J Cosmet Dermatol. 2022;21:5657-64.
- [CrossRef] [Google Scholar]
- Effects of microwave technology on the subcutaneous abdominal fat and anthropometric indices of overweight adults: A clinical trial. J Cosmet Dermatol. 2021;21:1482-8.
- [CrossRef] [Google Scholar]






